Abstract
Cyclic AMP stimulates hepatic bile acid uptake by translocating sodium-taurocholate (TC) cotransporting polypeptide (Ntcp) from an endosomal compartment to the plasma membrane. Rab4 is associated with early endosomes and involved in vesicular trafficking. This study was designed to determine the role of Rab4 in cAMP-induced TC uptake and Ntcp translocation. HuH-Ntcp cells transiently transfected with empty vector, GTP locked dominant active Rab4, Rab4(GTP), or GDP locked dominant inactive Rab4, Rab4(GDP) were used to study the role of Rab4. Neither Rab4(GTP) nor Rab4(GDP) affected either basal TC uptake or plasma membrane Ntcp level. However, cAMP-induced increases in TC uptake and Ntcp translocation were enhanced by Rab4(GTP) and inhibited by Rab4(GDP). In addition, cAMP increased GTP binding to endogenous Rab4 in a time-dependent, but phosphoinositide-3-kinase (PI3K)-independent manner. Taken together, these results suggest that cAMP-mediated PI3K-independent activation of Rab4 facilitates Ntcp translocation in HuH-Ntcp cells.
Keywords: HuH-Ntcp cells, Rab4 mutants, LY294002, PI3K, GTP overlay assay
INTRODUCTION
Cyclic AMP rapidly stimulates hepatic taurocholate (TC) uptake by translocating Ntcp from an endosomal pool to the sinusoidal membrane (1). Previous studies indicate that the effect of cAMP on Ntcp is mediated via the PI3K signaling pathway and involves PI3K/PKB and PI3K/PKCζ pathways (2). Similar signaling pathways have also been suggested for insulin-induced rapid translocation of GLUT4 to plasma membrane in myocytes and adipocytes (3, 4). It is now evident that rapid translocation of transporters involves recruitment of transporter containing vesicles to the plasma membrane. A number of proteins, including Rab proteins, have been suggested to play an important role in this process.
Rab proteins, which belong to the Ras GTPase superfamily and consist of over 60 members (5), are involved in endocytosis, protein trafficking and apically directed transcytosis in hepatocytes (6–8). Rab proteins cycle between GDP-bound inactive form and a GTP-bound active form. The general mechanism by which Rab proteins stimulate vesicular transport involves the binding of these proteins to the donor membrane (on target vesicle), attachment of the Rab-associated vesicle to cytoskeletal motor proteins through various effectors molecules, and docking and fusion of the vesicle to the acceptor membrane (plasma membrane) through the SNARE complex (9, 10). This process can also occur in reverse, allowing transport of proteins from the plasma membrane to the intracellular compartment.
Rab proteins have been shown to regulate a number of transporters. Thus, insulin-mediated GLUT4 translocation is facilitated by Rab proteins, specifically Rab4, Rab5, and Rab11 (11, 12). Rab4 has been reported to regulate CFTR function in HT-29 cells (13) and amiloride-sensitive sodium channel in colonic epithelia (14). In hepatocytes, Rab11 has been proposed to be involved in the translocation of ABC transporters to the apical membrane, and Rab4 plays a role in endocytosis and vesicle fusion in hepatocytes (15, 16). However, whether Rab proteins are involved in cAMP-mediated Ntcp translocation is not known.
Cyclic AMP is known to stimulate vesicular trafficking in hepatocytes (16, 17). Rab4 has been found to colocalize with Ntcp vesicles in rat hepatocytes (18). Since Rab4 is involved in endosomal trafficking and receptor recycling (19, 20), it is possible that cAMP-mediated translocation of Ntcp involves Rab4. The aim of the present study was to investigate whether Rab4 facilitates/mediates cAMP-mediated Ntcp translocation. Results of our studies using dominant active and dominant inactive Rab4 suggest that Rab4 facilitates cAMP-mediated Ntcp translocation.
MATERIALS AND METHODS
Materials
LipofectAMINE® reagent kit was purchased from Life Technologies/GIBCO, Carlsbad, CA. 8-Chlorophenylthio-cAMP (CPT-cAMP), TC (Na-salt), LY294002, wortmannin, aprotinin, leupeptin, okadaic acid and collagenase were obtained from Sigma Chemical Company, St. Louis, MO. [3H]TC (56 mCi/mmol), and [α-32P]GTP were purchased from Perkin-Elmer (Boston, MA). Anti-fusion protein antibodies to the cloned Ntcp were prepared as previously described (21). Rab4 antibody was obtained from BD Transduction Laboratories. Wild type (wt-Rab4) and mutant Rab4s were cloned in Clontech C1-CFP vector; Rab4(GTP) and Rab4(GDP) represent dominant active, GTP locked, GTPase deficient, Rab4Q67L and dominant inactive, GDP locked, Rab4S22N, respectively(19). HuH-Ntcp cells are HuH-7 cells, a human hepatocellular carcinoma cell line, stably transfected with rat Ntcp (22) and were generously provided by Dr. Gores (Rochester, MN). Male Wistar rats (200–300g) obtained from Charles River Laboratories (Wilmington, MA) served as liver donors.
