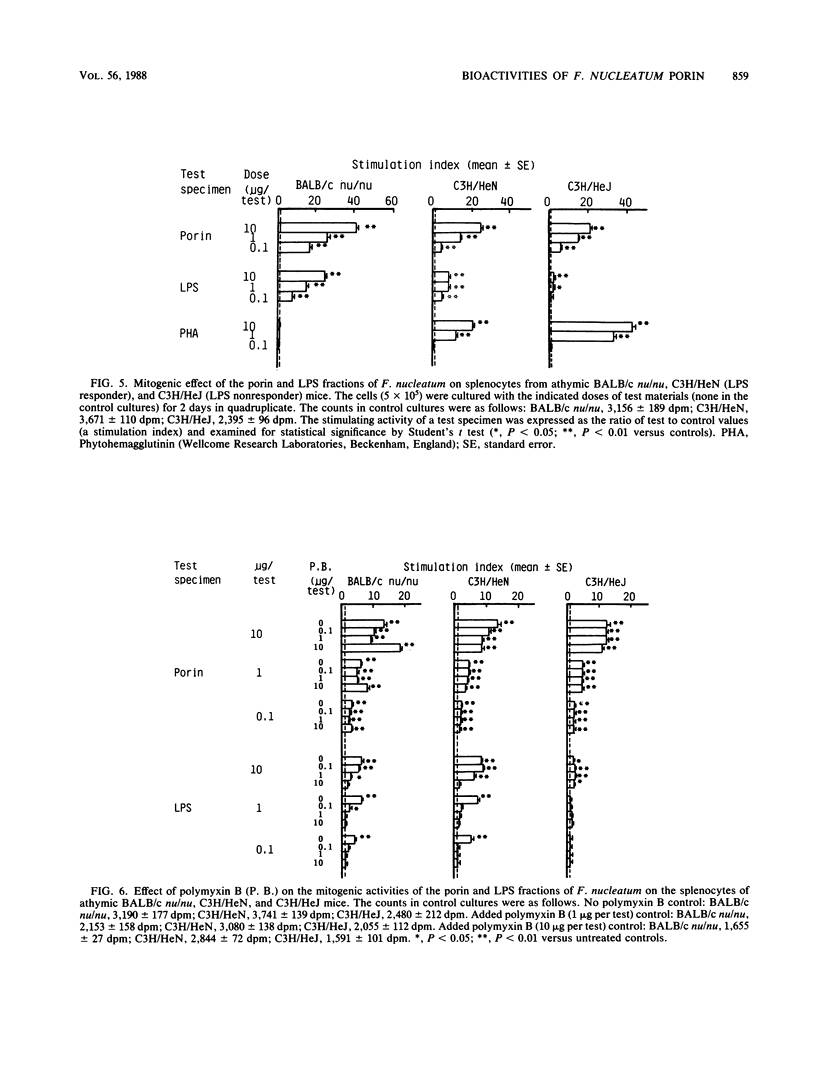
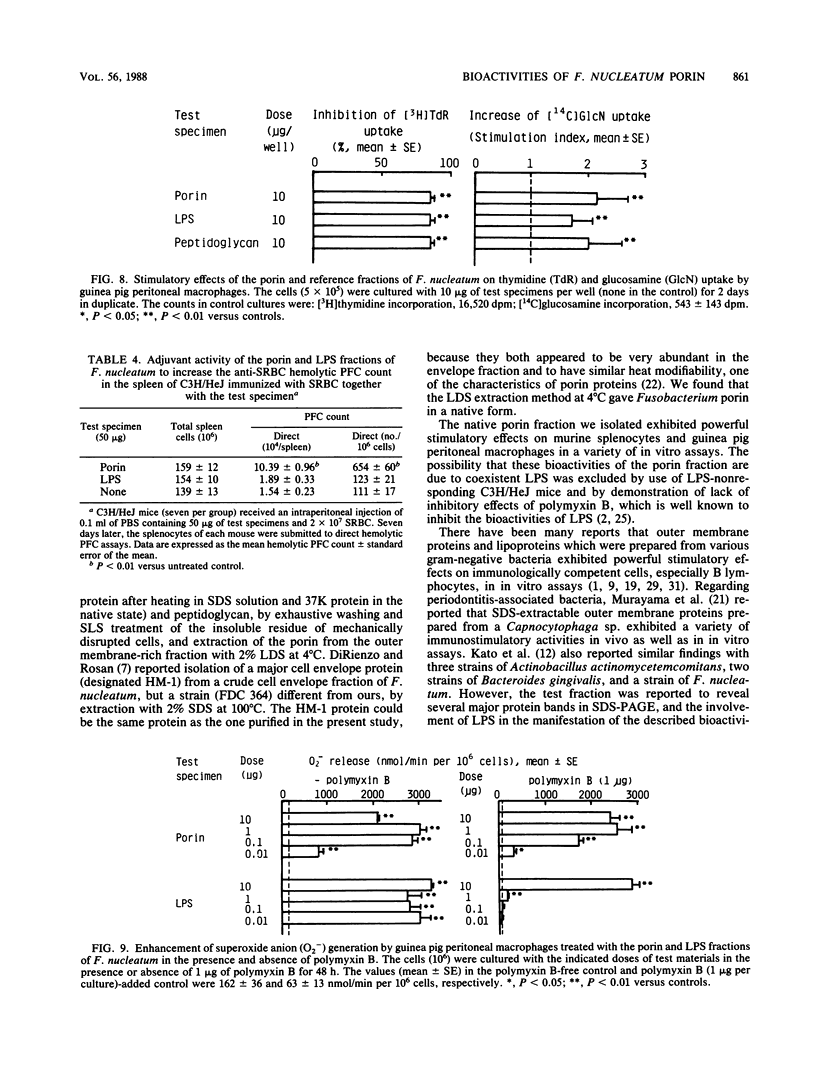
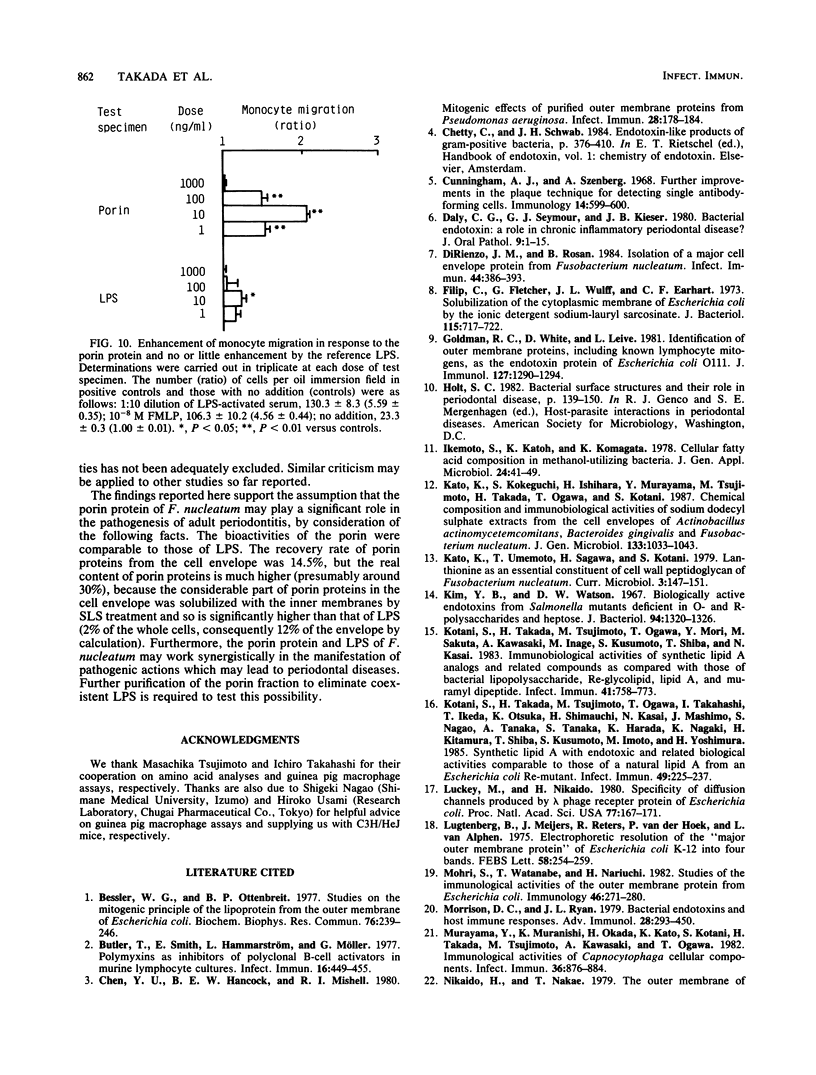
Abstract
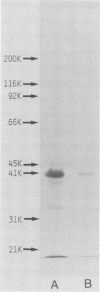
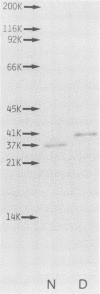
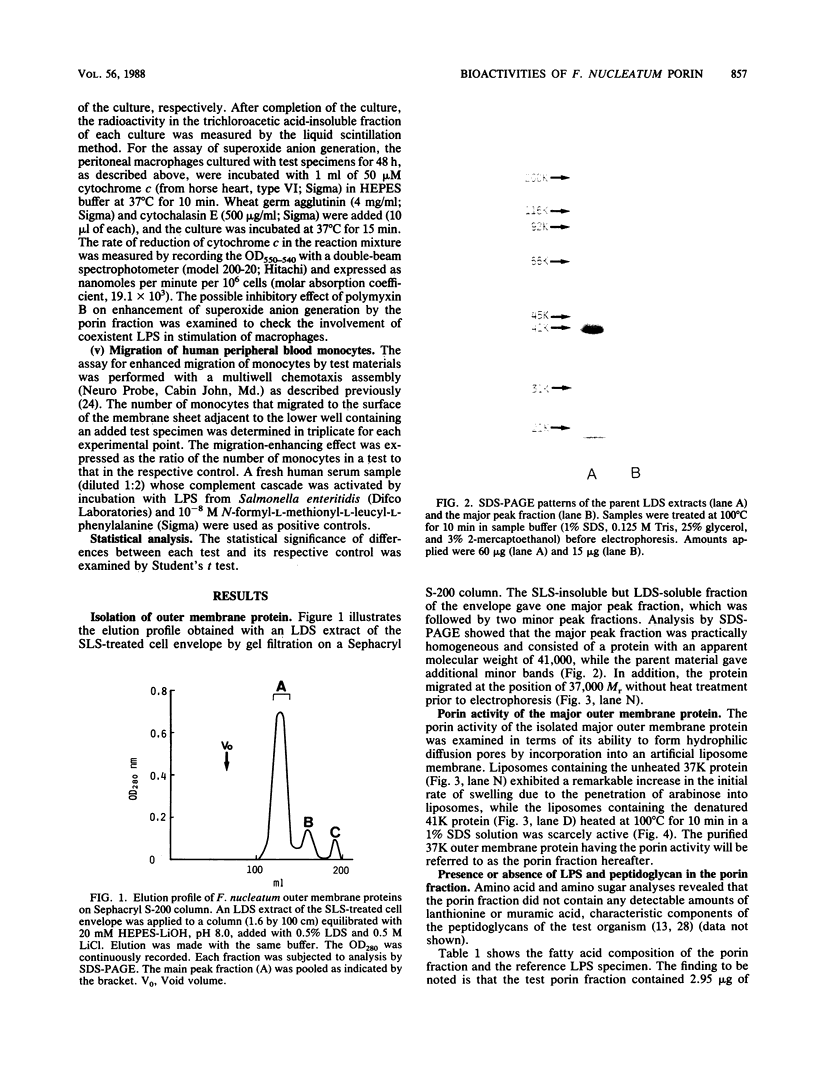
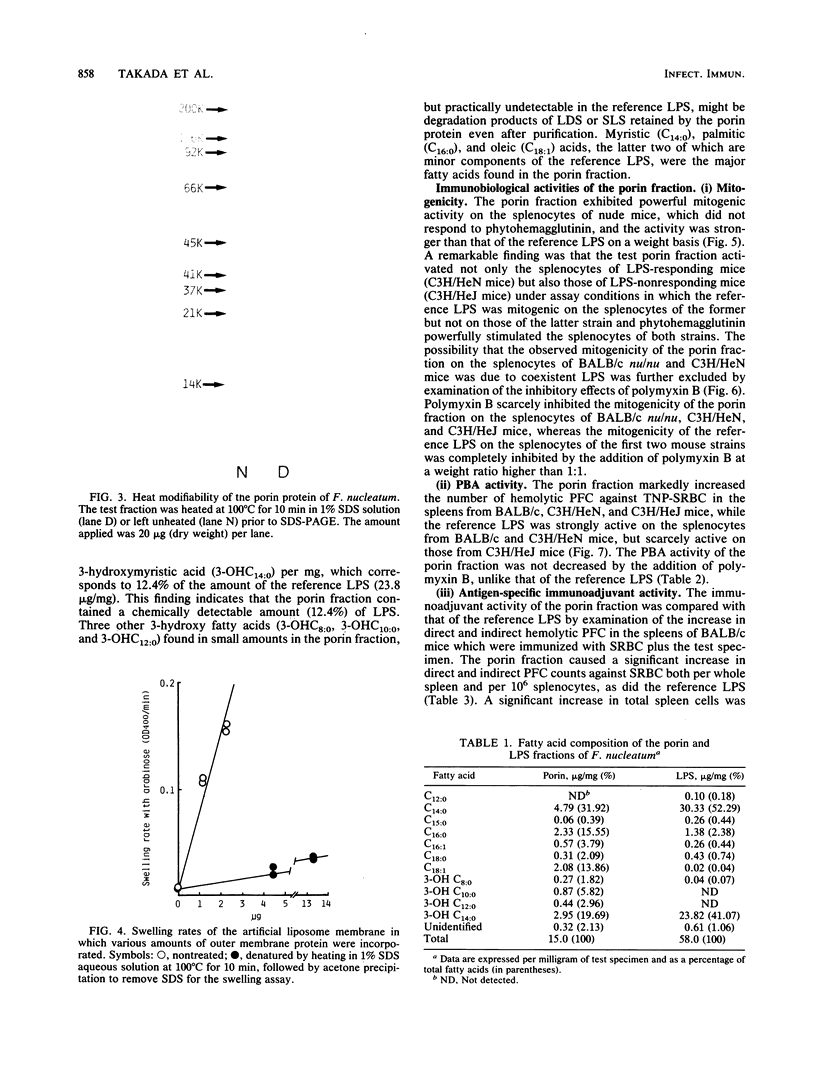
From Fusobacterium nucleatum ATCC 10953 cell envelope fraction whose inner membranes had been removed by treatment with sodium N-lauroyl sarcosinate, an outer membrane protein (37,000 Mr in a native state) was prepared by extraction with lithium dodecyl sulfate. The protein thus obtained showed distinct porin activity, namely, the ability to form hydrophilic diffusion pores by incorporation into the artificial liposome membrane. The porin fraction exhibited strong immunobiological activities in the in vitro assays: B-cell mitogenicity and polyclonal B-cell activation on murine splenocytes, stimulatory effects on guinea pig peritoneal macrophages, and enhancement of the migration of human blood monocytes. The porin fraction also exhibited immunoadjuvant activity to increase the antibody production against sheep erythrocytes in the spleen of mice that were immunized by sheep erythrocytes with porin. Although chemical analyses revealed that the test porin fraction contained a considerable amount of lipopolysaccharide (LPS) (around 12% of the fraction), the studies