Abstract
Purpose
In view of the genetic risks for the next generation, the importance of careful evaluation of karyotypes and AZF microdeletions in male infertility prior to assisted reproduction is evident. In the present study, it is aimed to investigate the frequency and types of both major chromosomal abnormalities by using standard cytogenetic methods and Y chromosome microdeletions of infertile males with azoospermia and oligozoospermia to give appropriate genetic counseling before assisted reproduction techniques in southeast Turkey.
Methods
A total of 80 infertile males (52 were azoospermic, 25 oligospermic and 3 asthenospermic) were studied for the cytogenetic evaluation and molecular AZF screening program prior to use of assisted reproduction techniques. A detailed history was taken for each man. Karyotyping was performed on peripheral blood lymphocytes according to standard methods. Polymerase chain reaction (PCR) amplification by using 15 Y-specific sequence-tagged sites of AZF region was performed to screen the microdeletions in the AZF region of Y chromosome.
Results
Of 80 cases, 71 had normal karyotype (46,XY). The total prevalence of chromosomal abnormalities was found to be 11.2% (9/80), including seven patients with Klinefelter syndromes and two patients with balanced autosomal rearrangements. All of the patients with Klinefelter Syndrome had azoospermia, but carriers with translocation had oligospermia. The deletions of Y chromosome were seen in one patient (1.3%) with features of normal karyotype and azoospermia. Microdeletions were seen in the AZFc and AZFd regions. Neither AZFa nor AZFb microdeletions were detected.
Conclusions
The occurrence of chromosomal anomalies and Y chromosome microdeletions among infertile males strongly suggests the need for routine genetic testing and counseling prior to employment of assisted reproduction techniques.
Keywords: Infertility, Chromosome, AZF, Azoospermia, Oligozoospermia
Introduction
Infertility is best defined as the inability to conceive after one year of unprotected intercourse [1]. There are many cases of infertility, and this problem affects approximately 10–15% of all married couples attempting pregnancy in the general population, and male factor is responsible for approximately 40–50% of the cases [2–8]. Male infertility can be caused by a variety of factors, such as infection, varicose, endocrine disorders, spermatic duct obstruction, antisperm antibodies, cryptorchidism, retrograde ejaculation, systemic diseases, testicular cancer, testicular trauma, etc. Apart from these, in 30–40% of male infertile cases that are referred to as idiopathic, genetic abnormality is suspected [4–9].
Male infertility with genetic factors might be the result of carriers of chromosomal abnormality and/or Y chromosomal microdeletions within the Yq11 region, where the genes that control spermatogenesis, known as AZF, are located [1–4, 7–11]. The incidence of cytogenetic abnormality has been estimated as 2.1 to 28.4% in infertile men and only 0.7–1% in the general male population [5, 8, 12–14]. Somatic chromosomal anomalies in the infertile male may be numerical or structural and involve sex chromosomes (e.g., 47,XXY) or autosomes (e.g., balanced Robertsonian translocations) [3–5, 8, 12–14]. Approximately 5–10% of oligozoospermic cases and 15–20% of azoospermic cases harbor genetic abnormalities [9].
After the Klinefelter syndrome, Y chromosomal microdeletions are the most frequent genetic cause of male infertility [2, 10, 13]. Analysis of these deletions demonstrates in the AZF region four nonoverlaping loci, AZFa, AZFb, AZFc and AZFd, localized between AZFb and AZFc [2, 3, 6, 8–11, 15–17]. Deletion of these loci results in spermatogenic arrest and is associated with azoospermia and oligozoospermia [2, 3, 6, 8–11, 15–17]. These AZF genes code for RNA binding proteins and may be involved in regulation of gene expression, RNA metabolism, packaging and transport to cytoplasm, and RNA splicing [7, 10, 15, 17].