Cell culture and Transfections
HuH-Ntcp cells were cultured in Eagle’s minimum essential medium supplemented with 10% fetal bovine serum, 100,000 units/liter penicillin, 100 mg/liter streptomycin, 1.2 g/liter G418 and 25 µg/ml amphotericin B at 37°C in a 5% CO2/95% O2 air incubator. The cells were transfected with CFP(cyan fluorescent protein)-tagged empty vector, wild type-Rab4, Rab4(GTP), and Rab4(GDP) using Lipofectamine® as described previously (23). The transfection efficiency based on cells exhibiting CFP immunofluorescence ranged from 25–48%. Immunoblot analysis for CFP was performed with anti-GFP antibody to verify protein expression of the Rab4 mutants.
TC uptake and Ntcp translocation
TC uptake in HuH-Ntcp cells was determined as previously described (23). Briefly, following pretreatment with 100 µM CPT-cAMP for 15 min, cells were incubated with 20 µM TC containing 3H-TC (100 dpm/pmol) for 2 min. Cells were then washed twice with 0.5 ml ice-cold HEPES buffer and lysed in 0.25 ml of a lysis buffer. Aliquots of cell lysate were counted for radioactivity and TC uptake rate was expressed in pmol/min/mg protein. A cell surface protein biotinylation method as previously described (23, 24) was used to quantitate Ntcp translocation in HuH-Ntcp cells. Briefly, following various treatments cell surface proteins were biotinylated by exposing HuH-Ntcp cells to sulfo-NHS-LC-Biotin (0.5mg/ml, Pierce) followed by preparation of cell lysate used to determine biotinylated and total Ntcp mass. Biotinylated proteins were isolated using streptavidin-agarose beads and then subjected to immunoblot analysis to determine plasma membrane Ntcp using rat Ntcp antibody (1:2000 dilution).
Rab4 GTP overlay assay
GTP binding ability of endogenous and mutant Rab4 was determined using a blot overlay assay for GTP binding proteins, as described by others (13, 19, 25, 26) with the exception that Rab4 was first immunoprecipitated. Briefly, Rab4s immunoprecipitated from cell lysates were subjected to SDS-PAGE. For GTP binding assays, gels were first soaked in 50 mM Tris pH 7.5–20% glycerol for one hour followed by transfer to nitrocellulose membranes. The membranes were then rinsed twice in wash buffer (50 mM Tris pH 7.6, 10 µM MgCl2, and 0.3% Tween-20) for 10 minutes at room temperature followed by incubation with an ATP overlay buffer (wash buffer + 100 mM DTT and 100 µM ATP) for 10 minutes at room temperature. Finally, the ATP overlay buffer was spiked with 1 µCi/ml of [α-32P]GTP and the membrane was incubated for 1 hour at room temperature. The membrane was washed, air-dried for 4 hours and then autoradiographed to determine GTP bound Rab4. The GTP blot was stripped and subjected to immunoblot analysis with Rab4 antibody to detect immunoprecipitated Rab4. Each assay was run in triplicate. To minimize variations due to differences in immunoprecipitated Rab4, GTP bound Rab4 was expressed as a fraction of total immunoprecipitated Rab4.
Other Methods
PKB (Akt) activity was determined from the ratio of phosphorylated PKB (active form) to total PKB as previously described (27). The Lowry method was used to determine cell protein (28). The blots were scanned in gray scale using Adobe Photoshop® (Adobe System Incorporated, San Jose, CA) and the relative band densities were quantitated using Sigmal Gel® (Jandel Scientific Software, San Rafael, CA). All values are expressed as mean ± SEM. Analysis of variance followed by Fisher’s LSD test was used to statistically analyze data, with p<0.05 considered significant.
RESULTS AND DISCUSSION
Role of Rab4 in cAMP-stimulated TC uptake and Ntcp translocation
We studied the effect of Rab4(GTP) and Rab4(GDP) to determine the role of Rab4, an approach used by others to define regulation of other transporters by Rab4 (13, 14, 29). If Rab4 is involved, Rab4(GTP) should facilitate while Rab(GDP) should inhibit cAMP-stimulated TC uptake and Ntcp translocation. To test this, HuH-Ntcp cells were transiently transfected with CFP tagged Rab4(GTP) or Rab4(GDP) followed by determination of TC uptake and Ntcp translocation in the presence and absence of cAMP.
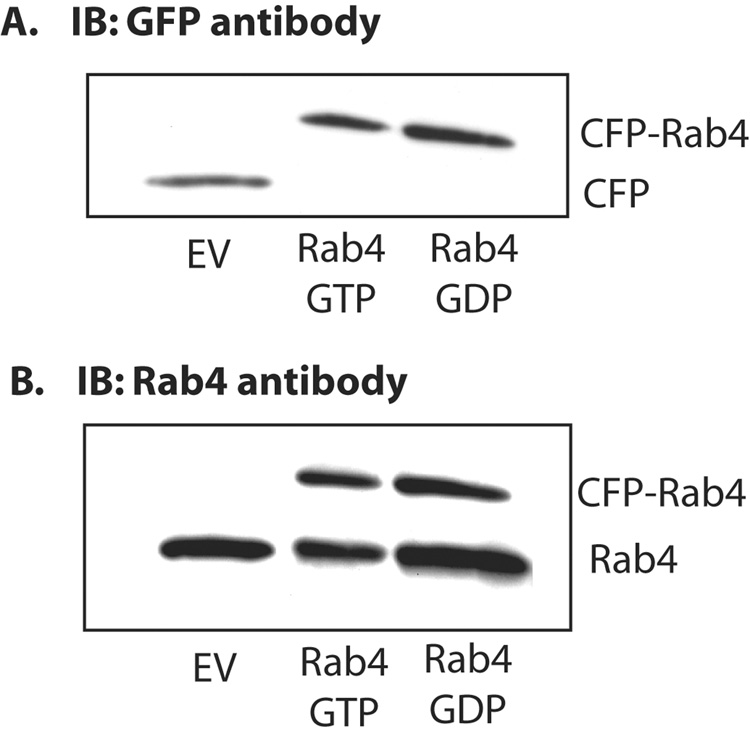
The expression of Rab4 mutants was confirmed by immunoblot analysis with GFP antibody, which detects CFP. The result showed expression of CFP tagged Rab4 in Rab4(GTP) and Rab4(GDP) transfected cells, while only CFP was detected in cells transfected with the empty vector (Fig. 1A). Immunoblot analysis with Rab4 antibody detected both endogenous and expressed CFP-tagged rab4 in cells transfected with Rab4(GTP) and Rab4(GDP), while only endogenous Rab4 was detected in cells transfected with the empty vector (Fig. 1B). The CFP tagged Rab4(GTP) and Rab4(GDP) represented 40 ± 4.5% and 35 ± 5.2% (mean ± SEM, n=4), respectively, of total Rab4 (endogenous + expressed). Thus, both Rab4 mutants were adequately expressed.
