Abstract
Serotonin (5HT) is a powerful modulator of respiratory circuitry in vitro but its role in the development of breathing behavior in vivo is poorly understood. Here we show, using 5HT neuron-deficient Pet-1 (Pet-1−/−) neonates, that serotonergic function is required for the normal timing of postnatal respiratory maturation. Plethysmographic recordings reveal that Pet-1−/− mice are born with a depressed breathing frequency and a higher incidence of spontaneous and prolonged respiratory pauses relative to wild type littermates. The wild type breathing pattern stabilizes by postnatal day 4.5, while breathing remains depressed, highly irregular, and interrupted more frequently by respiratory pauses in Pet-1−/− mice. Analysis of in vitro hypoglossal nerve discharge indicates that instabilities in the central respiratory rhythm generator contribute to the abnormal Pet-1−/− breathing behavior. In addition, the breathing pattern in Pet-1−/− neonates is susceptible to environmental conditions, and can be further destabilized by brief exposure to hypoxia. By postnatal day 9.5, however, breathing frequency in Pet-1−/− animals is only slightly depressed compared to wild type, and prolonged respiratory pauses are rare, indicating that the abnormalities seen earlier in the Pet-1−/− mice are transient. Our findings provide unexpected insight into the development of breathing behavior by demonstrating that defects in 5HT neuron development can extend and exacerbate the period of breathing instability that occurs immediately after birth during which respiratory homeostasis is vulnerable to environmental challenges.
1. Introduction
Normal development and maturation of the neural circuitry that underlies breathing behavior in mammals is essential for postnatal survival and occurs over a prolonged time frame encompassing the perinatal period (Hilaire and Duron, 1999; Abadie et al., 2000; Viemari et al., 2003; Thoby-Brisson et al., 2005). In the mouse, breathing rhythmogenesis begins around embryonic day (E) 15 and can be localized bilaterally in the ventrolateral medulla in the area of the preBötzinger Complex (Abadie et al., 2000; Thoby-Brisson et al., 2005). By E18, the respiratory control system is well developed and is capable of supporting survival ex utero (Viemari et al., 2003), but respiratory cycle durations are highly irregular (Di Pasquale et al., 1992; Greer et al., 1992; Viemari et al., 2003), and breathing frequency and minute ventilation are relatively depressed (Viemari et al., 2003). Between E18 and postnatal day (P) 2, breathing cycles stabilize but ventilation remains depressed, whereas respiratory frequency and ventilation subsequently increase dramatically from P3 to P9 (Viemari et al., 2003), indicating that breathing behavior continues to mature well into the postnatal period.
Several neurotransmitters including glutamate, substance P, GABA, and noradrenaline are known to influence respiratory network activity during these periods of respiratory maturation (Ritter and Zhang, 2000; Zhang et al., 2002; Hilaire et al., 2004; Thoby-Brisson et al., 2005; Hilaire, 2006; Zanella et al., 2006). In addition, a preponderance of physiological data indicate that respiratory drive is increased by exogenously applied serotonin (5HT) agonists or stimulation of endogenous 5HT release during the perinatal period, both in vivo and in vitro (for review, see Hilaire and Duron, 1999; Richerson, 2004). Moreover, either increased or decreased serotonergic activity can alter the pattern of respiratory rhythm in the isolated neonatal spinal cord and brainstem (Hilaire and Duron, 1999; Bou-Flores et al., 2000), suggesting that 5HT also serves to establish and/or maintain respiratory stability within developing respiratory control circuits. However, whether serotonergic activity is required for the maturation of breathing behavior in the intact newborn is not known.
A critical limitation for investigating the developmental role of 5HT in neonatal breathing in vivo has been the lack of a genetic loss of function approach that can establish specific and stable deficiencies of central 5HT neuron function during the early stages of respiratory maturation. Such an approach has provided insights into noradrenergic and ret signaling pathway contributions to the development of respiratory control (Shirasawa et al., 2000; Dauger et al., 2001; Viemari and Hilaire, 2003; Viemari et al., 2004, 2005a, 2005b). Several characteristics of Pet-1 ETS transcription factor expression suggest Pet-1 homozygous null (Pet-1−/−) mice may provide an effective experimental system for understanding serotonergic requirements for respiratory maturation in vivo. Specifically, Pet-1 expression in the brain is restricted to 5HT neurons and their undifferentiated embryonic precursors (Hendricks et al., 1999). In Pet-1−/− mice the majority of 5HT neuron precursors fail to synthesize 5HT while those that do express the transmitter show defects in serotonergic gene expression (Hendricks et al., 2003). This results in a severe deficiency of 5HT neurons in all midbrain, pontine and medullary raphe nuclei. Consequently, 5HT levels are only 10-15% of wild type in developing and adult Pet-1−/− brains while other monoaminergic neurons do not appear to be affected (Hendricks et al., 2003).
Here we report, using plethysmography and the in vitro rhythmic brainstem slice preparation (Smith et al., 1991), a transient respiratory depression and instability in Pet-1−/− neonates, the severity of which is susceptible to environmental conditions. These findings suggest that Pet-1−/− mice provide a novel genetic approach to investigate the role of serotonergic function in the development of breathing behavior, as well as a tractable model system for exploring how serotonergic defects might contribute to developmental disorders of respiratory control such as Sudden Infant Death Syndrome (SIDS).
2. Methods
2.1. Pet-1−/− mice
All animals used in this study were derived from a single colony established at Case Western Reserve University (CWRU). Pet-1−/− mice were produced on a mixed C57BL/6 and 129 background (Hendricks et al., 2003). Following completion of the initial plethysmographic studies at CWRU, sentinel screening revealed that these mice had been exposed to several murine pathogens, including mouse hepatitis virus, mouse parvovirus, Pseudomonas and Syphacia. Subsequently, 8 founder mice that had been re-derived from the pathogen-exposed (PE) stock were used to generate a second pathogen-free (PF) colony at The College of New Jersey (TCNJ). Since March 2004, mice from the TCNJ colony have tested negative (Charles River Mouse Tracking Test) for murine pathogens. The most recent test was in June 2006, following completion of all data collection. In both colonies, mice were maintained using standard animal husbandry procedures (12:12 hour light-dark cycle, food and water ad libitum). The animal care and experimental procedures used in this study were consistent with US National Institutes of Health Animal Care and Use Guidelines and were approved by the Institutional Animal Care and Use Committees (IACUC) at both CWRU and TCNJ.
2.2. Body weight and survival data
Pet-1+/− mice were mated and pregnant dams were checked twice daily for pups during the perinatal period. A birth date of postnatal age (P) 0.5 was assigned to the day in which pups were first detected with the dam. All pups were weighed, toe-clipped to distinguish individuals, and a tail tissue sample was taken for Pet-1 genotyping using standard PCR protocols (Hendricks et al., 2003). Birth weight and genotype data were collected from 86 different litters (PE: 61; PF: 25). However, only litters with indications of attentive maternal care (e.g. pups kept warm in a huddle, evidence of successful suckling in at least some of the pups) were used to monitor survival and obtain daily weights.
2.3. In vivo body plethysmography
Ventilatory measurements were obtained on postnatal days 0.5, 4.5 and 9.5 using a small pneumotachograph and a 20 ml body plethysmograph chamber in the “head out” configuration (Mortola and Noworaj, 1983; Mortola, 1984). The plethysmograph consisted of two compartments, one serving as a body chamber and the other for gas delivery to the animal. The two compartments were separated by a parafilm-latex-parafilm “sandwich” that served both to seal the body chamber to the gas chamber (parafilm) and to form an airtight seal around the animal's neck. Holes were cut through the two sheets of parafilm that were larger than the head of the animal. Two slits perpendicular to one another were cut through the thin (6.7 mil) latex to form a cross-shaped opening through which the animal's head was inserted until the latex rested just behind the ears. Particular care was taken to size the opening to minimize neck constriction. Temperature within the chamber was measured and kept within the thermo-neutral range (32-34°C) for neonatal rodents (Mortola and Dotta, 1992) via an infrared lamp. The side-arms of the pneumotachograph were connected to a differential pressure transducer (Model DP-103, Validyne Engineering, Northridge, CA) and the analog signal from the transducer was demodulated (model CD-15 carrier demodulator, Validyne), amplified and integrated (Gould model 11-4113-01, Cleveland, OH), digitized (TL-1 DMA interface, Axon Instruments, Inc., Foster City, CA), and captured to disk (Axotape, v.1.2, Axon Instruments) for subsequent analysis. Breathing gas was delivered continuously to the animal (30 ml/min) through a baffle to minimize stimulation of facial sensory receptors, and the gas composition could be changed without flow interruption. Effluent gas from the plethysmograph was monitored with O2 and CO2 analyzers (model FC-10/CA-10, Sable Systems International, Las Vegas, NV).
Once within the plethysmograph, each animal was allowed to acclimate for a period of at least five minutes prior to measurements while breathing 21% O2 (balance N2; normoxia). After the acclimation period, resting ventilation was recorded continuously under normoxic conditions for at least 10 minutes. To measure ventilatory responses to either decreased environmental O2 or elevated CO2, a control period of quiet resting breathing in normoxia was obtained for 5 minutes immediately prior to a 2 minute exposure to either a hypoxic (10% O2, balance N2) or hypercapnic (5% CO2, 21% O2, balance N2) gas mixture, followed by a 5 minute recovery period in normoxia. During both the initial control and final recovery periods, 50 μl of air was injected at least three times into the body chamber with a syringe (model 1710, Hamilton Company, Reno, NV) to serve as calibration pulses for tidal volume measurements (Mortola, 1984).
