Abstract
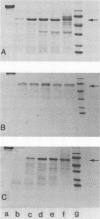
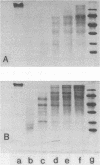
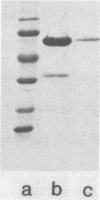
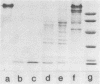
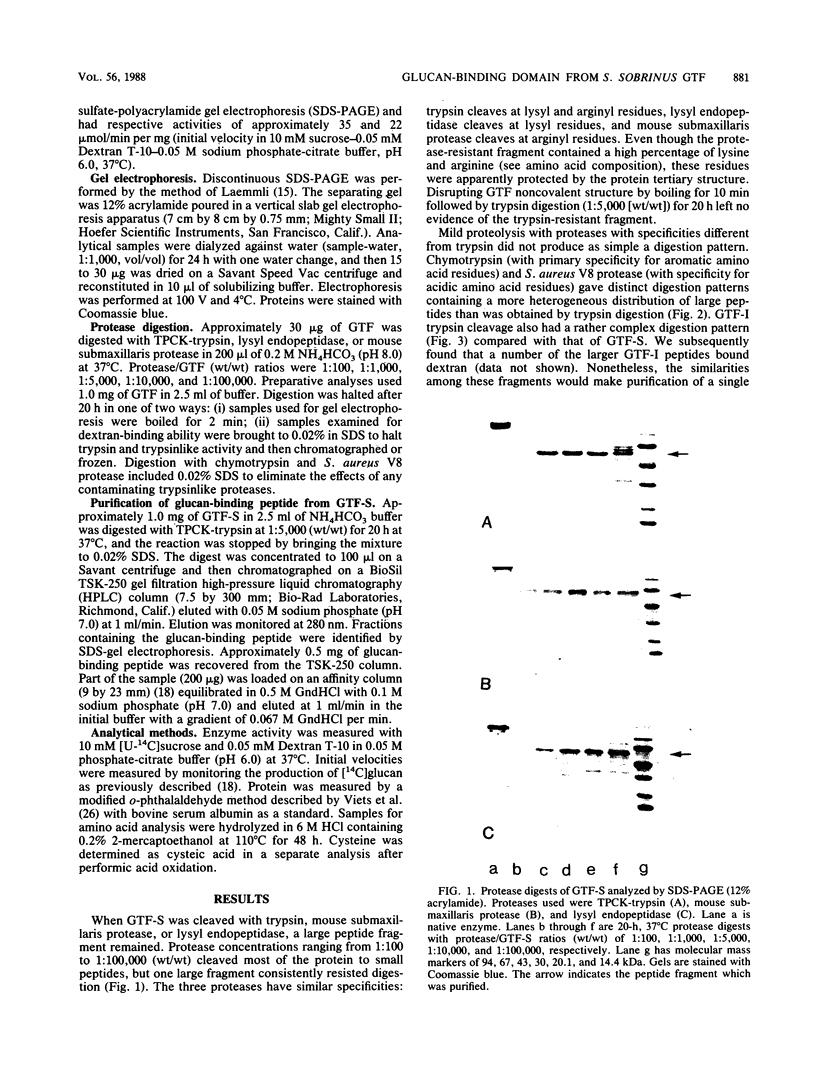
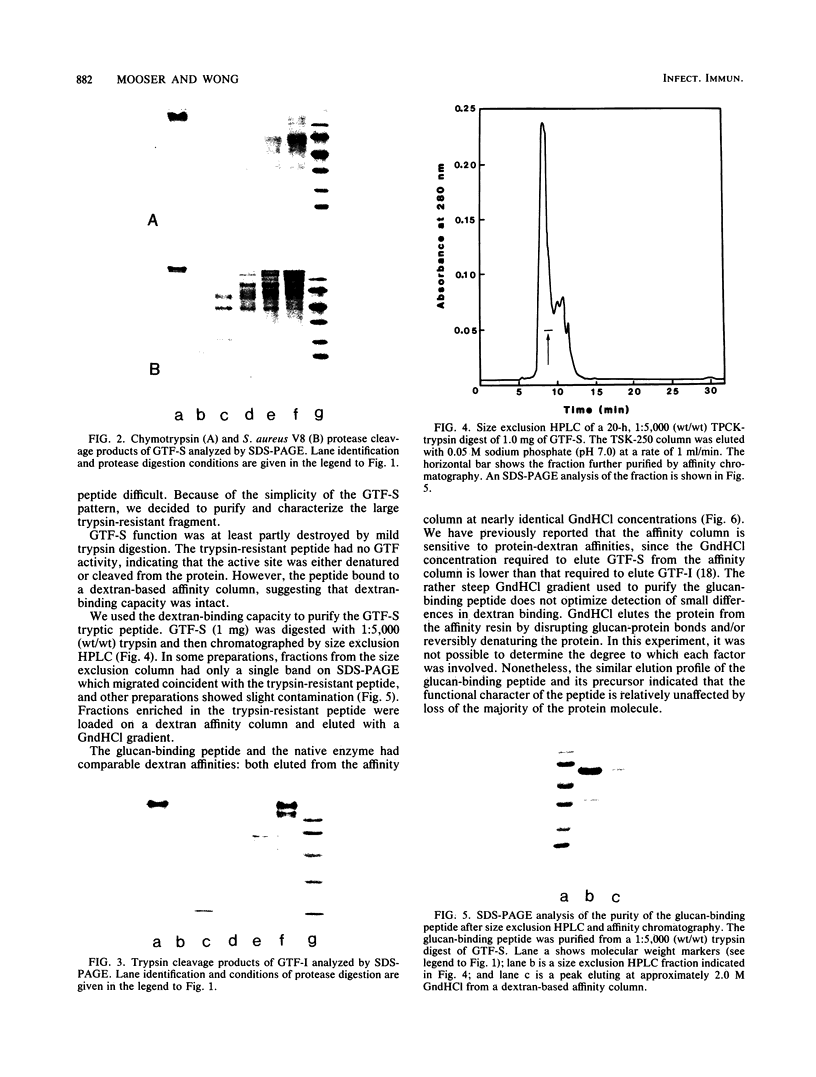
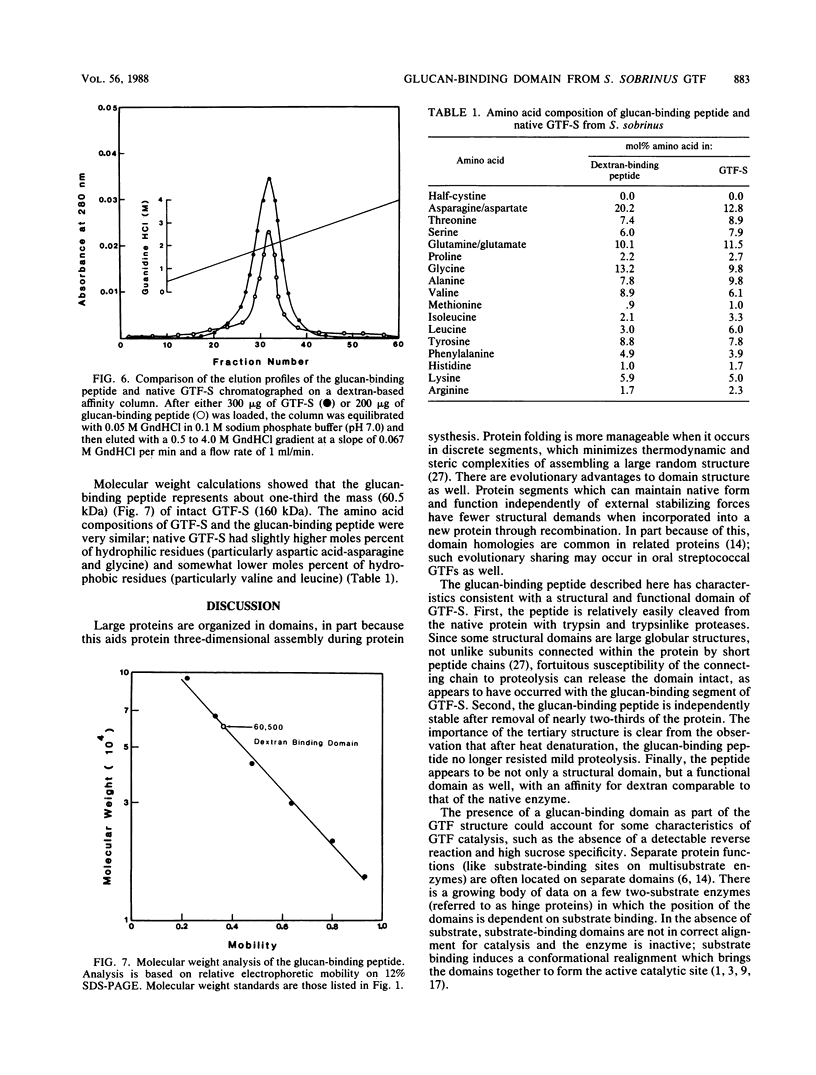
A glucan-binding domain of 1,6-alpha-glucan synthase (dextransucrase) (GTF-S) was isolated from a trypsin digest of the Streptococcus sobrinus enzyme. The large 60.5-kilodalton peptide had an affinity for dextran comparable to that of the native enzyme, but had no glucan synthesis activity. The domain was produced in high yield compared with other large cleavage products, which allowed easy purification by size exclusion high-pressure liquid chromatography and affinity chromatography. Two other proteases (mouse submaxillaris protease and lysyl endopeptidase) with specificities similar to trypsin generated a distribution of GTF-S peptides that was also greatly enriched in the glucan-binding peptide. Proteases with markedly different specificities (chymotrypsin and Staphylococcus aureus V8 protease) produced a family of peptides with some evidence of the glucan-binding domain but in far lower yield. The tertiary structure of the domain was critical to its resistance to proteolysis; heat denaturation of GTF-S before trypsin digestion resulted in cleavage of the enzyme to small limit peptides leaving no evidence of the glucan-binding domain. The amino acid composition of the peptide was very similar to that of the native enzyme. The common occurrence of proteases in oral streptococcus cultures and reports of glucosyltransferase degradation during purification and storage raises the