Figure 1.
Expression of Rab4(GTP) and Rab4(GDP) in HuH-Ntcp cells. Cells were transiently transfected with 0.5 µg empty vector (EV), Rab4(GTP) or Rab4(GTP) as described in “Materials and Methods” Cell lysates were subjected to immunoblotting with GFP antibody (Panel A) or Rab4 antibody (Panel B). Results show representative blots from four different experiments.
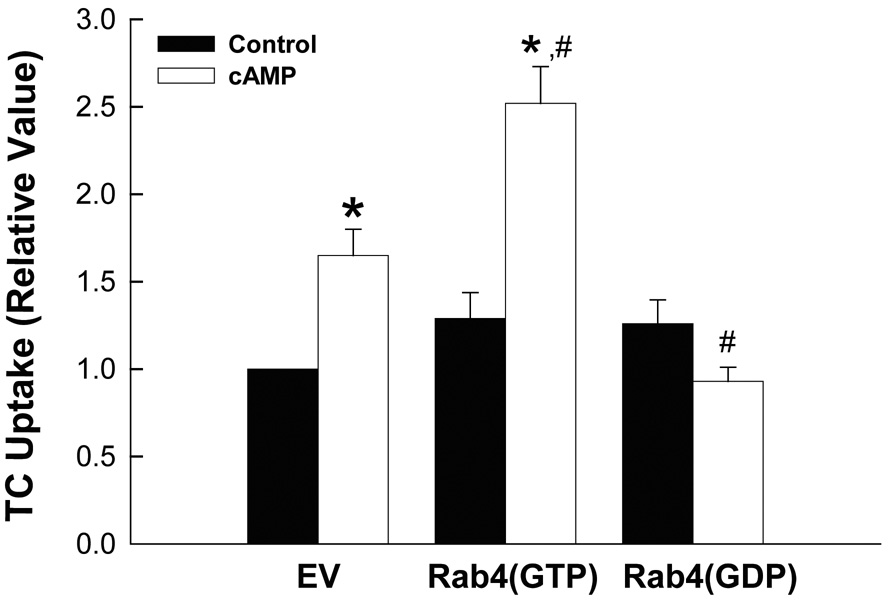
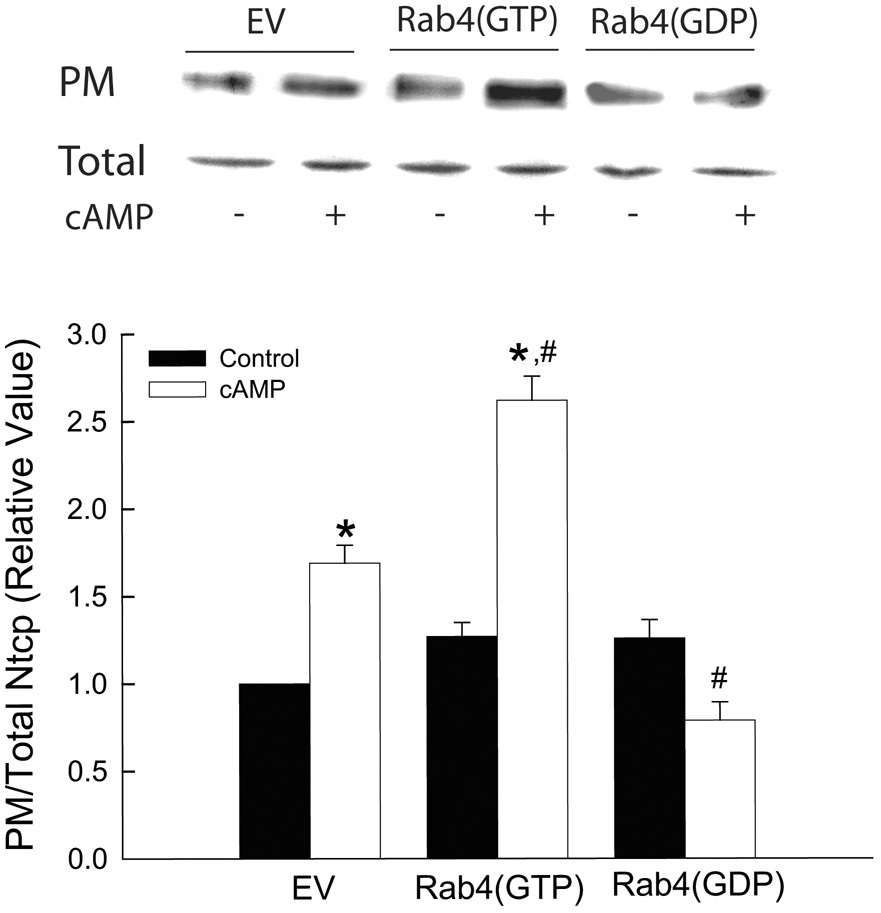
Next we determined the effect of Rab4 mutants on TC uptake and Ntcp translocation in HuH-Ntcp cells. As we previously reported (23), cAMP increased TC uptake (Fig. 2A) and Ntcp translocation (Fig. 2B) by 65% and 69%, respectively, in cells expressing the empty vector. Expression of neither Rab4(GTP) nor Rab4(GDP) affected basal level of TC uptake and plasma membrane Ntcp compared to cells expressing the empty vector. On the other hand, expression of Rab4(GTP) further augmented cAMP-induced stimulation of TC uptake and Ntcp translocation from 65% to 152% and from 69 to 162%, respectively (Fig. 2A & B). In contrast, cAMP failed to stimulate TC uptake and Ntcp translocation in cells expressing Rab4(GDP). These findings are similar to a previous study showing that expression of a dominant negative Rab4 does not affect basal, but inhibits insulin stimulated Glut4 translocation in 3T3-L1 cells (29). Since Rab4(GTP) and Rab4(GDP) do not affect basal TC uptake and Ntcp translocation, but augment and inhibit, respectively, the effect of cAMP, these results would suggest that Rab4 activation is necessary for cAMP to stimulate TC uptake and Ntcp translocation.
Figure 2.
Effect of Rab4(GTP) and Rab4(GDP) on TC uptake. HuH-Ntcp cells transiently transfected with 0.5 µg empty vector (EV), Rab4(GTP) or Rab4(GTP) were treated with 100 µM CPT-cAMP (cAMP) or buffer (control) for 15 min before determining TC uptake (A) or plasma membrane (PM) and total Ntcp (B) as described in “Materials and Methods” section. A: TC uptake values (mean ± SEM; n=4) are