In recent years, the introduction of testicular sperm extraction (TESE) and intracytoplasmic sperm injection (ICSI) into the treatment of male factor infertility has permitted the use of sperm from oligospermic or azoospermic patients to achieve successful fertilization and pregnancies [2, 4–6, 8, 10, 11, 13, 16–19]. However, this procedure might have a potential risk of transmitting genetic abnormalities to the offspring, since the natural selection process of sperm cells is bypassed [5–8, 11, 13, 17]. In view of the genetic risks for the next generation, the importance of careful evaluation of karyotypes and AZF microdeletions in male infertility prior to assisted reproduction by ICSI is evident [5, 6, 17]. Therefore, in the present study, it is aimed to investigate the frequency and types of both major chromosomal abnormalities by using standard cytogenetic methods and Y chromosome microdeletions by using gene-based multiplex polymerase chain reaction (PCR) of infertile men with azoospermia and oligozoospermia to give appropriate genetic counseling before assisted reproduction techniques in southeastern Turkey.
Materials and methods
Patients
Eighty infertile men were selected prior to employment of assisted reproduction techniques because of severe male-factor infertility and past history of infertility for more than one year. Patients were referred to Human Genetic Department from Urology Department of the University Hospital, School of Medicine, University of Dicle. Participants in this study were informed of the nature of the study, and provided informed consent. General physical and genital examination was performed by a specialist (Dr Abdullah Gedik). All of them underwent an andrological work-up, which included medical history, physical examination, and hormonal estimation. Serum concentrations of follicle stimulating hormone (FSH), luteinizing hormone (LH), prolactin (PRL) and testosterone were measured by electrochemiluminescence immunoassays (ECLIA), using Roche Elecsys 1010 (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions. Semen analyses (sperm concentration, motility, and morphology) were performed according to the World Health Organization Guidelines [20] for the diagnosis of azoospermia and oligozoospermia. Light microscopic evaluation of sperm concentration, motility, viability and morphology was performed. Specimens originally considered as azoospermic were centrifuged (1000 g for 20 min) and the pellets were examined for spermatozoa, before confirming azoospermia. The volunteers were requested to abstain from sexual activity for 48h to 7 days before the investigation. There were 52 men with non-obstructive azoospermia, 25 with oligospermia and 3 with asthenospermia. The diagnosis of non-obstructive azoospermia was based on clinical findings (small testicles plus elevated FSH). None of them had any history of childhood disease, environmental exposure, and radiation exposure or prescribed drug usage that could account for their infertility.
Somatic cytogenetic analysis
All patients were analyzed cytogenetically. The somatic karyotypes of the 80 patients were studied in cultured peripheral blood lymphocytes according to the macroculture technique described by Moorhead et al. [21]. At least, 20 metaphases per subject were analyzed. The chromosomal abnormalities were described according to the norms established by the International System for Human Cytogenetic Nomenclature [22].
Analysis of the AZF genes by PCR-sequence-tagged sites
Analysis of microdeletions in the AZF (Yq11) region was performed in these 80 clinically and cytogenetically studied patients for the presence of microdeletions in the AZF region (Yq11). Genomic DNA was extracted from peripheral blood leucocytes by standard procedures [23, 24] before being analyzed by multiplex PCR. The DNA was amplified by multiplex PCR by using 15 Y chromosome-specific STS (sequence- tagged sites according to the method described by Henegariu et al [25]. The STS primers used were sY81, sY82, sY84 (AZFa); sY127, sY142, sY164, RBM1 (AZFb); sY254, sY255, sY257, CDY, BPY2 (AZFc); and sY145, sY152, sY153 (AZFd) (Table 1). The PCR was carried out in a 25-μL reaction volume containing: 200ng genomic DNA, 1.5mmol/L MgCl2, 200μmol/L dNTP, 0.1–0.5μmol/L of each primer, PCR buffer with adjuvants (Q-solution; Qiagen, Hilden, Germany) and 1 U HotStarTaq DNA polymerase (Qiagen, Hilden, Germany). Thermocycling (Techne Cambridge Ltd, Duxford-Cambridge U.K.) were used for multiplex PCR set. Multiplex PCR sets No. 1, No. 2, No. 3 No. 4 and No. 5 were performed together under the same PCR conditions as follows: initial denaturation at 94°C for 5 min; followed by 40 cycles of denaturation at 94°C for 40 s, annealing at 62°C for 1 min, extension at 72°C for 1.5 min and a final extension at 72°C for 10 min.