possibility that some accounts of glucan-binding receptors are peptides derived from glucosyltransferase. Kinetic implications of a glucan-binding domain are discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. M., Zucker F. H., Steitz T. A. Space-filling models of kinase clefts and conformation changes. Science. 1979 Apr 27;204(4391):375–380. doi: 10.1126/science.220706. [DOI] [PubMed] [Google Scholar]
- Asem K., Cornish-Bowden A. J., Cole J. A. A comparative study of the extracellular glucosyl- and fructosyltransferases from cariogenic and non-cariogenic Streptococcus mutans strains of two different serotypes. Microbios. 1986;47(190):53–66. [PubMed] [Google Scholar]
- Bennett W. S., Jr, Steitz T. A. Glucose-induced conformational change in yeast hexokinase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4848–4852. doi: 10.1073/pnas.75.10.4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee M. K., Mayer R. M. Interaction of deoxyhalosucrose derivatives with dextransucrase. Carbohydr Res. 1985 Oct 15;142(2):277–284. doi: 10.1016/0008-6215(85)85029-1. [DOI] [PubMed] [Google Scholar]
- Binder T. P., Robyt J. F. Inhibition of Streptococcus mutans 6715 glucosyltransferases by sucrose analogs modified at positions 6 and 6'. Carbohydr Res. 1985 Jul 1;140(1):9–20. doi: 10.1016/0008-6215(85)85045-x. [DOI] [PubMed] [Google Scholar]
- Cowman R. A., Perrella M. M., Fitzgerald R. J. Caseinolytic and glyoprotein hydrolase activity of Streptococcus mutans. J Dent Res. 1976 May-Jun;55(3):391–399. doi: 10.1177/00220345760550031701. [DOI] [PubMed] [Google Scholar]