expressed relative to EV in the absence of cAMP. B: A typical immunoblot is shown in the upper panel and results of densitometric analysis (mean ± SEM; n=4) are shown in the lower panel. The values of PM Ntcp corrected for total Ntcp are expressed relative to EV in the absence of cAMP. *Significantly different from respective control values and #significantly different from the cAMP value in cells expressing the empty vector.
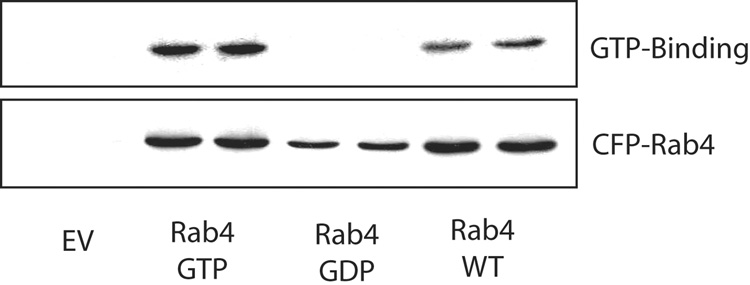
We then verified the dominant active and inactive properties of Rab4(GTP) and Rab4(GDP) by determining GTP binding using GTP overlay assay. For this study, Rab4 immunoprecipitated from cells transfected with CFP-tagged wt-Rab4, Rab4(GTP) and Rab4(GDP) was subjected to GTP overlay assay and immunoblotting with anti-Rab4 antibody (Fig. 3). While wt-Rab4 and Rab4(GTP) efficiently bound GTP, Rab4(GDP) failed to bind GTP. The Rab4 immunoblot revealed expression of all CFP-tagged Rab4 constructs. As expected, cells expressing the empty vector did not show GTP binding or expression of CFP-Rab4. These results confirmed active and inactive status of Rab4(GTP) and Rab4(GDP), respectively, as reported by others (13, 14, 19). Analysis of the extent of GTP binding (GTP binding/CFP-Rab4 expression) showed that wt Rab4 bound 50 ± 8.4 % (mean ± SEM, n=3) less GTP than Rab4(GTP). This is expected since Rab4(GTP) can only bind GTP, while wt Rab4 will be in configurations allowing binding of GTP or GDP. This result suggests that about 50% of Rab4 is in active form in HuH-Ntcp cells and that GTP overlay assay can be used to determine whether a stimulant can activate Rab4.
Figure 3.
GTP binding of wt-Rab4, Rab4(GTP) and Rab4(GDP). Cells transfected with CFP-tagged Rab4 mutants were lysed followed by GTP overlay assay and immunoblotting for CFP-Rab4 as described in “Materials and Methods”. Representative blots from three different studies showing GTP binding to and expression of CFP-tagged Rab4 in the upper and lower panel, respectively.