Table 1.
Sequence-Tagged Sites (STS) and gene-specific primer sequences for deletion analysis
| Multiplex PCR set No | STS | Region | Primer sequence | Size (bp) |
|---|---|---|---|---|
| 1 | sY142 | AZFb | Forward 5′ AGC TTC TAT TCG AGG GCT TC-3 | 182 bp |
| Reverse 5′ CTC TCT GCA ATC CCT GAC AT-3′ | ||||
| sY164 | AZFb | Forward 5′ AAT GTG CCC ACA CAG AGT TC-3 | 590 bp | |
| Reverse 5′ TGG AAG ACC AGG ATT TCA TG-3′ | ||||
| sY277 | AZFc | Forward 5′ GGG TTT TGC CTG CAT ACG TAA TTA-3′ | 275 bp | |
| DAZ3 | Reverse 5′CCT AAA AGC AAT TCT AAA CCT CCA G-3′ | |||
| sY153 | AZFd | Forward 5′ GCA TCC TCA TTT TAT GTC CA-3′ | 135 bp | |
| Reverse 5′ CAA CCC AAA AGC ACT GAG TA-3 | ||||
| 2 | RBM1 | AZFb | Forward 5′ ATG CAC TTC AGA GAT ACG G-3′ | 800 bp |
| Reverse 5′ CCT CTC TCC ACA AAA CC A ACA-3′ | ||||
| sY254 | AZFc | Forward 5′ GGG TGT TAC CAG AAG GCA AA-3′ | 350 bp | |
| DAZ1 | Reverse 5′GAA CCG TAT CTA CCA AAG CAG C-3′ | 380 bp | ||
| sY82 | AZFa | Forward 5′ ATC CTG CCC TTC TGA ATC TC-3′ | 280 bp | |
| Reverse5′ CAG TGT CCA CTG ATG GAT GA-3′ | ||||
| sY152 | AZFd | Forward 5′ AAG ACA GTC TGC CAT GTT TCA-3′ | 120 bp | |
| Reverse 5′ ACA GGA GGG TAC TTA GCA GT-3′ | ||||
| 3 | sY127 | AZFb | Forward 5′GGC TCA CAA ACG AAA AGA AA-3′ | 282 bp |
| Reverse 5′CTG CAG GCA GTA ATA AGG GA-3′ | ||||
| sY81 | AZFa | Forward 5′ AGG CAC TGG TCA GAA TGA AG-3′ | 200 bp | |
| Reverse 5′ AAT GGA AAA TAC AGC TCC CC-3 | ||||
| sY145 | AZFd | Forward 5′ CAA CAC AAA AAC ACT CAT ATA CTC G-3′ | 125 bp | |
| Reverse 5′ TTG AGA ATA ATT GTA TGT TAC GGG-3 | ||||
| 4 | sY84 | AZFa | Forward 5′ AGA AGG GTC TGA AAG CAG GT-3′ | 295 bp |
| Reverse 5′ GCC TAC TAC CTG GAG GCT TC-3′ | ||||
| BPY2 | AZFc | Forward 5′ CAG CGT ATC ATA GAA AAT GT-3′ | 142 bp | |
| Reverse 5′ AGT ACT TTA TTT GCA GGT TCT G-3′ | ||||
| sY255 | AZFc | Forward 5′ GTT ACA GGA TTC GGC GTG AT-3′ | 120 bp | |
| DAZ2 | Reverse 5′ CTC GTC ATG TGC AGC CAC-3′ | |||
| 5 | CDY | AZFc | Forward 5′ TCA TAC AAT CCA ATT GTA CTG G-3′ | 132 bp |
| Reverse 5′ TTC TAT CCC TCG GGC TGA GCT C-3′ |
PCR products were separated on 3% agarose gel electrophoresis (Metaphor, FMC Bioproducts, Rocklands, ME, USA), stained with ethidium bromide, and visualized using gel documentation system. The deletion of one PCR fragment was confirmed in single-primer pair PCR under the same experimental conditions. This analysis was performed at least three times on microdeleted sample. In each multiplex PCR assay, one sample from healthy female was used as negative control, and healthy fertile male was used as positive control.