Each record of ventilation was analyzed using commercially available software (Aquis1 v4.0, Bio-Logic Science Instruments SA, Claix, France) modified to detect minima and maxima of individual breaths, calculate tidal volume (VT), and measure inspiratory time (TI), expiratory time (TE), and the total time for each breath (TTOT), from which instantaneous breathing frequency (f) was determined (1/TTOT × 60). All detected breaths were hand-checked for accuracy. Coefficients of variation for these ventilatory parameters were calculated as the standard deviation SD/mean × 100. Minute ventilation (VE) was calculated as VT × f. Tidal volume was measured based on the mean voltage deflections arising from the 50 μl calibration air injections. Calibration values were used to calculate VT only if the beginning and final mean values were within ±10% of one another, indicating no substantial change in neck seal characteristics during the recording. Average resting ventilatory parameters were determined from 10 minute segments of continuous plethysmographic recordings from which movement artifacts had been eliminated prior to analysis. There was no significant difference between wild type and Pet-1−/− animals in the amount of movement artifact (percentage of total record) during any of the experimental procedures reported here (J.T.E., unpublished data). The duration and frequency (number per unit time) of prolonged respiratory pauses (here defined as an absence of breathing movements ≥1 sec in duration occurring either at the end of inspiration or at the end of expiration) were measured during resting ventilation under normoxic control conditions and during the 5 min recovery period after exposure to hypoxia. These pauses were excluded from estimates of mean f, TI, TE, VT and their coefficients of variation. Acute ventilatory responses to hypoxia were assessed by averaging ventilatory parameters over the first 15 seconds of the two minute hypoxic exposure, and were expressed as percent change from the 5 min pre-stimulus control level. Similarly, ventilatory parameters to elevated CO2 were averaged over the last minute of the two minute CO2 exposure and expressed as percent change from the average values obtained during the 5 min pre-stimulus control period. All ventilatory measurements and data analyses were performed without a priori knowledge of genotype.
2.4. In vitro hypoglossal nerve activity
Activity in hypoglossal nerve rootlets, which carry inspiratory-related motor discharge, was recorded to assess central respiratory rhythm from brainstem slices containing the preBötzinger Complex (preBötC), using previously described methods (Smith et al., 1991). Briefly, P4.5 wild type and Pet-1−/− mice from the PE colony were deeply anesthetized with isoflurane and the brainstem and cervical spinal cord were rapidly removed and placed in chilled artificial cerebrospinal fluid (ACSF) containing (in mM) 124 NaCl, 25 NaHCO3, 3 KCl, 1.5 CaCl2·2H2O, 1.0 MgSO4·7H2O, 0.5 NaH2PO4·H20, 30 D-glucose, and equilibrated with 95% O2 and 5% CO2 (pH: 7.35-7.40 at 5°C). Thick (∼500 μm) transverse slices containing the preBötC and rostral end of the hypoglossal (XIIth) motor nucleus with intact XIIth nerve rootlets were cut from the medulla oblongata (Vibratome Co. 1000, St. Louis, MO), placed in a polycarbonate recording chamber (0.2 ml volume; Warner Instruments, Hamden, CT) and viewed using a fixed-stage upright electrophysiology microscope (Leica DM LFS; Leica Microsystems, Inc., Bannockburn, IL). The slices were superfused continuously (4 ml/min) with ACSF at 27°C. Rhythmic respiratory network activity was recorded extracellularly from XIIth nerve rootlets via fire-polished glass suction electrodes (tip diameters 40–140μm). The nerve signal was amplified 20,000-50,000x (Axon Instruments CyberAmp 380; Molecular Devices Corporation, Sunnyvale, CA), bandpass filtered (0.3-1 kHz), digitized (10 kHz), then full-wave rectified and averaged over a 200 ms window (∫XII) with Chart software (ADInstuments, Colorado Springs, CO). Extracellular potassium concentration was changed from 3 to 8 mM to alter network excitability in the slice in a controlled fashion. The stability of integrated XIIth nerve activity from both wild type and Pet-1−/− brainstem slices was assessed initially by plotting n versus n+1 Poincaré plots (Nayfeh and Balachandran, 1995; Garfinkel et al., 1997) of interburst interval (analogous to TE in vivo) from a 10 minute continuous recording, as described by Del Negro et al. (2002). Subsequently, a measurement of the interval entropy (Reeke and Coop, 2004) was applied to the phasic network XIIth nerve to quantify variability in the respiratory timing signal.
2.5. Immunohistochemistry
Newborn (P4.5) animals were anesthetized deeply and perfused through the heart with 4% paraformaldehyde in 0.1M sodium phosphate buffer (PB), pH 7.4. The head of the animal was removed, post-fixed overnight (4% paraformaldehyde/0.1M PB), infiltrated with 30% sucrose in 0.1M PB for 24-48 hr, placed in a 1:1 mixture of 30% sucrose and Tissue Tek embedding medium (Baxter Scientific, McGraw Park, Il) for 24 hr, embedded and frozen in Tissue Tek over dry ice, and stored at −80°C until use. Frozen serial sections (20 μm) through the head were cut in the transverse or sagittal plane, thaw-mounted onto microscope slides, then processed immunohistochemically to detect either tyrosine hydroxylase (TH) or 5HT. TH staining was performed as previously described (Erickson et al., 1998) using a polyclonal rabbit anti-TH antibody (1:600; Pel-Freeze, Rogers, AR) and a goat anti-rabbit IgG-FITC secondary antibody (1:200; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). 5HT immunostaining was performed using a polyclonal rabbit anti-5HT antibody (ImmunoStar, Inc., Hudson, WI; #20080) and a goat anti-rabbit IgG-FITC secondary antibody (1:200; Jackson ImmunoResearch). Sections were equilibrated to room temperature, immersed in PB-Triton (PBT; 0.1 M PB with 10% Triton X-100) for 15 min, soaked in blocking solution (PBT with 2% sheep serum) for 1 hr at room temperature, then incubated with primary antibody (diluted 1: 8,000 in blocking solution) overnight at 4°C in a humidified chamber. The next day, the sections were thoroughly rinsed (6 × 5 min) with PBT, incubated with secondary antibody (diluted in PBT/2% sheep serum) in the dark at room temperature for 2 hrs, rinsed (6 × 5 min, in the dark), exposed to freshly made ρ-phenylenediamine (1 mg/ml; Sigma-Aldrich, Inc., St. Louis, MO; P6001) for 1 min, rinsed in PB (2 × 10 min), then cover-slipped with glycerol gel. Sections were stored at −20°C until use.
2.6. Cell counts
The total number of 5HT cells in the medulla oblongata (raphe pallidus, raphe obscurus, raphe magnus, and their lateral extensions) was estimated from serial sagittal sections in P4.5 wild type and Pet-1−/− null mice from both the PE and PF colonies (n=5 for each genotype in each colony) using an unbiased stereological technique, the optical fractionator (West et al., 1991). Counting was performed using a Nikon Eclipse E800 microscope, a Nikon DXM1200F digital camera, and Act-1 imaging software (v2.62). Individual estimates of the total number of 5HT-stained neurons (N) were calculated using the formula: N = ∑Q− × 1/ssf × 1/asf × 1/tsf where ∑Q− is the sum of the counted neurons for each sample, ssf is the section sampling fraction, asf is the area sampling fraction, and tsf is the thickness sampling fraction. For each animal, all brainstem sections containing the structures of interest were identified and were sampled systematically using a uniform random sampling procedure. Briefly, the initial section to be measured was selected randomly from the first four sections in the series and every fourth section thereafter through the entire series was analyzed (i.e., ssf =1/4). Within each selected section, the specific area containing 5HT cells to be counted was selected using a random number generator in conjunction with a numbered grid applied to the computer monitor that covered the entire area of interest when captured at 10x. Once randomly selected, the area was viewed at 40x for counting, centered on a second onscreen grid containing regularly spaced counting (disector) frames. The size of the disector frame and the distances separating the frames in both the x and y direction was calibrated with a stage micrometer imaged at the same (40x) magnification. These procedures set the area sampling fraction (frame area = 269 μm2, step area = 1076 μm2, asf = 269/1076 = ¼). The full thickness of the section was used as the height of the optical disector (i.e., tsf = 1). 5HT-stained cells could be distinguished clearly from unstained cells, however, the cell nucleus was often indistinct. Therefore, the cell body was used as the object counted. While focusing down through the tissue section, all cell bodies that either touched the inclusion lines of the disector frame or fell within the frame were counted, whereas cell bodies that fell outside the frame or touched the exclusion lines of the frame were not. Each onscreen image (at 40x) encompassed 30 disector frames, each of which was examined for the presence of 5HT-stained cells.
2.7. Statistical analyses
Weight data were analyzed within each colony using one-way ANOVA at each age, followed by post-hoc testing with the Bonferroni correction for multiple comparisons. Unpaired t-tests were used for weight comparisons between either wild type or Pet-1−/− mice at each age across colonies. To test for genotype differences in neonatal mortality and to assess genotype frequencies, Chi-square analyses were performed and the Yates correction factor was applied to 2×2 contingency tables. Unpaired t-tests were used to compare mean ventilatory parameters (f, TE, TI, VT, etc.) between wild type and Pet-1−/− null mice of the same age, either within or between colonies. Similarly, unpaired t-tests were used to compare mean ventilatory responses to elevated oxygen or CO2 levels (expressed as % change from baseline control) between genotypes. When comparing mean ventilatory parameters at different ages, paired t-tests were employed. One-way ANOVA was used test for differences in interburst intervals or interval entropy values derived from in vitro recordings of XIIth nerve discharge from wild type and Pet-1−/− brainstem slices. 5HT cell counts were analyzed using a two-way ANOVA (2 × 2 design) with genotype (levels = wild type and Pet-1−/− null) and location (levels = PE and PF) as the tested factors. Unless specified otherwise, all values are reported as the mean ± the standard deviation (SD). For all statistical tests, α = 0.05.
3. Results
3.1. 5HT neuron-deficient Pet-1−/− neonates have a low birth weight and experience increased early postnatal mortality
We reported previously that adult wild type and Pet-1−/− mice do not differ significantly in body weight (Hendricks et al., 2003). However, measurements taken on the day of birth indicated that Pet-1−/− neonates in both the PE and PF colonies had significantly reduced body weights compared to wild type littermates and these weight differences persisted through the second postnatal week (Supplementary Table 1). No age-specific differences in body weight were detected between wild type and heterozygous animals in either colony. When comparisons were made between wild types or Pet-1−/− mutants across colonies, no significant difference in body weight was found at any age through P10.5.
We also reported non-Mendelian numbers of Pet-1−/− mice at weaning, suggesting increased mutant mortality (Hendricks et al., 2003). To determine at what point in development the increased mortality occurred, we counted the numbers of live wild type, Pet-1+/−and Pet-1−/− mice present at birth and each day thereafter for 10 days. Pet-1−/− mice were born in expected Mendelian ratios (Table 1A), indicating that they survived to birth. Significantly, nearly a third of the mutant mice died within five days of birth in the PE colony (Table 1B, PE), and 23% died in the PF colony (Table 1B, PF). By contrast, in both colonies, wild type and Pet-1+/− animals had similar and significantly lower mortality rates than Pet-1−/− mice. After postnatal day 5 the number of Pet-1−/− deaths was not different from that of their Pet-1+/− or wild type littermates. These findings suggest an early postnatal critical period during which low birth weight Pet-1−/− neonates are at increased risk of death.