PI3K-independent activation of Rab4 by cAMP
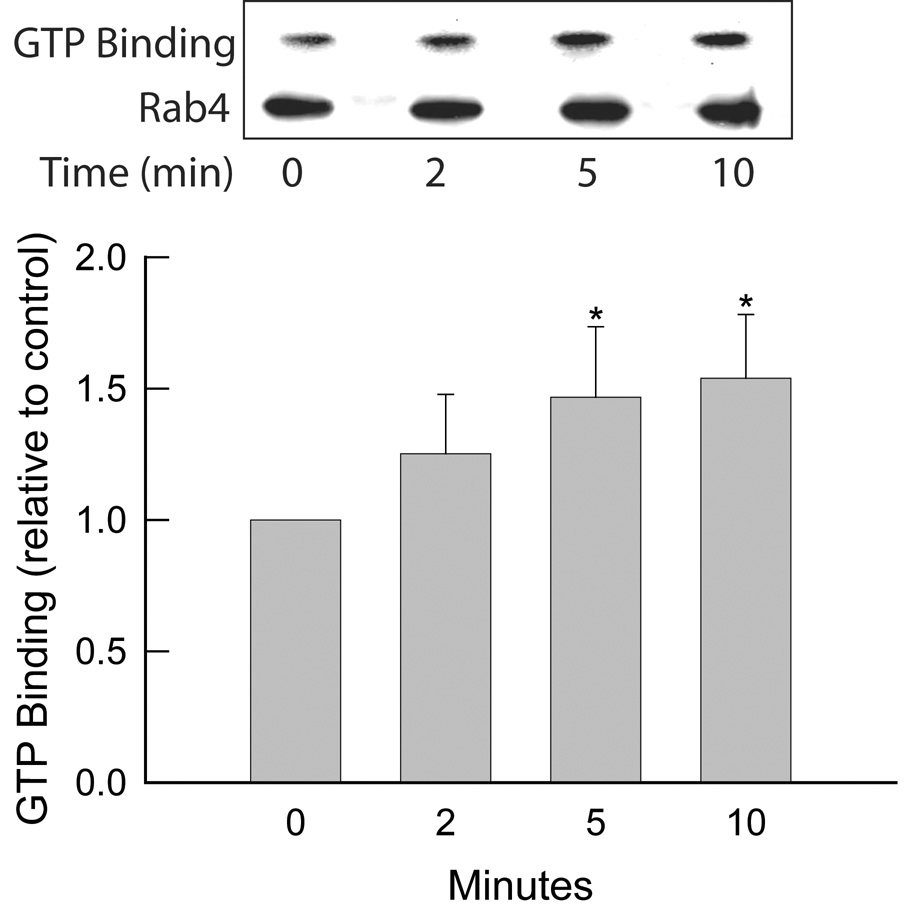
Above studies suggest a regulatory role for Rab4 in cAMP-stimulated TC uptake and Ntcp translocation. However, it is not known whether cAMP activates Rab4 (i.e., increases GTP binding of Rab4) and thereby stimulates Ntcp translocation. Alternatively, cAMP may not activate Rab4, but the presence of active Rab4 may be required to facilitate the effect of cAMP. It may be noted that insulin activates Rab4 in rat adipocytes (29, 30). We have used the GTP overlay assay to determine whether cAMP stimulates GTP binding of Rab4 in HuH-Ntcp cells. Treatment of cells with cAMP resulted in a time dependent increase in Rab4 GTP binding (Fig. 4); GTP-binding of Rab4 was increased significantly by cAMP as early as 5 min and remained increased at 10 min (Fig. 4). We determined the effect of cAMP on Rab4 GTP binding only up to 10 min to ascertain that the Rab4 activation was within the time frame (15 min) when cAMP stimulates TC uptake. Whether this effect of cAMP is sustained beyond 10 min is not known. This result would suggest that cAMP activates Rab4 by stimulating guanine nucleotide exchange on Rab4 and that the effect of cAMP on Ntcp translocation may be mediated via Rab4.
Figure 4.
Time-dependent activation of Rab4 by cAMP. Rab4 immunoprecipitated from HuH7-Ntcp cells treated with 100 µM CPT-cAMP (cAMP) was subjected to SDS-PAGE followed by GTP-overlay assay as described in “Materials and Methods”. Upper Panel: GTP binding represents a typical autoradiogram of GTP bound Rab4 while Rab4 represents Rab4 immunoblots. Lower Panel: Results of densitometric analysis (mean ± SEM, n=7). *Significantly different from pretreatment (0 min) value.