Patients who were diagnosed to have chromosomal abnormalities or Y chromosome microdeletions were given genetic counseling.
Statistical analysis was carried out by the Statistical Package for Social Science for Windows, version 11.0 (SPSS; Chicago, IL, USA). The unpaired t-test, Mann-Whitney U-test and Chi-squared test were used appropriately. P < 0.05 was considered significantly different.
Results
A total of 80 infertile men were studied, 52 (52/80 = 65%) of whom were azoospermic males, 25 (25/80 = 31.2%) oligozoospermic males, and 3 (3/80 = 3.8%) asthenospermic males, presenting with motility alteration. The age (mean ± SD) of infertile males was 32.79 year (range 24–45 year). There was no significant difference in the mean ages of men with oligozoospermia, azoospermia and asthenospermia (31.6 ± 3.2 and 33.1 ± 5.3 and 33.50 ± 12.02 years, respectively). The average duration of infertility was 8.85 years (range 1–28 years). None of the cases reported previous exposure to gonadotoxins, such as radiation treatment or cancer chemotherapy.
The levels of FSH and LH in azoospermic males (16.4 ± 9.2 and 10.3 ± 7.5.0 IU /L, respectively) were significantly higher than those in the oligozoospermic males (8.9 ± 8.1 and 7.5 ± 6.8, respectively, P = 0.001). However, the levels of testosterone (3.9 ± 1.52 vs. 4.1 ± 2.9 ng/mL; P = 0.749) were not significantly different.
Of 80 cases with male infertility, 71 (88.8%) had normal karyotype (46,XY) and nine cases (11.2%) had abnormal karyotype. Numerical and structural chromosomal abnormalities, which were detected in nine patients, are summarized in Table 2. Seven of the chromosomal abnormalities were gonosomal in patients with Klinefelter Syndrome and two were balanced reciprocal translocations involving autosomes. One of the translocations was 46,XY,t(3;7) (q24 ;q36) and the other was 46,XY,t(5;6) (q35 ;q21). All of those with Klinefelter Syndrome had azoospermia, but translocation carriers had oligospermia. Seven of 52 azospermic patients (13.5%) and two of 25 oligozoospermic patients (8%) had chromosomal abnormalities.
Table 2.
Cytogenetic abnormalities observed in infertile men with their Y chromosome microdeletion and semen analysis and hormone profiles and, A:azoospermia, B:oligozoospermia
| Patient number | 3 | 45 | 48 | 53 | 58 | 71 | 72 | 77 | 79 | 80 |
|---|---|---|---|---|---|---|---|---|---|---|
| Karyotyping | 47,XYY | 47,XXY | 46,XY,t(3;7) | 47,XXY | 46,XY | 46,XY,t(5;6) | 47,XXY | 47,XXY | 47,XXY | 47,XXY |
| AZF deletion | None | None | None | None | sY277 sY153 | None | None | None | None | None |
| Semen analysis | A | A | O | A | A | O | A | A | A | A |
| Testosteron (ng/ml) | 0.59 | 0.48 | 3.14 | 0.49 | 2.26 | 2.87 | 2.41 | 0.48 | 0.78 | 5.32 |
| FSH (IU/L) | 43.49 | 44.35 | 3.15 | 37.72 | 8.77 | 72.97 | 29.75 | 55.04 | 39.61 | 23.42 |
| LH (mIU/ml) | 27.32 | 26.15 | 4.28 | 18.26 | 72.91 | 3.47 | 13.70 | 30.63 | 19.54 | 16.31 |
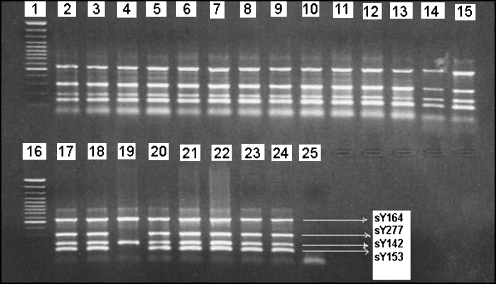
In the current study, Yq microdeletion was detected in 1 (1.9%) of 52 azoospermic cases, but not seen in other cases. Azoospermic patient Md-58 presented the loss of sY277, DAZ3 and sY153 in AZFc and AZFd regions, respectively (Fig. 1). Neither AZFa nor AZFb deletions were detected in any participant. The case with deletions had normal karyotype (46,XY). Combination of chromosomal abnormality and Y chromosome microdeletion was not seen in any case. The father and brothers of deleted patient were also investigated, and no deletions were found. It is possible to conclude that these represent de novo deletions and may be considered to be an etiological factor for the spermatogenic defect.