- Eklund H., Samma J. P., Wallén L., Brändén C. I., Akeson A., Jones T. A. Structure of a triclinic ternary complex of horse liver alcohol dehydrogenase at 2.9 A resolution. J Mol Biol. 1981 Mar 15;146(4):561–587. doi: 10.1016/0022-2836(81)90047-4. [DOI] [PubMed] [Google Scholar]
- Figures W. R., Edwards J. R. alpha-D-Glucopyranosyl fluoride as a D-glucopyranosyl donor for a glycosyltransferase complex from Streptococcus mutans FA1. Carbohydr Res. 1976 Jun;48(2):245–253. doi: 10.1016/s0008-6215(00)83220-6. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Banghart S. B. Synthesis of extracellular dextran by cariogenic bacteria and its presence in human dental plaque. Arch Oral Biol. 1967 Jan;12(1):11–23. doi: 10.1016/0003-9969(67)90137-9. [DOI] [PubMed] [Google Scholar]
- Guggenheim B., Schroeder H. E. Biochemical and morphological aspects of extracellular polysaccharides produced by cariogenic streptococci. Helv Odontol Acta. 1967 Oct;11(2):131–152. [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janin J., Wodak S. J. Structural domains in proteins and their role in the dynamics of protein function. Prog Biophys Mol Biol. 1983;42(1):21–78. doi: 10.1016/0079-6107(83)90003-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCabe M. M., Hamelik R. M., Smith E. E. Purification of dextran-binding protein from cariogenic Streptococcus mutans. Biochem Biophys Res Commun. 1977 Sep 9;78(1):273–278. doi: 10.1016/0006-291x(77)91250-5. [DOI] [PubMed] [Google Scholar]
- McDonald R. C., Steitz T. A., Engelman D. M. Yeast hexokinase in solution exhibits a large conformational change upon binding glucose or glucose 6-phosphate. Biochemistry. 1979 Jan 23;18(2):338–342. doi: 10.1021/bi00569a017. [DOI] [PubMed] [Google Scholar]