Table 1.
Pet-1 mutant mice are born in normal Mendelian ratios but a significant subset die prematurely during the first postnatal week. (A) Genotype frequencies of wild type (+/+), heterozygous (+/−) and homozygous (−/−) Pet-1 mice at birth. The frequencies of +/+, +/− and −/− Pet-1 mice observed on the day of birth were not significantly different from expected Mendelian ratios in either the pathogen-exposed (PE) or pathogen-free (PF) colonies (χ2; PE, P=0.66, 2 df; PF, P=0.79, 2 df). (B) In both colonies, postnatal mortality rates of −/− mice were significantly greater than either +/+ or +/− animals, while the mortality rates of +/+ and +/− mice were not significantly different from one another. In addition, there was no significant difference in mutant mortality rates between the two colonies (χ2, P=0.83, 2 df).
| A Genotype Frequency | |||
|---|---|---|---|
| Genotype | Number born (n) | Frequency (%) | |
| PE | |||
| (+/+) | 96 | 23 | |
| (+/−) | 224 | 52 | |
| (−/−) | 107 | 25 | |
| PF | |||
| (+/+) | 71 | 25 | |
| (+/−) | 150 | 52 | |
| (−/−) | 65 | 23 | |
| B Mortality Rate | |||
| Genotype | Number born (n) |
Number of deaths |
Mortality (%) |
| PE | |||
| (+/+) | 81 | 4 | 5 |
| (+/−) | 174 | 12 | 7 |
| (−/−) | 80 | 25 | 31 |
| PF | |||
| (+/+) | 60 | 2 | 3 |
| (+/−) | 107 | 7 | 7 |
| (−/−) | 47 | 11 | 23 |
3.2. Breathing frequency is depressed and interrupted more frequently by prolonged respiratory pauses in Pet-1−/− neonates from the PE colony
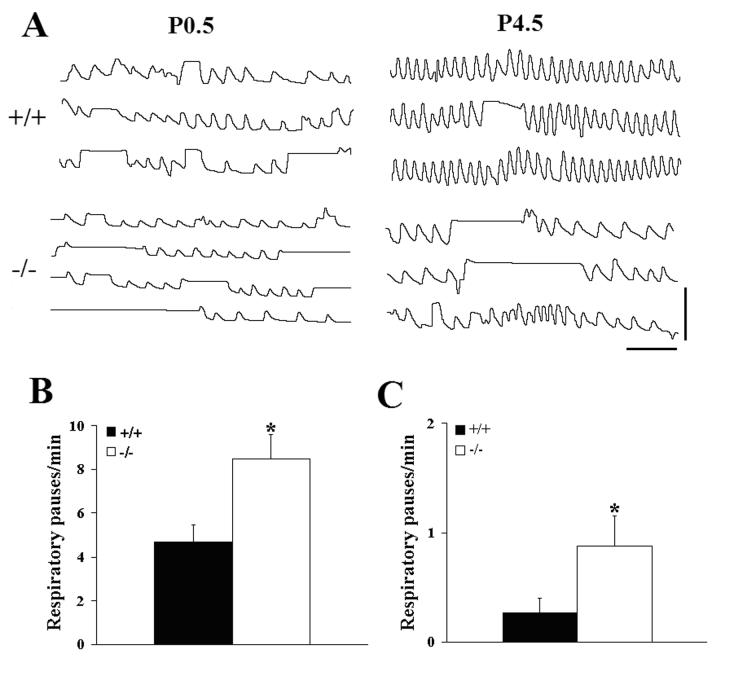
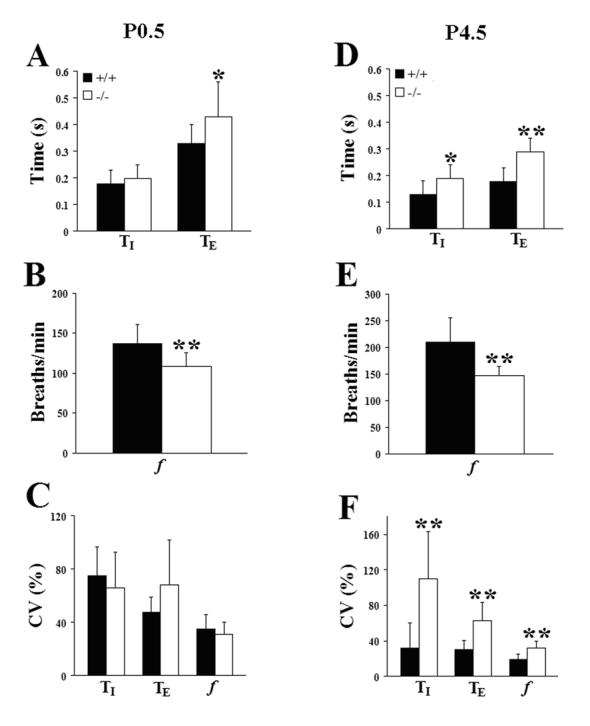
Plethysmographic recordings on the day of birth revealed a highly variable breathing pattern in both wild type and Pet-1−/− mice (Fig. 1A, P0.5), as is typical of newborn mammals (Mortola, 1984). The Pet-1−/− null mice could sustain periods of rhythmic breathing (Fig. 1A −/−, top trace), however, compared to wild type littermates, this was interrupted more frequently by prolonged (≥1s) respiratory pauses (Fig. 1B) of longer mean duration (+/+, 2.18 ± 0.21s, n = 13 vs −/−, 2.93 ± 0.21s, n = 13, P=0.0183). Both wild type and Pet-1−/− neonates experienced prolonged respiratory pauses during expiration, however, the majority of these pauses occurred at the end of inspiration (Fig. 1A; 105/145 = 72% were end-inspiratory in +/+ and 193/238 = 81% were end-inspiratory in −/− animals). When these long respiratory pauses were excluded from the analysis, the breathing frequency (f) of the Pet-1 mutants was found to be significantly depressed by 21% compared to wild type controls, due to a 30% increase in expiratory time (TE), while no genotype-specific differences in breath-to-breath variability of f, TE, and inspiratory time (TI) were observed (Fig. 2A,B,C; Table 2A). The baseline ventilatory pattern in the Pet-1−/− null was therefore characterized by an underlying depression of breathing frequency that was exacerbated by a higher incidence of prolonged respiratory pauses, compared to wild type controls.
Figure 1.
Breathing behavior is compromised in Pet-1−/− mice during the early postnatal period. (A) Plethysmographic recordings of resting ventilation in wild-type (+/+) and Pet-1 null (−/−) mice made within 12 hrs of birth (P0.5) and on postnatal day 4.5 (P4.5) while breathing 21% O2. The −/− recordings reflect the finding that the prolonged respiratory pauses occurred predominantly during inspiration. (B,C) Comparison of the incidence of prolonged (≥1 sec duration) respiratory pauses in wild type (+/+) and Pet-1 mutant (−/−) mice on the day of birth (B; +/+, 4.7 ± 0.78/min, n = 13 vs −/−, 8.5 ± 4.1/min, n = 13, P=0.0114) and on postnatal day 4.5 (C; +/+, 0.27 ± 0.13/min, n = 19 vs −/−, 0.88 ± 0.27/min, n = 10, P=0.0288). Values are means ± SE. *P<0.05. Scale bars: horizontal, 2 sec; vertical, 50 μL.
Figure 2.
Comparison of mean inspiratory (TI) and expiratory (TE) time, breathing frequency (f), and the coefficients of variation (CV=SD/mean × 100) of these parameters, respectively, on the day of birth (P0.5; A,B,C) and on postnatal day 4.5 (D,E,F). Values are means ± SD. *P<0.05; **P<0.01. See Table 2A,B for sample sizes and P values.
Table 2.
Comparison of ventilatory parameters between wild type (+/+) and Pet-1 homozygous (−/−) mice in the PE colony at three postnatal ages. Values are means (± SD) of inspiratory time (TI, sec), expiratory time (TE, sec), breathing frequency (f, breaths/min), and their coefficients of variation (CvTI, CvTE, Cvf; CV=SD/mean × 100) on postnatal days 0.5 (A), 4.5 (B) and 9.5 (C). n = sample size. Two-tailed t-tests, α=0.05;
| A P0.5 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Genotype | n | TI | TE | f | CvTI | CvTE | Cvf | |
| (+/+) | 12 | .18 ±.05 | .33 ± .07 | 137 ± 24 | 75 ± 22 | 48 ± 11 | 35 ± 11 | |
| (−/−) | 12 | .20 ± .05 | .43 ± .13 | 108 ± 17 | 66 ± 27 | 68 ± 34 | 31 ± 9 | |
| P | 0.2584 | 0.0227* | 0.0030** | 0.4102 | 0.0606 | 0.4023 | ||
| B P4.5 | ||||||||
| Genotype | n | TI | TE | f | CvTI | CvTE | Cvf | |
| (+/+) | 11 | .13 ±.05 | .18 ± .05 | 210 ± 45 | 32 ± 28 | 30 ± 11 | 19 ± 6 | |
| (−/−) | 7 | .19 ± .05 | .29 ± .05 | 147 ± 17 | 110 ± 53 | 63 ± 20 | 32 ± 8 | |
| P | 0.0448* | 0.0003** | 0.0026** | 0.0002** | 0.0003** | 0.0017** | ||
| C P9.5 | ||||||||
| Genotype | n | TI | TE | f | CvTI | CvTE | Cvf | |
| (+/+) | 9 | .11 ± .02 | .16 ± .02 | 222 ± 26 | 22 ± 6 | 19 ± 5 | 14 ± 3 | |
| (−/−) | 5 | .12 ± .02 | .20 ± .01 | 193 ± 14 | 29 ± 7 | 27 ± 4 | 16 ± 3 | |
| P | 0.2437 | 0.0080** | 0.0387* | 0.0953 | 0.0079** | 0.4479 | ||
P<0.05
P<0.01
Similar measurements on postnatal day (P) 4.5 revealed a worsening respiratory phenotype in the Pet-1−/− mice, relative to wild type littermates. At this age, f was decreased by 30% in the mutants, due to significant increases in both TI (46%) and TE (61%) (Fig. 1A, P4.5; Fig. 2D, E; Table 2B). Tidal volume did not differ between genotypes at this age (+/+, 28 ± 1 μL, n = 11 vs −/−, 23 ± 3 μL, n = 7; P=0.0538), however, minute ventilation was depressed by 28% in the Pet-1−/− neonates (+/+, 2133 ± 207, μL/min/g, n = 11 vs −/−, 1539 ± 102 μL/min/g, n = 7; P=0.0462). Importantly, the breathing pattern remained highly variable in Pet-1−/− mice, while the variability of f, TE and TI decreased significantly in wild type animals between the day of birth and P4.5 (Fig. 2F; paired t-tests between P0.5 and P4.5, CVf, P=0.0004; CVTI, P=0.0002; CVTE, P=0.0036). In addition, the depressed rhythmic ventilation in the Pet-1−/− neonates continued to be interrupted more frequently by prolonged respiratory pauses than in wild type littermates (Fig. 1C). Similar to P0.5, the majority of these pauses occurred at the end of inspiration (+/+: 39/48 = 81%; −/− 92/112 = 82%). Occasional sighs were observed, however, the frequency of these augmented breaths was not different between genotypes (+/+, 0.40 ± 0.06/min vs −/−, 0.36 ± 0.09/min; P=0.6736). Taken together, these data indicate that 5HT neuron-deficient Pet-1−/− mice are born with an underlying abnormality in ventilatory control and are unable to stabilize their breathing pattern as well as wild types during the early postnatal period.