Fig. 1.
Results from multiplex polymerase chain reaction analysis; lanes 1 and 16 show 100-bp molecular weight markers; lanes 2–15 and 17–18 and 20–23 show undeleted samples, lane 19 show deletions of sY277 and sY153 in the patient Md-58; lane 24 show positive control (normal male); lane 25 show negative control sample of female DNA
Including Y chromosome microdeletions and chromosomal abnormality, a total genetic abnormality rate was detected in 13.5% of azoospermic cases (7/52) and 8% of oligospermic cases (2/25), but not seen among the cases of oligoastenospermic.
Cytogenetic abnormalities observed in infertile men with their semen analysis as well as Y chromosome microdeletion status are shown in Table 2.
Discussion
Among numerous etiologic factors, genetic plays a primary-key role in male infertility with abnormal semen parameters [5, 6, 9]. Spermatogenesis is regulated by a number of genes on the Y chromosome and by autosomes that act at different stages of germ cell development [9]. In the present study, the frequency of chromosomal aberrations (11.2%) exceeded the incidence of microdeletions of the Y chromosome (1.3%). While nine cases had numerical or structural chromosomal anomaly, only one case had Yq microdeletions. These findings are very similar to those reported in earlies studies [2, 13, 14].
Sex chromosome abnormalities, among which Klinefelter syndrome is the commonest, are the most frequent chromosome-related cause of hypogonadism and infertility [4, 5, 26, 27]. This abnormality is associated with severe spermatogenic failure, causing a marked reduction in testicular size and azoospermia resulting in infertility [4]. In our study, we found seven men with 47,XXY karyotype i.e., Klinefelter’s syndrome and azoospermia. It has been shown that the possibility to retrieve sperm by TESE in a proportion of XXY patients who can be candidates for ICSI and preimplantation genetic diagnosis [18, 19].
A relationship between balanced autosomal translocation and infertility has been reported among severely oligozoospermic and azoospermic men [4–6, 8]. In our study, reciprocal translocations [t(3;7) and t(5;6)] were seen in two oligozoospermic male cases. It is hypothesized that balanced translocations interfere with normal chromosome pairing and segregation at meiosis I, thus providing a potential for formation of unbalanced gametes and subsequent unbalanced abnormal offspring [4, 27]. Another hypothesis acts on the assumption of potential autosomal genes involved in male gametogenesis that might be deregulated by chromosome breakpoints. The relation between chromosomal breakpoints and male infertility has been investigated, and it has been found that there is a non-random distribution of breakpoints associated with infertility [3, 27]. Further research in this direction is necessary.
The frequency of autosomal chromosome anomalies detected in the present study was 2.5% (2/80 patients), only in the oligospermic group. These anomalies were balanced reciprocal translocations. The frequency of sex chromosome anomalies (47,XXY) was seen in the group of azoospermic patients as 8.75% (7/80 patients). These findings are compatible with those of previous studies [5, 10, 12, 13, 26–29].
In conclusion, the occurrence (11.3%) of chromosomal anomalies among infertile males strongly suggests genetic testing and counseling prior to the ICSI treatment.
