Abstract
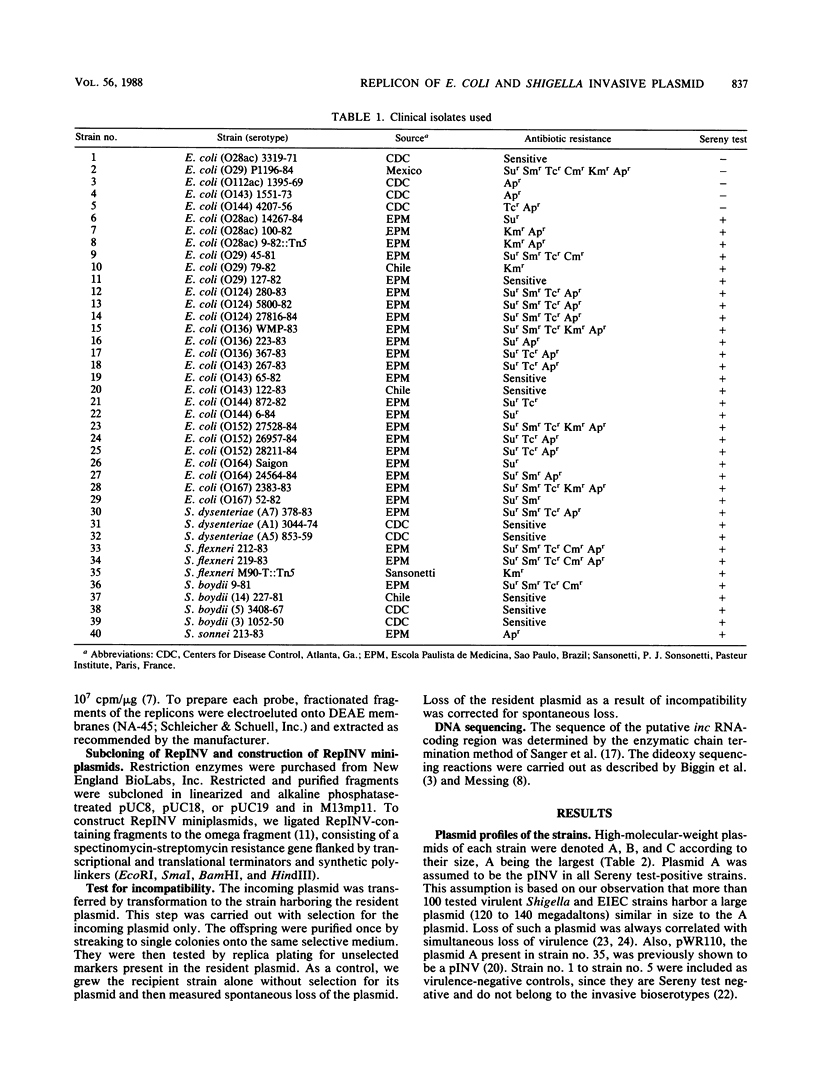
Shigella species and enteroinvasive Escherichia coli strains carry a large (120- to 140-megadalton) plasmid called pINV, which contains genes essential for the invasiveness of these pathogens. Hybridization with specific probes derived from the RepFIC and RepFIB replicons of the IncF1 Ent plasmid P307 showed that pINVs present in 35 clinical isolates are homologous with RepFIC but not RepFIB, regardless of the serogroup of the Shigella or E. coli strain. RepFIC of P307, in turn, is very similar to RepFIIA replicons of IncFII R plasmids. These and other related replicons constitute the RepFIIA family. With one pINV, pWR110, a plasmid of Shigella flexneri 5, we demonstrated the existence of a functional replicon, RepINV, with a restriction map similar to that of RepFIIA of plasmid R1. We isolated the putative inc RNA coding region of RepINV, which is a major determinant of incompatibility. The nucleotide sequence of the RepINV-inc RNA-coding region was determined and compared with the corresponding sequences of RepFIC and RepFIIA. The differences were small, but apparently were sufficient to affect the target specificity of the inc RNAs, thus rendering the replicons compatible with each other. We conclude that pINVs present in Shigella spp. and enteroinvasive E. coli constitute a homogeneous group, containing one basic replicon that belongs to the RepFIIA family of replicons.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist P. L., Saadi S., Maas W. K. Distribution of basic replicons having homology with RepFIA, RepFIB, and RepFIC among IncF group plasmids. Plasmid. 1986 Jan;15(1):19–34. doi: 10.1016/0147-619x(86)90011-9. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORMAL S. B., LABREC E. H., KENT T. H., FALKOW S. ABORTIVE INTESTINAL INFECTION WITH AN ESCHERICHIA COLI-SHIGELLA FLEXNERI HYBRID STRAIN. J Bacteriol. 1965 May;89:1374–1382. doi: 10.1128/jb.89.5.1374-1382.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal S. B., Gemski P., Baron L. S., Labrec E. H. A Chromosomal Locus Which Controls the Ability of Shigella flexneri to Evoke Keratoconjunctivitis. Infect Immun. 1971 Jan;3(1):73–79. doi: 10.1128/iai.3.1.73-79.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas R., Silva R. M., Gomes T. A., Trabulsi L. R., Maas W. K. Detection of genes for heat-stable enterotoxin I in Escherichia coli strains isolated in Brazil. Infect Immun. 1985 Jul;49(1):46–51. doi: 10.1128/iai.49.1.46-51.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Nordström K., Molin S., Light J. Control of replication of bacterial plasmids: genetics, molecular biology, and physiology of the plasmid R1 system. Plasmid. 1984 Sep;12(2):71–90. doi: 10.1016/0147-619x(84)90054-4. [DOI] [PubMed] [Google Scholar]