3.3. The respiratory phenotype of Pet-1−/− mice improves with increasing postnatal age
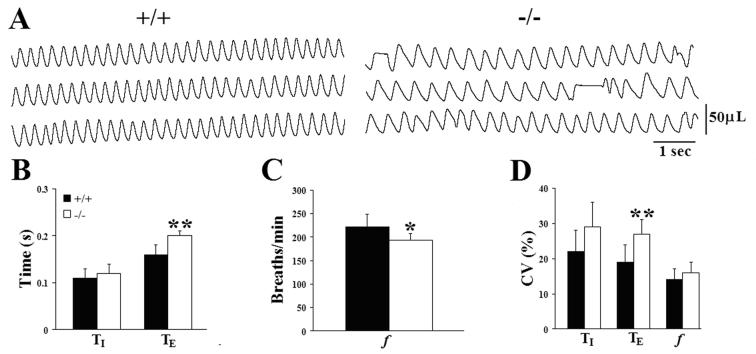
To determine whether the breathing deficits observed in Pet-1−/− mice on P4.5 were permanent, we repeated our measurements on postnatal day 9.5. By this age, breathing behavior in the Pet-1−/− neonates from the PE colony was markedly improved (Fig. 3A, −/−; compare with Fig. 1A, P4.5 −/− recorded from the same animal). Mean f was depressed by only 13% relative to the wild type (Fig. 3C; Table 2C), due to a 25% increase in TE (Fig. 3B). Tidal volume was significantly greater in wild type animals (+/+, 46 ± 2 μL, n=9 vs −/−, 35 ± 4 μL, n=5; P=0.0210) due to a pronounced difference in body size at this age (Supplementary Table 1). However, weight-specific minute ventilation was not different between the two groups (+/+, 1723 ± 102 μL/min/g, n = 9 vs −/−, 1660 ± 118 μL/min/g, n = 5; P=0.7083). We found similar variability in f and TI between wild type and Pet-1−/− mice at P9.5, whereas the coefficient of variation of TE remained greater in the mutants (Fig. 3D). Variability of TE was, however, significantly less than at P4.5 (Table 2B,C; paired t-test, P=0.0307). In addition, although we continued to detect occasional short respiratory pauses in Pet-1−/− mice (see, e.g. Fig 3A), we rarely observed prolonged (≥1s) pauses in the Pet-1−/− animals at this age. The significant improvement in breathing behavior in the Pet-1 mutants by P9.5, relative to wild type controls, indicates that a regular and stable breathing pattern can eventually develop in the 5HT-deficient Pet-1−/− mice. However, the loss of serotonergic modulation delays the normal time course of postnatal respiratory maturation, significantly prolonging the early neonatal period that is characterized by highly unstable ventilation.
Figure 3.
Breathing deficits improve in Pet-1−/− mice with increasing postnatal age. (A) Plethysmographic recordings of resting ventilation in a wild type (+/+) and Pet-1 mutant (−/−) mouse breathing 21% O2 on postnatal day 9.5. Recordings are from the same P4.5 animals presented in Fig. 1. (B,C,D) Comparison of mean inspiratory (TI) and expiratory (TE) time (B), breathing frequency (f; panel C), and the coefficients of variation (CV=SD/mean x 100) of these parameters (D) between genotypes on P9.5. At this age prolonged respiratory pauses were rarely seen in the serotonin-deficient mice. Values are means ± SD. *P<0.05; **P<0.01. See Table 2C for sample sizes and P values.
3.4. Irregular central respiratory rhythm in Pet-1−/− neonates
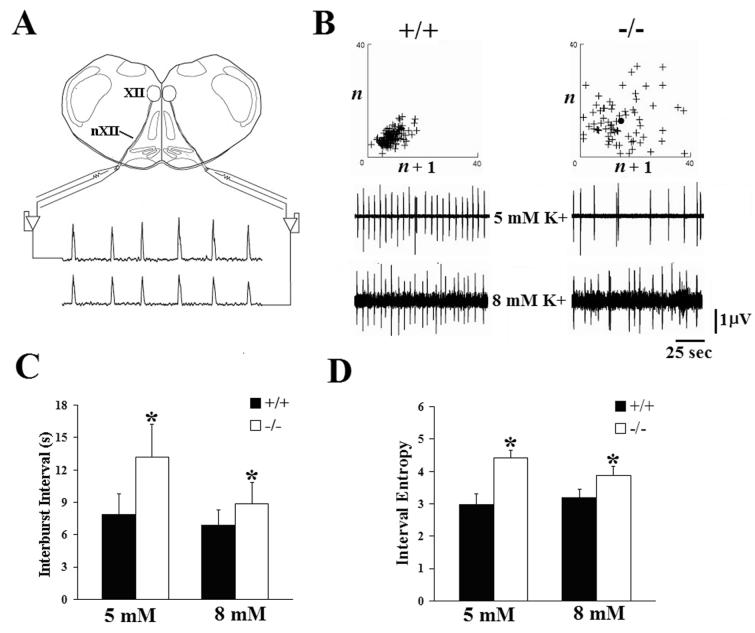
We wondered whether the depressed and irregular breathing we observed in Pet-1−/− mice in vivo might reflect deficiencies at the level of the central respiratory rhythm generator. Previous in vitro studies indicated that application of serotonergic agonists or stimulation of endogenous 5HT release in brainstem tissue has excitatory effects on developing neural networks responsible for respiratory rhythmogenesis (see Hilaire and Duron, 1999). We therefore predicted that respiratory activity in vitro would be relatively depressed in 5HT-deficient Pet-1−/− mice. To test this prediction, we compared hypoglossal (XIIth) nerve discharge from brainstem slice preparations taken from P4.5 wild type and Pet-1−/− mice in the PE colony (Fig. 4A). These slices contained the preBötC and all necessary and sufficient neural circuitry to generate fictive inspiratory rhythm (Smith et al., 1991). We found no genotype-specific differences in burst duration (5mM, +/+, 0.506 ± 0.083s, n=11 vs −/−, 0.596 ± 0.284s, n=10, P=0.3265; 8 mM, +/+, 0.617 ± 0.183s, n=16 vs −/−, 0.683 ± 0.244s, n=15, P=0.3992) or burst area (5mM, +/+, 0.370 ± 0.068 volt x seconds, n=11 vs −/−, 0.449 ± 0.141 volt x seconds, n=10, P=0.1132; 8 mM, +/+, 0.340 ± 0.078 volt x seconds, n=16 vs −/−, 0.447 ± 0.286 volt × seconds, n=15, P=0.1601) of XIIth nerve discharges at either level of extracellular potassium tested (Fig. 4B), suggesting that inspiratory drive in vitro is not significantly affected by 5HT deficiency. Construction of Poincarè plots (Del Negro et al., 2002) derived from interburst intervals (analogous to TE in vivo) measured in wild type brainstem slices revealed nerve discharges that were consistently regular and predictable, and characterized by a compact distribution of points in a dense cluster (Fig. 4B, +/+). By contrast, similar plots derived from Pet-1−/− slices displayed fewer points over the same time period in a much more diffuse pattern (Fig. 4B, −/−), suggesting that 5HT plays a role in the timing of the discharges. Quantitative analysis of these data indicated that XIIth nerve discharges from Pet-1−/− slices had a significantly longer interburst interval (and therefore lower discharge frequency) than wild type slices, when tested at two different levels of extracellular potassium concentration (Fig. 4C). Potassium levels were altered to uniformly change the excitability of all neuronal populations contained within the slice. The Poincarè analysis suggested that the timing of successive XIIth nerve discharges were poorly correlated in the Pet-1−/− slices. We used interval entropy analysis (Reeke and Coop, 2004) to quantify and compare the variability of interburst intervals in the respiratory neural network between genotypes. This analysis demonstrated that the inter-burst intervals between XIIth nerve discharges were significantly more variable in Pet-1−/− than wild type slices (Fig. 4D), particularly at near physiologic extracellular potassium levels (Brockhaus et al., 1993). Combined with our in vivo studies, these data suggest that the ventilatory abnormalities in resting ventilation we observed in intact 5HT-deficient Pet-1−/− neonates derive, at least in part, from depressed and irregular output from the central respiratory rhythm generator.
Figure 4.