The levels of FSH and LH in azoospermic males were significantly higher than those in the oligozoospermic males. However, the levels of testosterone were not significantly different. This is consistent with a large study by Vutyavanich et al. [8].
The frequency of AZF microdeletions detected in our sample was only 1.3% (1/80 patients), a value similar to that in the studies previously reported [16, 29–31]. However, our finding is lower than that in some previous studies that indicated it between 3 and 55% [2, 15, 17, 27, 31].
Yq microdeletion was detected in 1 (1.9%) of 52 azoospermic cases, but not seen in other cases. This result is similar to those of previous studies [29, 31]. It has been shown by Ferlin et al [15] that chromosome Y microdeletions are much less frequent when sperm conscentration is less than 2 millions/ml. This may be one of the reasons for the failure to detect Y microdeletions in the non-azoospermic groups. An effect of the small size and slightly unbalanced nature of our sample (azoospermic patients = 52, oligospermic patients = 25, asthenospermic = 3), ethnic differences, different patient selection criteria, methodological aspects, other additional genetic, epigenetic, nutritional and local factors can contribute to the difference between the present and previous studies. Furthermore, the type and number of markers used in the studies may have further contributed to the variation [8].
The DAZ gene family is located in the AZFc region, and it is reported to be the most frequently deleted AZF candidate gene in infertile males [1, 2, 10, 15]. The DAZ genes are encoding proteins in testicular tissue that contain RNA-binding motive, which has a regulatory role in RNA metabolism [2, 10, 15]. AZFc deletions appear to remove the DAZ gene cluster and have been associated with a variety of spermatogenic alterations, ranging from azoospermia due to Sertoli cell-only to oligozoospermia with different testicular phenotype [1, 2, 10, 15]. AZFd deletions may present with mild oligospermia or even normal sperm counts with abnormal sperm morphology such as severe teratozoospermia phenotype [2, 10]. In the present study, the azoospermic patient presented the loss of sY277 (DAZ3) and sY153 in AZFc and AZFd regions, respectively. Neither AZFa nor AZFb deletions were detected in any participant. Our result confirms the role of deletions in the AZFc region overlapping the DAZ gene family among other Yq regions in determining male infertility.
Most deletions are de novo in origin, and the transmission of these deletions to the male offspring is 100% [6]. The deletions in our study were de novo too, and were not found in the father and brothers of deleted patient.
However, Y chromosome microdeletions were found only in one azoospermic patient in our population; the occurrence of Y chromosome microdeletions among infertile men strongly suggests genetic testing and counseling prior to the ICSI treatment, because studies have indicated that deletions on the long arm of the Y chromosome involving a particular and consistent segment might lead to azoospermia and sometimes to severe oligozoospermia [4].
In conclusion, the occurrence of chromosomal anomalies and Y chromosome microdeletions among infertile males strongly suggests genetic testing and counseling prior to employment of assisted reproduction techniques in southeastern Turkey.
Footnotes
Capsule In the present study, it is aimed to investigate the frequency and types of both major chromosomal abnormalities by using standard cytogenetic methods and Y chromosome microdeletions of infertile males with azoospermia and oligozoospermia to give appropriate genetic counseling before assisted reproduction techniques in southeast Turkey.