with LPS-nonresponding C3H/HeJ mice and on the inhibitory effects of polymyxin B strongly suggest that most of the above bioactivities are due to porin protein itself, not to coexistent LPS in the porin fraction.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bessler W. G., Ottenbreit B. P. Studies on the mitogenic principle of the lipoprotein from the outer membrane of Escherichia coli. Biochem Biophys Res Commun. 1976 May 23;76(2):239–246. doi: 10.1016/0006-291x(77)90717-3. [DOI] [PubMed] [Google Scholar]
- Butler T., Smith E., Hammarström L., Möller G. Polymyxins as inhibitors of polyclonal B-cell activators in murine lymphocyte cultures. Infect Immun. 1977 May;16(2):449–455. doi: 10.1128/iai.16.2.449-455.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. H., Hancock R. E., Mishell R. I. Mitogenic effects of purified outer membrane proteins from Pseudomonas aeruginosa. Infect Immun. 1980 Apr;28(1):178–184. doi: 10.1128/iai.28.1.178-184.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Daly C. G., Seymour G. J., Kieser J. B. Bacterial endotoxin: a role in chronic inflammatory periodontal disease? J Oral Pathol. 1980 Jan;9(1):1–15. doi: 10.1111/j.1600-0714.1980.tb01383.x. [DOI] [PubMed] [Google Scholar]