Disorder of central respiratory rhythm may contribute to the breathing deficits of Pet-1 null mutant mice. (A) In vitro recording arrangement. Output from the central respiratory rhythm generator was assessed via recordings from hypoglossal nerve (nXII) rootlets arising from neurons in the hypoglossal nucleus (XII). (B) XIIth nerve recordings from wild type (+/+, left panel) and Pet-1−/− (−/−, right panel) medullary slices at two different levels of extracellular potassium (5 and 8 mM K+). No significant differences in either burst duration or burst area were detected. Above each set of recordings are representative Poincaré plots (see Materials and Methods) of interburst intervals (analogous to TE in vivo) derived from recordings in +/+ and −/− slices at 8 mM K+. Each cross (+) represents a single nerve discharge. If interburst intervals were identical for every fictive breath, the graph would reduce to a single point representing that identical time between two discharges. (C) Comparison of mean interburst interval of XIIth nerve discharges from +/+ and −/− brainstem slices exposed to either 5 or 8 mM potassium. (D) Comparison of interval entropy values derived from XIIth nerve discharges in +/+ and −/−brainstem slices exposed to two different levels of extracellular potassium. For both panels (C) and (D): +/+: n=13 at 5 mM, n=19 at 8 mM; −/−: n=13 at 5 mM, n=15 at 8 mM. All values are means ± SD. *P<0.05.
3.5. The respiratory phenotype of Pet-1−/− neonates is susceptible to environmental conditions
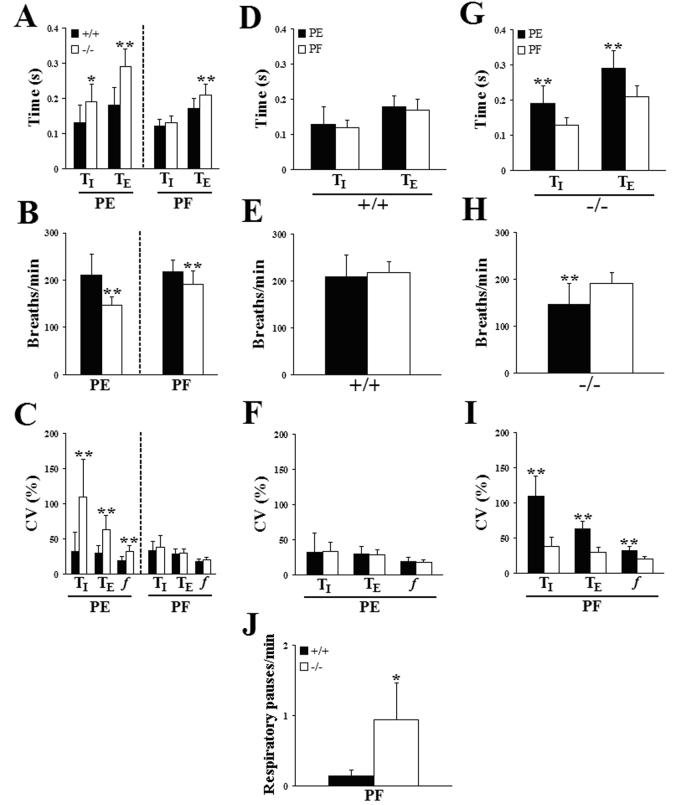
After completion of our initial plethysmographic study, sentinel testing of the original Pet-1 colony revealed exposure to several murine pathogens (see Materials and Methods). We therefore established a second Pet-1 mutant colony under pathogen-free (PF) conditions and repeated our in vivo plethysmographic studies. Similar to PE Pet-1−/−mice, the incidence of prolonged respiratory pauses during resting ventilation in normoxia was significantly increased in P4.5 Pet-1−/− animals in the PF colony, compared to PF wild type controls (Fig. 5J). Resting ventilation was also significantly impaired in P4.5 Pet-1−/− neonates from the PF colony but this impairment was less severe than in the PE colony (Fig. 5A,B,C; data from the PE colony presented in Fig. 2 is repeated here for easy comparison. See also Table 2B, Table 3). Specifically, the mutant f in the PF colony was depressed by only 12% relative to wild type controls (Fig. 5B) due to a 24% increase in TE but no difference in TI (Fig. 5A). In addition, no significant differences in variability of f, TI or TE were observed between wild type and Pet-1−/− mutants in the PF colony (Fig. 5C), whereas the Pet-1−/− mice in the PE colony displayed greater variability than the wild type in all three parameters. Importantly, when ventilatory parameters from wild type animals from each colony were compared directly (Fig. 5D,E,F) no significant differences were observed in any of the measured parameters, highlighting the fact that the breathing pattern in wild type animals was not influenced by any differences in environmental conditions within the PE and PF colonies. In stark contrast, however, all ventilatory parameters measured in Pet-1−/− neonates from the PE colony were significantly different when compared directly with the PF colony (Fig. 5G,H,I). Thus, breathing frequency was significantly lower (Fig. 5H), both TI and TE were greater (Fig. 5G) and the coefficients of variation for all three parameters were greater (Fig. 5I) in Pet-1−/−mice reared in the PE colony. Taken together, these findings confirm an underlying abnormality of breathing behavior in Pet-1−/− neonates and demonstrate that the magnitude of these deficits is susceptible to environmental influences.
Figure 5.
The severity of breathing abnormalities in Pet-1−/− mice can be influenced by environmental conditions. (A,B,C) Comparison of inspiratory (TI) and expiratory (TE) time (A), breathing frequency (B) and the coefficients of variation of these ventilatory parameters (C) in P4.5 wild type (+/+, dark bars) and Pet-1 null mutant (−/−, light bars) mice reared in either a pathogen-exposed (PE) or pathogen-free (PF) environment. (D,E,F) Direct comparison of inspiratory (TI) and expiratory (TE) time (D), breathing frequency (E) and the coefficients of variation of these ventilatory parameters (F) in P4.5 +/+ mice from the PE and PF colonies. (G,H,I) Direct comparison of inspiratory (TI) and expiratory (TE) time (G), breathing frequency (H) and the coefficients of variation of these ventilatory parameters (I) in P4.5 Pet-1−/− mice from the PE and PF colonies. (J) Pet-1−/− mice from the PF colony also suffered an increased incidence of prolonged respiratory pauses, relative to wild type littermates (compare with Fig. 1C). Values are means ± SD. *P<0.05; **P<0.01. See Table 2B, Table 3 for sample sizes and P values.
Table 3.
The severity of breathing deficits in Pet-1−/− mice is influenced by environmental conditions. (A) Mean values (± SD) of inspiratory time (TI, sec), expiratory time (TE, sec), breathing frequency (f, breaths/min), and their coefficients of variation (CvTI, CvTE, Cvf ; SD/mean × 100) for P4.5 wild type (+/+) and Pet-1 knockout (−/−) mice reared in a pathogen-free (PF) colony. Statistical comparisons (P) between genotypes within the colony for each parameter reveal a milder respiratory phenotype compared to the pathogen-exposed colony (see Table 1B). (B) Statistical comparisons of ventilatory measurements made on P4.5 between similar genotypes (+/+ or −/−) across-colonies (PE vs PF). No differences were detected between PF and PE wild type animals. By contrast, mutant mice from the PF and PE colonies differed significantly in all ventilatory parameters tested. n = sample size. P = two-tailed t-tests, α=0.05;
| A P4.5 PF | ||||||||
|---|---|---|---|---|---|---|---|---|
| Genotype | n | TI | TE | f | CvTI | CvTE | Cvf | |
| (+/+) | 19 | .12 ±.02 | .17 ± .03 | 218 ± 24 | 34 ± 13 | 29 ± 7 | 18 ± 4 | |
| (−/−) | 12 | .13 ± .02 | .21 ± .03 | 191 ± 28 | 38 ± 17 | 30 ± 6 | 20 ± 4 | |
| P (P4.5 PF +/+ vs P4.5 PF −/−) | 0.2506 | 0.0019** | 0.0079** | 0.4649 | 0.7839 | 0.3412 | ||
| B | Comparison | TI | TE | f | CvTI | CvTE | Cvf | |
| P (P4.5 PF +/+ vs P4.5 PE +/+) | 0.3244 | 0.3957 | 0.5530 | 0.7525 | 0.8214 | 0.8361 | ||
| P (P4.5 PF −/− vs P4.5 PE −/−) | 0.0020** | 0.0005** | 0.0018** | 0.0003** | 0.0004** | 0.0004** | ||
P<0.05
P<0.01
3.6. The incidence of prolonged respiratory pauses can be increased in Pet-1−/− neonates by low environmental oxygen
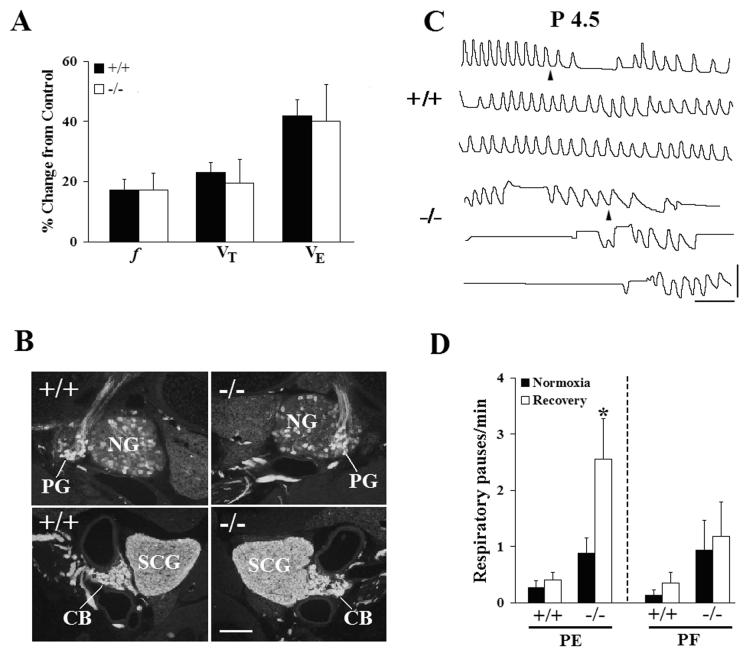
To further explore the influence of environmental conditions on breathing behavior in Pet-1−/− neonates, we measured the ventilatory responses of P4.5 wild type and Pet-1−/− mice to low environmental oxygen. During the initial 15 sec of a 2 min exposure to a 10% O2 test stimulus, wild type and Pet-1−/− mice from the PE colony increased f, tidal volume and minute ventilation to the same relative extent (Fig. 6A), indicating that Pet-1−/− neonates are able to detect low environmental O2 and respond acutely with an immediate increase in breathing. Consistent with this finding, immunohistochemical staining of the carotid body and petrosal ganglion with an antibody against tyrosine hydroxylase, a marker for both carotid body glomus cells (Erickson et al., 1998) and carotid body chemoafferent neurons (Katz and Black, 1986), revealed no obvious differences in these structures between wild type and Pet-1−/− mice (Fig. 6B), suggesting the presence of an intact carotid body chemoafferent pathway in the mutant. During the hypoxic exposure, the incidence of prolonged respiratory pauses in both wild type and Pet-1 mutants decreased (data not shown). However, when switched back to a normoxic gas mixture at the end of the hypoxic exposure, the PE mutants exhibited a nearly 3-fold increase in the incidence of prolonged respiratory pauses during the post-hypoxic recovery period (Fig. 6C −/−; Fig. 6D, PE −/−). Pet-1−/− mice from the PF colony, in contrast, were unaffected by the same hypoxic exposure (Fig. 6D, PF −/−). Importantly, the incidence of prolonged respiratory pauses during the recovery period was not increased in wild type animals from either colony following hypoxia (Fig 6D +/+ PE and PF). In the PE colony, mean respiratory pause duration was not different between genotypes during the prehypoxia control period (+/+, 2.43 ± 0.31s vs −/−, 1.99 ± 0.17s; P=0.1763), but increased significantly in the Pet-1−/− animals during the normoxic recovery period (2.64 ± 0.15s vs control value of 1.99 ± 0.17s; P=0.0257) and decreased significantly in the wild types (1.65 ± 0.09s vs control value of 2.43 ± 0.31s; P=0.0029). Moreover, the range of pause durations observed in the PE Pet-1−/− mice during the recovery period was substantially wider than in wild type controls (+/+: 1-3.5s vs −/−: 1-8.6s). Interestingly, a 2 min exposure to 10% O2 did not increase the incidence of prolonged respiratory pauses in P9.5 PE mice (data not shown). Taken together, these data show that a brief exposure to low environmental O2 during the early postnatal period can further destabilize breathing in Pet-1−/− mice in an environment-dependent manner.