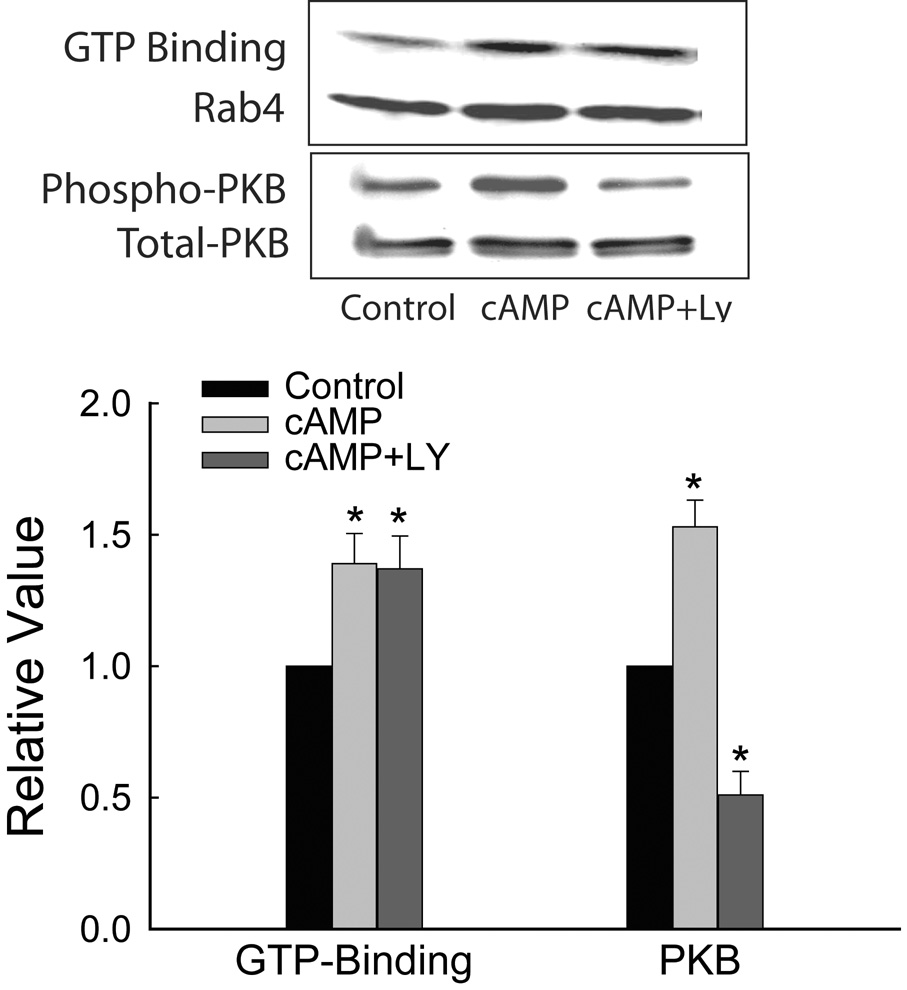
In a previous study, we reported that cAMP-stimulated Ntcp translocation is PI3K-dependent (27). Thus, it is possible that the activation of Rab4 by cAMP may also be PI3K-dependent. PI3K-dependent activation of Rab4 by insulin has been reported in adipocytes(30). However, inhibition of PI3K by LY 294002 did not affect cAMP-induced Rab4 GTP-binding (Fig. 5). LY294002 did inhibit cAMP-induced PI3K-dependent activation of PKB (27) in these cells (Fig. 5), indicating that PI3K was inhibited. Thus, cAMP-induced activation of Rab4 is not mediated via PI3K.
Figure 5.
PI3K-independent activation of Rab4 by cAMP. HuH-Ntcp cells were pretreated with 20 µM LY294002 (LY) for 30 min and then incubated with 100 µM CPT-cAMP for 10 min before performing the GTP-overlay and PKB assay. Upper Panel: GTP binding represents a typical autoradiogram of GTP bound Rab4 while Rab4 represents Rab4 immunoblots. Middle panel: Immunoblots of active S473 phosphorylated (Phospho) and total PKB. Lower Panel: Results of densitometric analysis (mean ± SEM, n=3). *Significantly different from respective control values.
The mechanism by which cAMP activates Rab4 in hepatocytes is not known and is currently under investigation. One possibility may be that cAMP activates guanine nucleotide exchange factor involved in GTP/GDP exchange on Rab4 or inhibit Rab4-GTPase activating protein. The present finding of PI3K-independent activation of Rab4 may suggest that cAMP-induced TC uptake and Ntcp translocation may require activation of two independent pathways, as discussed below.
The translocation of Ntcp by cAMP is mediated via PI3K signaling pathway; the downstream mediators being PKB, PKCζ and PKCδ (31, 32). A recent study showed that PKCζ is present in Ntcp-containing vesicles and is required for microtubule-based movement of these vesicles (18). However, the steps between cAMP→PI3K→PKB/PKCζ/PKCδ and membrane translocation are not known. One possibility may be that Rab4 is downstream of the cAMP→PI3K→PKB/PKCζ/PKCδ pathway. However, this seems unlikely since activation of Rab4 by cAMP is PI3K-independent (Fig. 5). This may also explain why Rab4(GTP) did not stimulate TC uptake or Ntcp translocation in the absence of cAMP (Fig. 2A & B); such an effect would have been expected if Rab4 was the downstream mediator of the above mentioned signaling pathway. Since cAMP-induced Ntcp translocation is enhanced by Rab4(GTP) and inhibited by Rab4(GDP), activation of Rab4 is necessary, but not sufficient for cAMP-induced Ntcp translocation. Thus, we propose that cAMP activates two parallel signaling pathways, namely cAMP→→Rab4 and cAMP→PI3K→PKB/PKCζ/PKCδ pathways, and the activation of both pathways is necessary for cAMP-induced Ntcp translocation.
The mechanism by which Rab4-GTP facilitates cAMP-induced increases in cell surface Ntcp is not known. Rab4 could stimulate translocation to or inhibit translocation from the plasma membrane. In as much as Rab4 has been suggested to be involved in vesicle trafficking from early endosome to plasma membrane in non-polarized cells (9, 33), it is likely that Rab4 stimulates translocation of Ntcp to the plasma membrane. However, the alternate possibility that Rab4-GTP decreases endocytosis can not be ruled out. Since Rab5 and Rab11 also colocalize with Ntcp vesicles (18), it is possible that Rab5 and Rab11 are also involved in cAMP-induced Ntcp translocation. Further studies similar to the present one will be needed to ascertain that possibility.