- Picken R. N., Mazaitis A. J., Saadi S., Maas W. K. Characterization of the basic replicons of the chimeric R/Ent plasmid pCG86 and the related Ent plasmid P307. Plasmid. 1984 Jul;12(1):10–18. doi: 10.1016/0147-619x(84)90062-3. [DOI] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robinson P., Bergquist P., Lane D. Analysis of a region in plasmid R386 containing two functional replicons. Plasmid. 1985 Jul;14(1):28–36. doi: 10.1016/0147-619x(85)90029-0. [DOI] [PubMed] [Google Scholar]
- Ryder T. B., Davidson D. B., Rosen J. I., Ohtsubo E., Ohtsubo H. Analysis of plasmid genome evolution based on nucleotide-sequence comparison of two related plasmids of Escherichia coli. Gene. 1982 Mar;17(3):299–310. doi: 10.1016/0378-1119(82)90146-9. [DOI] [PubMed] [Google Scholar]
- SERENY B. Experimental keratoconjunctivitis shigellosa. Acta Microbiol Acad Sci Hung. 1957;4(4):367–376. [PubMed] [Google Scholar]
- Saadi S., Maas W. K., Bergquist P. L. RepFIC, a basic replicon of IncFI plasmids that has homology with a basic replicon of IncFII plasmids. Plasmid. 1984 Jul;12(1):61–64. doi: 10.1016/0147-619x(84)90067-2. [DOI] [PubMed] [Google Scholar]
- Saadi S., Maas W. K., Hill D. F., Bergquist P. L. Nucleotide sequence analysis of RepFIC, a basic replicon present in IncFI plasmids P307 and F, and its relation to the RepA replicon of IncFII plasmids. J Bacteriol. 1987 May;169(5):1836–1846. doi: 10.1128/jb.169.5.1836-1846.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Hale T. L., Dammin G. J., Kapfer C., Collins H. H., Jr, Formal S. B. Alterations in the pathogenicity of Escherichia coli K-12 after transfer of plasmid and chromosomal genes from Shigella flexneri. Infect Immun. 1983 Mar;39(3):1392–1402. doi: 10.1128/iai.39.3.1392-1402.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Shigella sonnei plasmids: evidence that a large plasmid is necessary for virulence. Infect Immun. 1981 Oct;34(1):75–83. doi: 10.1128/iai.34.1.75-83.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva R. M., Toledo M. R., Trabulsi L. R. Biochemical and cultural characteristics of invasive Escherichia coli. J Clin Microbiol. 1980 May;11(5):441–444. doi: 10.1128/jcm.11.5.441-444.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva R. M., Toledo M. R., Trabulsi L. R. Correlation of invasiveness with plasmid in enteroinvasive strains of Escherichia coli. J Infect Dis. 1982 Nov;146(5):706–706. doi: 10.1093/infdis/146.5.706. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Weber P. C., Palchaudhuri S. Incompatibility repressor in a RepA-like replicon of the IncFI plasmid ColV2-K94. J Bacteriol. 1986 Jun;166(3):1106–1112. doi: 10.1128/jb.166.3.1106-1112.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womble D. D., Dong X., Wu R. P., Luckow V. A., Martinez A. F., Rownd R. H. IncFII plasmid incompatibility product and its target are both RNA transcripts. J Bacteriol. 1984 Oct;160(1):28–35. doi: 10.1128/jb.160.1.28-35.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]