Figure 6.
Breathing behavior in Pet-1−/− mice can be destabilized following a short-term exposure to low environmental oxygen. (A) Pet-1−/− mice are able to detect a low oxygen stimulus. No differences in breathing frequency (f), tidal volume (VT) or minute ventilation (VE) were observed in P4.5 +/+ or −/− mice from the PE colony during the initial 15s of a 2 minute exposure to 10% O2. (B) Tyrosine hydroxylase (TH)-immunostained sections through the petrosal ganglion (upper panels) and carotid body (lower panels) from a +/+ and −/− mouse. (C) Comparison of plethysmographic recordings from a P4.5 wild type (+/+) and Pet-1 mutant (−/−) mouse during the transition (arrowhead) from 10% to 21% O2, following a 2 min hypoxic exposure. Several long respiratory pauses can be seen in the record from the mutant mouse immediately following the low O2 exposure. (D) Comparison of the incidence of long respiratory pauses in +/+ and −/− mice from either the pathogen-exposed (PE) or pathogen-free (PF) colony while breathing 21% O2 before (Normoxia) or during a 5 minute normoxic period following a 2 min exposure to 10% O2 (Recovery). A significant increase in the incidence of hypoxia-induced pauses was seen only in −/− mice from the PE colony. Values are means ± SE. Scale bars: panel B, 100 μm; panel C, horizontal, 2 sec, vertical, 50 μL. *P<0.05.
3.7. Pet-1−/− neonates respond normally to increased environmental CO2
Since serotonergic neurons in the brainstem have been proposed as central CO2 sensors (Wang et al., 2001; Bradley et al., 2002; Richerson, 2004), we compared the respiratory responses of P4.5 wild type and Pet-1−/− mice to high (5%) environmental CO2 (in 21% O2, balance N2). We found no significant differences in f (P=0.6575), tidal volume (P=0.5495) or minute ventilation (P=0.6259) between the genotypes when measured during the second minute of a two minute continuous exposure (Fig. 7).
Figure 7.
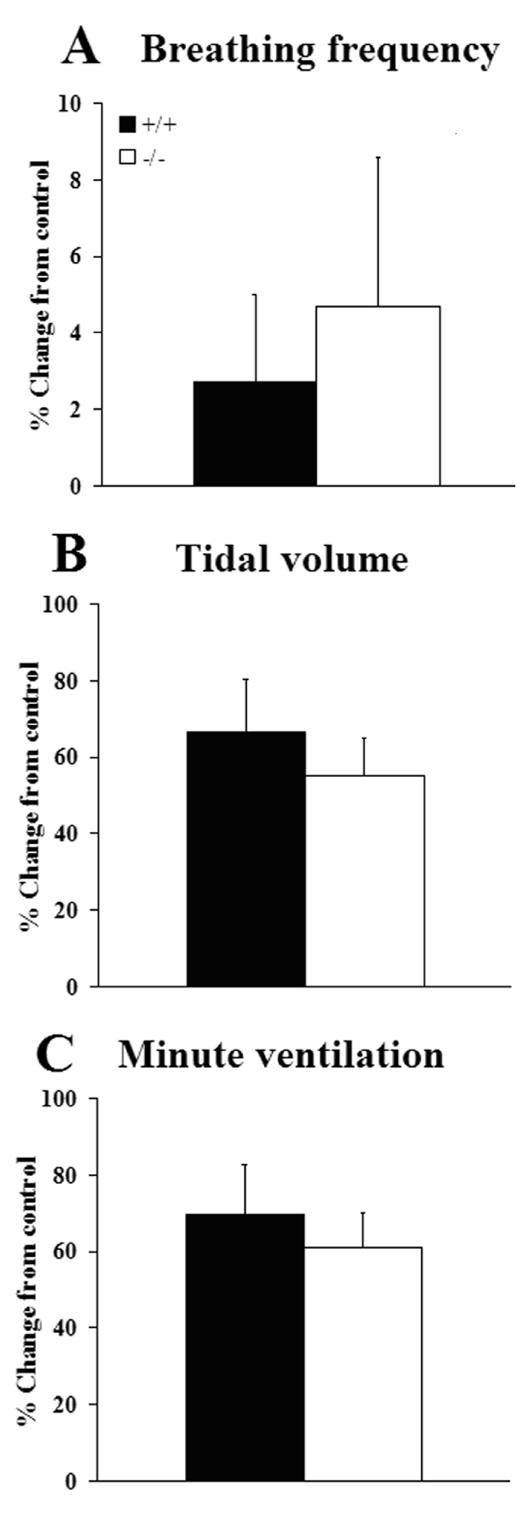
Breathing responses of P4.5 wild type (+/+, n=12) and Pet-1 null mutants (−/−, n=8) from the PF colony to 5% CO2 (in 21% O2, balance N2) expressed as mean percent change from pre-stimulus control levels. Measurements were made during the final minute of a two minute stimulation period. No significant differences (two-tailed t-test, α=0.05) were observed between genotypes in breathing frequency (A), tidal volume (B) or minute ventilation (C). Values are means ± SE.
3.8. The loss of 5HT cells in the medulla oblongata is identical in the PE and PF colonies
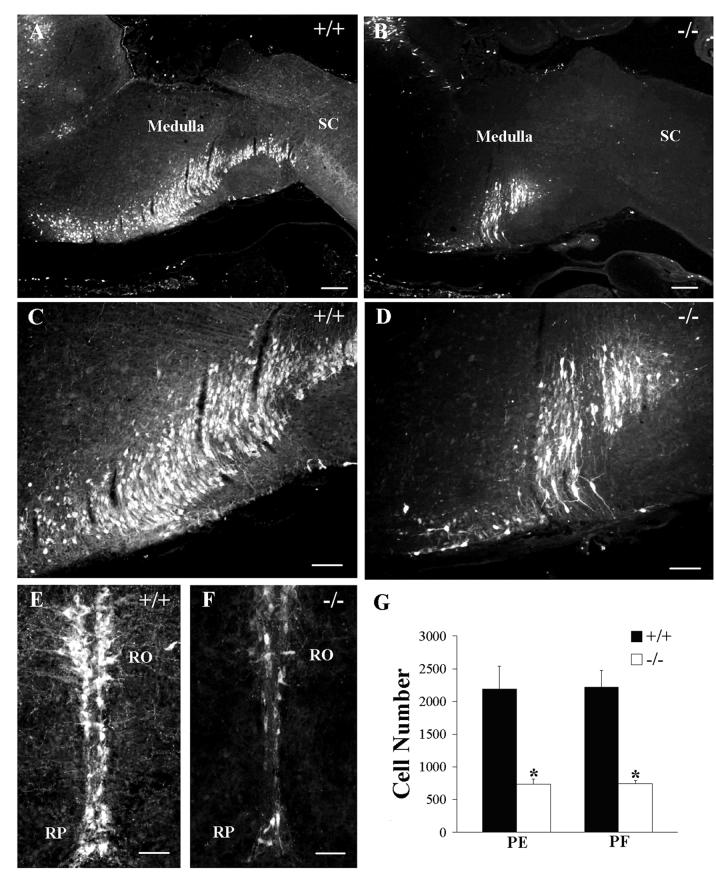
We considered the possibility that the significant differences in respiratory phenotype we detected between Pet-1−/− neonates in the PE and PF colony might be due to more extensive 5HT cell losses in knockouts from the PE colony. We therefore used an unbiased stereology-based technique, the optical fractionator (West et al., 1991), to estimate and compare total 5HT-immunostained cell number in the medulla oblongata in P4.5 wild type and Pet-1−/− mutant mice from each colony. We found a 67% decrease (+/+, 2189 ± 351, n = 5 vs −/−, 733 ± 80, n = 5) in the total number of medullary 5HT-labeled cells in PE null mutants, relative to wild type controls (Fig. 8), very close to the 70% overall loss of 5HT cells in these mice originally reported by Hendricks et al. (2003). In the PF colony, we found a virtually identical 66% decrease in medullary 5HT cell number (+/+, 2221 ± 253, n = 5 vs −/−, 746 ± 50, n = 5) in PF Pet-1−/− mice relative to PF wild types (Fig. 8). A two-way ANOVA revealed a highly significant treatment (genotype) effect (F(1,16) = 219.2, P<0.0001), but no significant location (F(1,16) = 0.051, P=0.824) or interaction (F(1,16) = 0.009, P=0.924) effects. These data indicate that the total number of 5HT cells in the medulla oblongata in wild type animals from the PE and PF colonies were identical, as was the magnitude of the 5HT cell losses sustained by the null mutants in each colony. Therefore, differences in 5HT cell number, per se, cannot account for the more severe respiratory phenotype that we observed in mutant mice from the PE colony.