In summary, the present study showed for the first time that cAMP activates Rab4 and activated Rab4 facilitates cAMP-induced Ntcp translocation.
ACKNOWLEDGEMENTS
We thank Holly Jameson for excellent technical assistance.
Financial support
This study was supported in part by National Institutes of Health Grants DK-33436 (MSA), DK-65975 (CRLW), DK-07635 & RR-18267 (KT), DK23026 (AWW) and by the Intramural Program of Eunice Kennedy Shriver, NICHD, NIH (YW).
List of abbreviations
- Rab4(GTP)
GTP locked dominant active Rab4
- Rab4(GDP)
GDP locked dominant inactive Rab4
- PI3K
Phosphoinositide-3-kinase
- TC
Taurocholate
- GLUT4
Glucose transporter 4
- Ntcp
Na+-taurocholate cotransporting polypeptide
- CPT-cAMP
8-Chlorophenylthio cAMP
- PKCζ
Protein kinase Cζ
Contributor Information
Christopher M. Schonhoff, Email: Christopher.Schonhoff@Tufts.edu.
Krishna Thankey, Email: Krisna.Thankey@gmail.com.
Cynthia R.L. Webster, Email: cynthia.leveille-webster@tufts.edu.
Yoshiyuki Wakabayashi, Email: wakabayo@mail.nih.gov.
Allan W. Wolkoff, Email: wolkoff@aecom.yu.edu.
M. Sawkat Anwer, Email: Sawkat.Anwer@tufts.edu.
REFERENCES
- 1.Mukhopadhayay S, Ananthanarayanan M, Stieger B, Meier PJ, Suchy FJ, Anwer MS. cAMP increases liver Na+-taurocholate cotransport by translocating transporter to plasma membranes. Am J Physiol. 1997;273:G842–G848. doi: 10.1152/ajpgi.1997.273.4.G842. [DOI] [PubMed] [Google Scholar]
- 2.Anwer MS. Cellular Regulation of hepatic bile acid transport in health and cholestasis. Hepatology. 2004;39:581–589. doi: 10.1002/hep.20090. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez R, Teruel T, Lorenzo M. Akt mediates insulin induction of glucose uptake and up-regulation of GLUT4 gene expression in brown adipocytes. FEBS Lett. 2001;494:225–231. doi: 10.1016/s0014-5793(01)02353-5. [DOI] [PubMed] [Google Scholar]
- 4.Standaert ML, Galloway L, Karnam P, Bandyopadhyay G, Moscat J, Farese RV. Protein kinase C-zeta as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes. Potential role in glucose transport. J Biol Chem. 1997;272:30075–30082. doi: 10.1074/jbc.272.48.30075. [DOI] [PubMed] [Google Scholar]
- 5.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 6.Juvet LK, Bert T, Gjoen T. The expression of endosomal rab proteins correlates with endocytic rate in rat liver cells. Hepatology. 1997;25:1204–1212. doi: 10.1002/hep.510250524. [DOI] [PubMed] [Google Scholar]
- 7.Goldenring JR, Smith J, Vaughan HD, Cameron P, Hawkins W, Navarre J. Rab11 is an apically located small GTP-binding protein in epithelial tissues. Am J Physiol. 1996;270:G515–G525. doi: 10.1152/ajpgi.1996.270.3.G515. [DOI] [PubMed] [Google Scholar]
- 8.Nagy LE, Lakshman MR, Casey CA, Bearer CF. Ethanol and membrane protein trafficking: diverse mechanisms of ethanol action. Alcohol Clin Exp Res. 2002;26:287–293. [PubMed] [Google Scholar]
- 9.Novick P, Zerial M. The diversity of Rab proteins in vesicle transport. Curr Opin Cell Biol. 1997;9:496–504. doi: 10.1016/s0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- 10.Jahn R, Lang T, Sudhof TC. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 11.Cormont M, Marchand-Brustel Y. The role of small G-proteins in the regulation of glucose transport (review) Mol Membr Biol. 2001;18:213–220. doi: 10.1080/09687680110077541. [DOI] [PubMed] [Google Scholar]
- 12.Kessler A, Tomas E, Immler D, Meyer HE, Zorzano A, Eckel J. Rab11 is associated with GLUT4-containing vesicles and redistributes in response to insulin. Diabetologia. 2000;43:1518–1527. doi: 10.1007/s001250051563. [DOI] [PubMed] [Google Scholar]
- 13.Saxena SK, Kaur S, George C. Rab4GTPase modulates CFTR function by impairing channel expression at plasma membrane. Biochem Biophys Res Commun. 2006;341:184–191. doi: 10.1016/j.bbrc.2005.12.170. [DOI] [PubMed] [Google Scholar]
- 14.Saxena SK, Singh M, Shibata H, Kaur S, George C. Rab4 GTP/GDP modulates amiloride-sensitive sodium channel (ENaC) function in colonic epithelia. Biochem Biophys Res Commun. 2006;340:726–733. doi: 10.1016/j.bbrc.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 15.Wakabayashi Y, Kipp H, Arias IM. Transporters on demand: intracellular reservoirs and cycling of bile canalicular ABC transporters. J Biol Chem. 2006;281:27669–27673. doi: 10.1074/jbc.R600013200. [DOI] [PubMed] [Google Scholar]