References
- 1.Hopps CV, Mielnik A, Goldstein M, Palermo GD, Rosenwaks Z, Schlegel PN. Detection of sperm in men with Y chromosome microdeletions of the AZFa, AZFb and AZFc regions. Hum Reprod. 2003;18(8):1660–5. doi:10.1093/humrep/deg348. [DOI] [PubMed]
- 2.Kleiman SE, Yogev L, Gamzu R, Hauser R, Botchan A, Lessing JB, et al. Genetic evaluation of infertile men. Hum Reprod. 1999;4:33–8. doi:10.1093/humrep/14.1.33. [DOI] [PubMed]
- 3.Bache I, Van Assche E, Cingoz S, Bugge M, Tümer Z, Hjorth M, et al. An excess ofchromosome 1 breakpoints in male infertility. Eur J Med Genet. 2004;12(12):993–1000. [DOI] [PubMed]
- 4.Nagvenkar P, Desai K, Hinduja I, Zaveri K. Chromosomal studies in infertile men with oligozoospermia and non-obstructive azoospermia. Indian J Med Res. 2005;122:34–42. [PubMed]
- 5.Şamlı H, Solak M, İmirzalioğlu N, Şamlı MM. Genetic anomalies detected in patients with non-obstructive Azoospermia and Oligozoospermia. Med J Kocatepe. 2005;6:7–11. [DOI] [PubMed]
- 6.Hellani A, Al-Hassan S, Iqbal M, Coskun S. Y chromosome microdeletions in infertile men with idiopathic oligo-or azoospermia. J Exp Clin Assist Reprod. 2006;30,3(1):1–6. [DOI] [PMC free article] [PubMed]
- 7.Zamani AG, Kutlu R, Durakbasi-Dursun HG, Gorkemli H, Acar A. Y chromosome microdeletions in Turkish infertile men. Indian J Hum Genet. 2006;12(2):66–71.
- 8.Vutyavanich T, Piromlertamorn W, Sirirungsi W, Sirisukkasem S. Frequency of Y chromosome microdeletions and chromosomal abnormalities in infertile Thai men with oligozoospermia and azoospermia. Asian J Androl. 2007;9:68–75. doi:10.1111/j.1745-7262.2007.00239.x. [DOI] [PubMed]
- 9.Dada R, Gupta NP, Kucheria K. Cytogenetic and molecular analysis of male infertility Y chromosome deletion during nonobstructive Azoospermia and severe Oligozoospermia. Cell Biochem Biophys. 2006;171(44):171–7. doi:10.1385/CBB:44:1:171. [DOI] [PubMed]
- 10.Briton-Jones C, Haines CJ. Microdeletions on the long arm of the Y chromosome and their association with male-factor infertility. Hong Kong Med J. 2000;6:184–9. [PubMed]
- 11.Ferlin A, Arredi B, Speltra E, Cazzadore C, Selice R, Garolla A, et al. Molecular and clinical characterization of y chromosome microdeletions in infertile men: a 10-year experience in Italy. J Clin Endocrinol Metab. 2007;92(3):762–70. doi:10.1210/jc.2006-1981. [DOI] [PubMed]
- 12.Van Assche E, Bonduelle M, Tournaye H, Joris H, Verheyen G, Devroey P, et al. Cytogenetics of infertile men. Hum Reprod. 1996;11:1–26. [DOI] [PubMed]
- 13.Vicdan A, Vicdan K, Günalp S, Kence A, Akarsu C, Işık AZ, et al. Genetic aspects of human male infertility: The frequency of chromosomal abnormalities and Y chromosome microdeletions in severe male factor infertility. Eur J Obstet Gynecol Reprod Biol. 2004;117:49–54. doi:10.1016/j.ejogrb.2003.07.006. [DOI] [PubMed]
- 14.Chiang HS, Wei HJ, Chen YT. Genetic screening for patients with azospermia and severe oligo-asthenospermia. Int J Androl. 2000;23:20–5. doi:10.1046/j.1365-2605.2000.00006.x. [DOI] [PubMed]
- 15.Ferlin A, Moro E, Garolla A, Foresta C. Human male infertility and Y chromosome deletions: role of AZF-candidate genes DAZ, RBM and DFFRY. Hum Reprod. 1999;14:1710–6. doi:10.1093/humrep/14.7.1710. [DOI] [PubMed]
- 16.Bor P, Hindkjær J, Kølvraa S, Jakob H, Ingerslev HJ. Y-Chromosome microdeletions and cytogenetic findings in unselected ICSI candidates at a Danish fertility clinic. J Assist Reprod Genet. 2002;19(5):224–31. doi:10.1023/A:1015358802577. [DOI] [PMC free article] [PubMed]
- 17.SaoPedro SL, Fraietta R, Spaine D, Porto CS, Srougi M, Cedenho AP, et al. Prevalence of Y chromosome deletions in a Brazilian population of nonobstructive azoospermic and severely oligozoospermic men. Braz J Med Biol Res. 2003;36:787–93. [DOI] [PubMed]
- 18.Tournaye H, Liu J, Nagy PZ, Camus M, Goossens A, Silber S, Van Steirteghem AC, Devroey P. Correlation between testicular histology and outcome after intracytoplasmic sperm injection using testicular spermatozoa. Hum Reprod. 1996;11(1):127–32. [DOI] [PubMed]
- 19.Vernaeve V, Staessen C, Verheyen G, Van Steirteghem A, Devroey P, Tournaye H. Can biological or clinical parameters predict testicular sperm recovery in 47,XXY Klinefelter’s syndrome patients? Hum Reprod. 2004;19(5):1135–9. doi:10.1093/humrep/deh253. [DOI] [PubMed]
- 20.World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction 4th ed. Cambridge: Cambridge University Press; 1999.