Figure 8.
Pet-1−/− mice reared in the PE and PF colonies have a similar degree of 5HT cell loss in the medulla oblongata. Photomicrographs of sagittal (A-D) or transverse (E,F) sections of the medulla oblongata of a wild type (A, 4x; C, 10x, E, 20x) and a Pet-1−/− (B, 4x; D, 10x, F, 20x) mouse from the PF colony that were immunostained to detect 5HT. (G) The total number of 5HT-immunostained cells was reduced by 67% in the null mutants from the PE colony and by 66% in mutants from the PF colony, relative to their respective wild type controls. Two-way ANOVA revealed significant differences between genotypes in each colony (treatment effect, F(1,16) = 219.2, P<0.0001), but no differences between genotypes across colonies (location effect, F(1,16) = 0.051, P=0.824) and no significant interaction effect (F(1,16) = 0.009, P=0.924). n = 5 for each group. RO, nucleus raphe obscurus; RP, nucleus raphe pallidus; SC, spinal cord. Scale bars: A,B = 250 μm; C,D = 100 μm; E,F = 50 μm.
4. Discussion
Numerous in vitro studies have demonstrated excitatory (Murakoshi et al., 1985; Di Pasquale et al., 1992; Lindsay and Feldman, 1993; Al-Zubaidy et al., 1996; Hilaire et al., 1997; Onimaru et al., 1998; Schwarzacher et al., 2002) and stabilizing (Bou-Flores et al., 2000; Peña and Ramirez, 2002) roles for 5HT neuromodulation during maturation of the respiratory control system, particularly during the perinatal period. However, it is not yet clear whether a specific developmental deficiency of 5HT influences maturation of breathing behavior in the intact mammalian neonate. Here we report, using 5HT neuron-deficient Pet-1−/− mice, that serotonergic neuron function is required for stabilization of ventilation after birth and proper timing of respiratory maturation during the early postnatal period. Of particular significance is our finding that breathing behavior in Pet-1−/− neonates can be adversely influenced by environmental conditions. Moreover, loss of 5HT neuron function is associated with increased neonatal mortality. Thus, our study provides unanticipated insights into the impact of serotonergic deficiencies on the maturation of breathing behavior, which may be relevant to understanding mechanisms underlying early childhood respiratory disorders such as SIDS.
4.1. Serotonergic function and breathing stability
The establishment of rhythmogenesis during embryonic development does not require a full complement of 5HT neurons since Pet-1−/− mice are capable of rhythmic ventilation at birth. However, the baseline breathing frequency is depressed at this age and ventilation is interrupted by prolonged respiratory pauses more frequently in Pet-1−/− animals than in wild type littermates, indicating that breathing behavior is already compromised in mutant mice very early in postnatal development. The respiratory phenotype of Pet-1−/− neonates further diverges from wild type during the first postnatal week. Whereas both wild type and Pet-1−/− mice initially display a high degree of variability in breathing pattern in vivo that is characteristic of all newborn mammals (Mortola, 1984), breathing irregularities in wild type animals diminish greatly by P4.5 while respiratory depression and instability persists in the knockouts at this age.
One function of the 5HT system may be to provide excitatory drive, perhaps through direct modulation of synaptic and/or intrinsic pacemaker properties, that promotes a dynamic and stable reconfiguration of the central respiratory network during a critical perinatal period (Peña and Ramirez, 2002; Ramirez and Viemari, 2005). Peña and Ramirez (2002) have shown that blockade of endogenously activated 5HT-2A receptors in mouse brainstem slices decreases the frequency and regularity of respiratory neuron activity, suggesting that 5HT acts directly on the rhythm-generating network to stabilize breathing rhythm. This could serve to smooth the transition from the intrauterine environment where inhibition of breathing movements is prominent (Jansen and Chernick, 1991), to the external environment where continuous and stable breathing movements are required. Our finding that central respiratory rhythm in Pet-1−/− mouse brainstem slices is depressed and more disordered compared to wild type controls, particularly at extracellular potassium concentrations (5 mM) that are close to normal physiologic levels (Brockhaus et al., 1993), supports this idea and suggests that endogenous 5HT normally provides excitatory drive during early postnatal development to stabilize output from the central respiratory rhythm generator.
Surprisingly, we found that by P9.5 the breathing phenotype of Pet-1−/− mice had improved significantly. Specifically, breathing frequency was nearly normal, the variability of f and TI were comparable to wild type, and the variability of TE was significantly reduced. In addition, the high incidence of prolonged respiratory pauses during resting ventilation that was characteristic of P0.5 and P4.5 Pet-1−/− mice was no longer detectable. One major consequence of loss of Pet-1 gene function, therefore, is to significantly prolong the initial ventilatory instability that is typical of the immediate postnatal period. Taken together, these data indicate that 5HT neuron function is crucial for achieving ventilatory stability immediately after birth and appears to be much less important, or not required, for maintaining this stability later in postnatal development. Burnet et al. (2001) have shown that increased levels of 5HT in MAO-A-deficient mice during the perinatal period alter respiratory network maturation, while high 5HT levels in adult mice impair reflex responses to hypoxia and lung inflation. Although breathing behavior appears to normalize in the Pet-1 mutants during the second postnatal week, the possibility remains that the loss of 5HT neuron function as a result of Pet-1 gene deletion could also impair specific aspects of respiratory control in older animals.
The specific underlying mechanisms whereby serotonergic neurons stabilize breathing postnatally remain to be determined. As discussed above, a direct effect of 5HT is likely. However the possibility that 5HT acts indirectly by modulating other neurotransmitter systems should also be considered. For example, endogenous noradrenaline can modulate neonatal respiratory rhythm (see Hilaire, 2006). In particular, A2/C2 neurons in the nucleus tractus solitarius have been implicated in exerting a stabilizing influence on respiratory rhythm (Zanella et al., 2006). Since catecholaminergic neurons in this region receive serotonergic synaptic input (Pickel et al., 1984), it is possible that the effects we have observed in 5HT neuron-deficient Pet-1−/− mice are indirect and result from the loss of 5HT modulation of noradrenergic influences on central rhythm generation. In addition, a developmental switch from excitatory to inhibitory γ-aminobutyric acid (GABA)-mediated neurotransmission has been shown to occur in mouse preBötC neurons during the first postnatal week (Ritter and Zhang, 2000; Zhang et al., 2002). Since serotonergic inputs have been shown to delay the maturation of inhibitory (GABAergic) influences on developing rhythmic circuits in the spinal cord (Branchereau et al., 2002; Allain et al., 2005), it is conceivable that the loss of 5HT neuron function results in premature switching to GABA-mediated inhibitory inputs to the respiratory central pattern generator, resulting in a longer period of depressed and unstable breathing in Pet-1−/− mice.
We observed prolonged respiratory pauses in both wild type and Pet-1 mutants during the early postnatal period that occurred predominantly at the end of inspiration. Respiratory pauses with a similar profile have been reported previously in a variety of different species, including humans, using several different recording methods (Farber, 1972, 1978; Farber and Marlow, 1978; Fisher et al., 1982; Radvanyi-Bouvet et al., 1982; Mortola, 1984; Mortola, 1987), suggesting that these pauses are a general and relatively common phenomenon during early postnatal respiratory maturation in mammals. These pauses are initially common in human infants (Fisher et al., 1982) and newborn rats (Mortola, 1987) but decline in frequency shortly after birth, similar to the pattern we have observed in newborn mice in the present study. Mortola (1984) has demonstrated that they occur in the absence of diaphragmatic EMG activity, suggesting closure of the upper airway as a probable cause, possibly at the laryngeal level, in agreement with earlier conclusions (Farber, 1972; 1978). The function of these occluded breaths is not known although it has been suggested that elevated airway pressure associated with these breaths may provide a “back force” to help clear pulmonary fluid from the lungs and promote even lung expansion in the initial postnatal period (Mortola, 1987). Since laryngeal muscles help maintain airway patency (Bartlett et al., 1973; Harding et al., 1986), we speculate that the increased incidence of end-inspiratory pauses in 5HT-deficient Pet-1 neonates may be due, at least in part, to decreased muscle tone in the upper airway, resulting in more frequent airway occlusions. This is consistent with the fact that motor neurons in the rodent nucleus ambiguus that drive laryngeal muscle contraction receive substantial serotonergic innervation (Sun et al., 2002) and can be excited by iontophoretically applied (Fenik et al., 1997) or endogenously released (Holtman et al., 1987) 5HT. Additional studies will be needed to document any deficiencies in motor control of upper airway musculature in the Pet-1 null mutants and to eliminate the possibility that neck seal constriction, in conjunction with decreased muscle tone, contributed to the increased frequency of occluded breaths in the Pet-1−/− mice.
4.2. Responsiveness to elevated CO2 or decreased O2 levels
P4.5 Pet-1−/− mice responded vigorously to a 5% CO2 challenge with an increase in both tidal volume and breathing frequency, despite the loss of 67% of caudal brainstem 5HT neurons. This is an interesting finding, given the recent proposal that 5HT neurons contribute significantly to central CO2 chemoreception (Richerson, 2004; Richerson et al., 2005). It may be that the remaining 5HT neurons in the Pet-1−/− brain are sufficient for normal CO2 responsiveness. Alternatively, 5HT neurons may constitute only one of several redundant neuronal populations (see Mulkey et al., 2004; Guyenet et al., 2005) that are capable of responding to changes in brain CO2/pH levels and initiating reflex changes in ventilation. In addition, we found that 10% O2 stimulated similar relative increases in tidal volume and breathing frequency in wild type and Pet-1−/− mice, and no obvious differences in the morphology of the carotid body or carotid body afferent neurons were observed in the mutant. It is therefore unlikely that the depressed and irregular resting ventilation we observed in newborn Pet-1−/− mice was due to functional impairment of the carotid body chemoafferent pathway (Erickson et al., 1996) or to a generalized loss of central neuronal excitability in the 5HT-deficient Pet-1−/− mice.