- Mooser G., Shur D., Lyou M., Watanabe C. Kinetic studies on dextransucrase from the cariogenic oral bacterium Streptococcus mutans. J Biol Chem. 1985 Jun 10;260(11):6907–6915. [PubMed] [Google Scholar]
- Porter R. R. Structural studies of immunoglobulins. Science. 1973 May 18;180(4087):713–716. doi: 10.1126/science.180.4087.713. [DOI] [PubMed] [Google Scholar]
- Russell R. R., Coleman D., Dougan G. Expression of a gene for glucan-binding protein from Streptococcus mutans in Escherichia coli. J Gen Microbiol. 1985 Feb;131(2):295–299. doi: 10.1099/00221287-131-2-295. [DOI] [PubMed] [Google Scholar]
- Russell R. R., Donald A. C., Douglas C. W. Fructosyltransferase activity of a glucan-binding protein from Streptococcus mutans. J Gen Microbiol. 1983 Oct;129(10):3243–3250. doi: 10.1099/00221287-129-10-3243. [DOI] [PubMed] [Google Scholar]
- Russell R. R. Glucan-binding proteins of Streptococcus mutans serotype c. J Gen Microbiol. 1979 May;112(1):197–201. doi: 10.1099/00221287-112-1-197. [DOI] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaniyavarn S., Singh S., Maynard C. M., Taylor K. G., Doyle R. J. Amino sugars: a new class of inhibitors of dextransucrase. Carbohydr Res. 1981 Oct 1;96(1):134–137. doi: 10.1016/s0008-6215(00)84706-0. [DOI] [PubMed] [Google Scholar]
- Viets J. W., Deen W. M., Troy J. L., Brenner B. M. Determination of serum protein concentration in nanoliter blood samples using fluorescamine or 9-phthalaldehyde. Anal Biochem. 1978 Aug 1;88(2):513–521. doi: 10.1016/0003-2697(78)90451-7. [DOI] [PubMed] [Google Scholar]
- Wetlaufer D. B. Nucleation, rapid folding, and globular intrachain regions in proteins. Proc Natl Acad Sci U S A. 1973 Mar;70(3):697–701. doi: 10.1073/pnas.70.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]