- DiRienzo J. M., Rosan B. Isolation of a major cell envelope protein from Fusobacterium nucleatum. Infect Immun. 1984 May;44(2):386–393. doi: 10.1128/iai.44.2.386-393.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., White D., Leive L. Identification of outer membrane proteins, including known lymphocyte mitogens, as the endotoxin protein of Escherichia coli 0111. J Immunol. 1981 Oct;127(4):1290–1294. [PubMed] [Google Scholar]
- Kato K., Kokeguchi S., Ishihara H., Murayama Y., Tsujimoto M., Takada H., Ogawa T., Kotani S. Chemical composition and immunobiological activities of sodium dodecyl sulphate extracts from the cell envelopes of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Fusobacterium nucleatum. J Gen Microbiol. 1987 Apr;133(4):1033–1043. doi: 10.1099/00221287-133-4-1033. [DOI] [PubMed] [Google Scholar]
- Kim Y. B., Watson D. W. Biologically active endotoxins from Salmonella mutants deficient in O- and R-polysaccharides and heptose. J Bacteriol. 1967 Nov;94(5):1320–1326. doi: 10.1128/jb.94.5.1320-1326.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Takada H., Tsujimoto M., Ogawa T., Mori Y., Sakuta M., Kawasaki A., Inage M., Kusumoto S., Shiba T. Immunobiological activities of synthetic lipid A analogs and related compounds as compared with those of bacterial lipopolysaccharide, re-glycolipid, lipid A, and muramyl dipeptide. Infect Immun. 1983 Aug;41(2):758–773. doi: 10.1128/iai.41.2.758-773.