- 16.Bananis E, Murray JW, Stockert RJ, Satir P, Wolkoff AW. Regulation of early endocytic vesicle motility and fission in a reconstituted system. J Cell Sci. 2003;116:2749–2761. doi: 10.1242/jcs.00478. [DOI] [PubMed] [Google Scholar]
- 17.Hayakawa T, Bruck R, Ng OC, Boyer JL. DBcAMP stimulates vesicle transport and HRP excretion in isolated perfused rat liver. Am J Physiol. 1990;259:G727–G735. doi: 10.1152/ajpgi.1990.259.5.G727. [DOI] [PubMed] [Google Scholar]
- 18.Sarkar S, Bananis E, Nath S, Anwer MS, Wolkoff AW, Murray JW. PKCzeta is required for microtubule-based motility of vesicles containing the ntcp transporter. Traffic. 2006;7:1078–1091. doi: 10.1111/j.1600-0854.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 19.McCaffrey MW, Bielli A, Cantalupo G, Mora S, Roberti V, Santillo M, et al. Rab4 affects both recycling and degradative endosomal trafficking. FEBS Lett. 2001;495:21–30. doi: 10.1016/s0014-5793(01)02359-6. [DOI] [PubMed] [Google Scholar]
- 20.Odley A, Hahn HS, Lynch RA, Marreez Y, Osinska H, Robbins J, et al. Regulation of cardiac contractility by Rab4-modulated beta2-adrenergic receptor recycling. Proc Natl Acad Sci U S A. 2004;101:7082–7087. doi: 10.1073/pnas.0308335101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ananthanarayanan M, NG O-C, Boyer JL, Suchy FJ. Characterization of cloned rat liver Na+ -bile acid cotransporter using peptide and fusion protien antibodies. Am J Physiol. 1994;267:G637–G643. doi: 10.1152/ajpgi.1994.267.4.G637. [DOI] [PubMed] [Google Scholar]
- 22.Higuchi H, Bronk SF, Takikawa Y, Werneburg N, Takimoto R, El Deiry W, et al. The bile acid glycochenodeoxycholate induces trail-receptor 2/DR5 expression and apoptosis. J Biol Chem. 2001;276:38610–38618. doi: 10.1074/jbc.M105300200. [DOI] [PubMed] [Google Scholar]
- 23.Webster CR, Srinivasulu U, Ananthanarayanan M, Suchy FJ, Anwer MS. Protein kinase B/Akt mediates cAMP- and cell swelling-stimulated Na+/taurocholate cotransport and Ntcp translocation. J Biol Chem. 2002;277:28578–28583. doi: 10.1074/jbc.M201937200. [DOI] [PubMed] [Google Scholar]
- 24.Webster CRL, Blanch CJ, Philips J, Anwer MS. Cell swelling-induced translocation of rat liver Na+/taurocholate cotransport polypeptide is mediated via the phosphoinositide 3-kinase signaling pathway. J Biol Chem. 2000;275:29754–29760. doi: 10.1074/jbc.M002831200. [DOI] [PubMed] [Google Scholar]
- 25.Gromov PS, Celis JE. Blot overlay assay for the identification of GTP-binding proteins. In: Celis JE, editor. Cell Biology: a laboratory handbook. 2nd ed. New York: Academic Press; 1998. pp. 454–457. [Google Scholar]
- 26.Wang T, Hong W. Assay and functional properties of Rab34 interaction with RILP in lysosome morphogenesis. Methods Enzymol. 2005;403:675–687. doi: 10.1016/S0076-6879(05)03058-2. [DOI] [PubMed] [Google Scholar]
- 27.Webster CRL, Anwer MS. Role of the PI3K/PKB signaling pathway in cAMP-mediated translocation of rat liver Ntcp. Am J Physiol. 1999;277:G1165–G1172. doi: 10.1152/ajpgi.1999.277.6.G1165. [DOI] [PubMed] [Google Scholar]
- 28.Lowry DH, Rosenberg NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 29.Imamura T, Huang J, Usui I, Satoh H, Bever J, Olefsky JM. Insulin-induced GLUT4 translocation involves protein kinase C-lambda-mediated functional coupling between Rab4 and the motor protein kinesin. Mol Cell Biol. 2003;23:4892–4900. doi: 10.1128/MCB.23.14.4892-4900.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shibata H, Omata W, Kojima I. Insulin stimulates guanine nucleotide exchange on Rab4 via a wortmannin-sensitive signaling pathway in rat adipocytes. J Biol Chem. 1997;272:14542–14546. doi: 10.1074/jbc.272.23.14542. [DOI] [PubMed] [Google Scholar]
- 31.Anwer MS, Webster CR. Signal Transduction in bile formation and cholestasis. In: Trauner M, Jansen PL, editors. Molecular Pathogenesis of Cholestasis. Austin, TX: Landes Bioscience; 2003. [Google Scholar]
- 32.Schonhoff CM, Gillin H, Webster CR, Anwer MS. Protein kinase Cdelta mediates cyclic adenosine monophosphate-stimulated translocation of sodium taurocholate cotransporting polypeptide and multidrug resistant associated protein 2 in rat hepatocytes. Hepatology. 2008;47:1309–1316. doi: 10.1002/hep.22162. [DOI] [PubMed] [Google Scholar]
- 33.Somsel RJ, Wandinger-Ness A. Rab GTPases coordinate endocytosis. J Cell Sci. 2000;113:183–192. doi: 10.1242/jcs.113.2.183. [DOI] [PubMed] [Google Scholar]