- 21.Moorhead PS, Nowell PC, Mellman WJ, Battips DM, Hungerford DA. Chromosome preparations of leukocytes cultured from human peripheral blood. Exp Cell Res. 1960;20:613–6. doi:10.1016/0014-4827(60)90138-5. [DOI] [PubMed]
- 22.Mitelman F, editor. ISCN 1995-An international system for human cytogenetic nomenclature Basel: S. Karger; 1995.
- 23.Poncz M, Solowiejczky D, Harpel B, Mory Y, Schwartz E, Surrey S. Construction of human gene libraries from small amount of peripheral blood. Haemoglobin. 1982;6:27–36. doi:10.3109/03630268208996930. [DOI] [PubMed]
- 24.Miller M, Dykes DD, Polsesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;18:1215. doi:10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed]
- 25.Henegariu O, Hirschmann P, Kilian K, Kirsch S, Lengauer C, Maiwald R, et al. Rapid screening of the Y chromosome in idiopathic sterile men, diagnostic for deletions in AZF, a genetic Y factor expressed during spermatogenesis. Andrologia. 1994;26:97–106. [DOI] [PubMed]
- 26.Gündüz G, Lüleci G, Baykara M. Cytogenetic study in 102 infertile men. Urol Int. 1998;61(1):32–4. doi:10.1159/000030280. [DOI] [PubMed]
- 27.Zhou-Cun A, Yang Y, Zhang SZ, Zhang W, Lin L. Chromosomal abnormality and Y chromosome microdeletion in Chinese patients with azoospermia or severe oligozoospermia. Yi Chuan Xue Bao. 2006;33(2):111–6. [DOI] [PubMed]
- 28.Bartels I, Starke H, Argyriou L, Sauter SM, Zoll B, Liehr T. An exceptional complex chromosomal rearrangement (CCR) with eight breakpoints involving four chromosomes (1;3;9;14) in an azoospermic male with normal phenotype. Eur J Med Genet. 2006;50(2):33–8. [DOI] [PubMed]
- 29.Sargin CF, Berker-Karauzum S, Manguoglu E, Erdogru T, Karaveli S, Gulkesen KH, et al. AZF microdeletions on the Y chromosome of infertile men from Turkey. Ann Genet. 2004;47:61–8. doi:10.1016/j.anngen.2003.09.002. [DOI] [PubMed]
- 30.Qureshi SJ, Ross AR, Ma K, Cooke HJ, Intyre MA, Chandley AC, et al. Polymerase chain reaction screening for Y chromosome microdeletions: a first step towards the diagnosis of genetically determined spermatogenic failure in men. Mol Hum Reprod. 1996;2:775–9. doi:10.1093/molehr/2.10.775. [DOI] [PubMed]
- 31.Osterlund C, Segersteen E, Arver S, Pousette A. Low number of Y-chromosome deletions in infertile azoospermic men at a Swedish andrology centre. Int J Androl. 2000;23(4):225–9. doi:10.1046/j.1365-2605.2000.00234.x. [DOI] [PubMed]