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Takada H., Tsujimoto M., Ogawa T., Takahashi I., Ikeda T., Otsuka K., Shimauchi H., Kasai N., Mashimo J. Synthetic lipid A with endotoxic and related biological activities comparable to those of a natural lipid A from an Escherichia coli re-mutant. Infect Immun. 1985 Jul;49(1):225–237. doi: 10.1128/iai.49.1.225-237.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey M., Nikaido H. Specificity of diffusion channels produced by lambda phage receptor protein of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):167–171. doi: 10.1073/pnas.77.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Mohri S., Watanabe T., Nariuchi H. Studies of the immunological activities of the outer membrane protein from Escherichia coli. Immunology. 1982 Jun;46(2):271–280. [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- Murayama Y., Muranishi K., Okada H., Kato K., Kotani S., Takada H., Tsujimoto M., Kawasaki A., Ogawa T. Immunological activities of Capnocytophaga cellular components. Infect Immun. 1982 Jun;36(3):876–884. doi: 10.1128/iai.36.3.876-884.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y. Effect on solute size on diffusion rates through the transmembrane pores of the outer membrane of Escherichia coli. J Gen Physiol. 1981 Feb;77(2):121–135. doi: 10.1085/jgp.77.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Kotani S., Fukuda K., Tsukamoto Y., Mori M., Kusumoto S., Shiba T. Stimulation of migration of human monocytes by bacterial cell walls and muramyl peptides. Infect Immun. 1982 Dec;38(3):817–824. doi: 10.1128/iai.38.3.817-824.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkind D. Prevention by polymyxin B of endotoxin lethality in mice. J Bacteriol. 1967 Apr;93(4):1463–1464. doi: 10.1128/jb.93.4.1463-1464.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart-Tull D. E. The immunological activities of bacterial peptidoglycans. Annu Rev Microbiol. 1980;34:311–340. doi: 10.1146/annurev.mi.34.100180.001523. [DOI] [PubMed] [Google Scholar]
- Vasstrand E. N., Hofstad T., Endresen C., Jensen H. B. Demonstration of lanthionine as a natural constituent of the peptidoglycan of Fusobacterium nucleatum. Infect Immun. 1979 Sep;25(3):775–780. doi: 10.1128/iai.25.3.775-780.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vordermeier M., Stäb K., Bessler W. G. A defined fragment of bacterial protein I (OmpF) is a polyclonal B-cell activator. Infect Immun. 1986 Jan;51(1):233–239. doi: 10.1128/iai.51.1.233-239.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. B., Ribi E., Wheat R. W. Enhancement of macrophage-mediated tumor cell killing by bacterial outer membrane proteins (porins). Infect Immun. 1983 Oct;42(1):219–223. doi: 10.1128/iai.42.1.219-223.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]