4.3. Susceptibility to environmental challenge
In the present study, specific features of the Pet-1−/− mutant phenotype were invariant, regardless of the environmental conditions under which the newborns were born and nurtured, while others were not. In both colonies Pet-1−/− mice were born smaller, lagged in body weight, experienced an increased incidence of prolonged respiratory pauses during resting ventilation, and were much more likely to die during the first postnatal week, compared to wild type littermates. However, additional aspects of the phenotype including baseline breathing frequency, the variability of breathing parameters (f, TI and TE) and the ability of short-term hypoxia to induce more frequent prolonged respiratory pauses, were clearly different (and worse) in Pet-1−/− mutants from the PE colony. It is important to note that differences in respiratory phenotype between colonies were observed only in the mutant mice; wild type animals from the PE and PF colonies were completely indistinguishable in all respects. These findings demonstrate that the arrest of 5HT neuron differentiation resulting from loss of Pet-1 function establishes an underlying deficit in respiratory control that is susceptible to environmental conditions. We do not yet know the specific environmental conditions responsible for these differences. The fact that the PE colony was exposed to several murine pathogens certainly raises the possibility that the pathogen load contributed, either directly or indirectly, to the more severe respiratory phenotype in the mutant PE mice. A direct effect would seem most likely, although the possibility that a pathogen-induced alteration in maternal behavior may have indirectly impacted the phenotype of the PE mutant neonates cannot be excluded completely. Additional studies will be needed to determine whether, for example, respiratory infection caused by specific pathogens can exacerbate the unstable resting ventilation or promote hypoxia-induced respiratory pauses in Pet-1−/− neonates. Nevertheless, these findings are important in that they establish, in principle, the 5HT-deficient Pet-1−/− mouse as a tractable model system for further understanding the interplay between environmental factors and 5HT neuron function on respiratory homeostasis and survival during early postnatal development.
4.4. Relevance to SIDS
Kinney and colleagues have hypothesized in the “triple risk” model of SIDS that at least some SIDS cases involve an underlying abnormality in 5HT neuron development which disrupts normal protective homeostatic responses to life-threatening environmental “trigger” factor(s) during a critical developmental period of cardiorespiratory control (Kinney, 2005; Kinney et al., 2005). Intriguing new neuropathological findings appear to support this model and suggest that 5HT neuron differentiation is disrupted in SIDS (Paterson et al., 2006). Specifically, the number of brainstem tryptophan hydroxylase (TPH)-immunoreactive cells is significantly greater in SIDS cases compared to controls, while SIDS cases have reduced levels of 5HT-1A receptor binding in the raphe nuclei, reduced levels of the 5HT transporter relative to 5HT neuron number, and altered morphology of 5HT neurons (Paterson et al., 2006), suggesting that defects in 5HT neuron differentiation may cause a compensatory increase in TPH immunoreactive cells in the brainstem. However, an open question in this study is whether the observed 5HT abnormalities in the SIDS cases, especially the number of TPH immunoreactive cells, were the outcome of abnormal serotonergic neuron development or resulted secondarily from some insult common to SIDS (e.g. hypoxia) experienced near the time of death. This question is of particular importance given the time interval between death and postmortem processing of tissue samples, and the impossibility of obtaining the most appropriate control tissue for comparison (i.e., brainstems from healthy individuals who would otherwise not die of SIDS, collected and processed without delay). Moreover, it is not possible to tell from this study whether the increased TPH-stained cell number is indicative of increased serotonergic neurotransmission in the SIDS brain.
In view of these uncertainties, we believe our findings complement those of Paterson et al. (2006) and provide genetic support for the 5HT “triple risk” model. Our data demonstrate that a specific developmental arrest in 5HT neuron differentiation delays respiratory maturation after birth, resulting in a prolonged postnatal period characterized by a depressed and unstable breathing pattern that is vulnerable to environmental conditions. The finding that Pet-1−/− neonates are at increased risk for early mortality is also consistent with this model. Given the strikingly SIDS-like phenotype of the Pet-1−/− mouse and the current uncertainty concerning the level of 5HT neuron activity, and 5HT synthesis, release, and synaptic clearance in the SIDS brainstem (Paterson et al., 2006), our study raises the possibility that deficient rather than excessive serotonergic neuromodulation may play a role in SIDS. Indeed, increased neonatal mortality has not been reported in monoamine oxidase A null mutant mice that develop with an excess of 5HT (Cases et al., 1995; Bou-Flores et al., 2000). Although our data does not yet allow us to establish a direct causal relationship between mortality and breathing deficits, they certainly suggest the possibility that the increased mortality of Pet-1−/− neonates may result from a genetically compromised cardiorespiratory homeostatic response to environmental factors. It is likely that other deficits, for example heart rate variability or abnormalities in arousal during changes in sleep state, contribute to neonatal Pet-1−/− deaths. A search for additional physiologic deficits in Pet-1−/− mice, as well as identification of specific environmental factors that exacerbate cardiorespiratory instability and/or increase neonatal mortality, should help to clarify this issue. In this regard, Pet-1−/− neonates appear to provide a powerful animal model for providing important additional insights into the interactions between 5HT neuron function and environment in the development of neonatal breathing disorders.
Supplementary Material
Mean body weights (± SD) of wild type (+/+), Pet-1 heterozygous (+/−) and Pet-1 homozygous (−/−) mice from the day of birth (P0.5) through postnatal day 10 (P10.5). (a) Mean body weights (± SD) of mice in the pathogen-exposed (PE) colony. (b) Mean body weights (± SD) of mice in the pathogen-free (PF) colony. (c) Results of post-hoc tests (Bonferroni (LSD) method) comparing body weights between genotypes within the PE colony following one-way ANOVA (α=0.05) at each age. (d) Results of post-hoc tests comparing body weights between genotypes within the PF colony following one-way ANOVA at each age. (e) Body weight comparisons between wild type or Pet-1−/− mice across colonies at each age (two-tailed t-test). *P<0.01. n=sample size.
Acknowledgements
We would like to thank Dr. David M. Katz for use of plethymographic equipment at CWRU, RoxAnne Murphy for care of the colony and genotyping at CWRU, and Nimit Saraiya, Jason Salamandra, and Brian Sposato for help with the plethysmography, survival studies, and immunohistochemistry at TCNJ. Supported by US National Institutes of Health grant MH62723 (E.S.D.) and Support for Scholarly Activity awards from The College of New Jersey (J.T.E.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abadie V, Champagnat J, Fortin G. Branchiomotor activities in mouse embryo. NeuroReport. 2000;11:141–145. doi: 10.1097/00001756-200001170-00028. [DOI] [PubMed] [Google Scholar]
- Allain AE, Meyrand P, Branchereau P. Ontogenetic changes of the spinal GABAergic cell population are controlled by the serotonin (5-HT) system: implication of the 5-HT1 receptor family. J. Neurosci. 2005;25:8714–8724. doi: 10.1523/JNEUROSCI.2398-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Zubaidy Z, Erickson R, Greer J. Serotonergic and noradrenergic effects on respiratory neural discharges in the medullary slice preparation of neonatal rats. Pflügers Arch. 1996;431:942–949. doi: 10.1007/s004240050089. [DOI] [PubMed] [Google Scholar]
- Bartlett D, Remmers JE, Jr., Gautier H. Laryngeal regulation of respiratory airflow. Respir. Physiol. 1973;18:194–200. doi: 10.1016/0034-5687(73)90050-9. [DOI] [PubMed] [Google Scholar]
- Bou-Flores C, Lajard AM, Monteau R, De Maeyer E, Seif I, Lanoir J, Hilaire G. Abnormal phrenic motoneuron activity and morphology in neonatal monoamine oxidase A-deficient transgenic mice: possible role of serotonin excess. J. Neurosci. 2000;20:4646–4656. doi: 10.1523/JNEUROSCI.20-12-04646.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley SR, Pieribone VA, Wang W, Severson CA, Jacobs RA, Richerson GB. Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nat. Neurosci. 2002;5:401–402. doi: 10.1038/nn848. [DOI] [PubMed] [Google Scholar]
- Branchereau P, Chapron J, Meyrand P. Descending 5-hydroxytryptamine raphe inputs repress the expression of serotonergic neurons and slow the maturation of inhibitory systems in mouse embryonic spinal cord. J. Neurosci. 2002;22:2598–2606. doi: 10.1523/JNEUROSCI.22-07-02598.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhaus J, Ballanyi K, Smith JC, Richter DW. Microenvironment of respiratory neurons in the in vitro brainstem-spinal cord of neonatal rats. J. Physiol. (Lond.) 1993;412:421–445. doi: 10.1113/jphysiol.1993.sp019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnet H, Bévengut M, Chakri F, Bou-Flores C, Coulon P, Gaytán S, Pásaro R, Hilaire G. Altered respiratory activity and respiratory regulations in adult monoamine oxidase A-deficient mice. J. Neurosci. 2001;21:5212–5221. doi: 10.1523/JNEUROSCI.21-14-05212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Pournin S, Müller U, Aguet M, Babinet C, Shih JC, De Maeyer E. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauger S, Guimiot F, Renolleau S, Levacher B, Boda B, Mas C, Népote V, Simonneau M, Gaultier C, Gallego J. MASH-1/RET pathway involvement in development of brain stem control of respiratory frequency in newborn mice. Physiol. Genomics. 2001;7:149–157. doi: 10.1152/physiolgenomics.00056.2001. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Wilson CG, Butera RJ, Rigatto H, Smith JC. Periodicity, mixed-mode oscillations, and quasiperiodicity in a rhythm-generating neural network. Biophys. J. 2002;82:206–214. doi: 10.1016/S0006-3495(02)75387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pasquale E, Morin D, Monteau R, Hilaire G. Serotonergic modulation of the respiratory rhythm generator at birth: an in vitro study in the rat. Neurosci. Lett. 1992;143:91–95. doi: 10.1016/0304-3940(92)90240-8. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Conover JC, Borday V, Champagnat J, Barbacid M, Yancopoulos GD, Katz DM. Mice lacking Brain-Derived Neurotrophic Factor exhibit visceral sensory neuron losses distinct from mice lacking NT4 and display a severe developmental deficit in control of breathing. J. Neurosci. 1996;16:5361–5371. doi: 10.1523/JNEUROSCI.16-17-05361.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JT, Mayer C, Jawa A, Ling L, Olson EB, Jr., Vidruk EH, Mitchell GS, Katz DM. Chemoafferent degeneration and carotid body hypoplasia following chronic hyperoxia in newborn rats. J. Physiol. (Lond.) 1998;509:519–526. doi: 10.1111/j.1469-7793.1998.519bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber JP. Development of pulmonary reflexes and pattern of breathing in the Virginia opossum. Respir. Physiol. 1972;14:278–286. doi: 10.1016/0034-5687(72)90034-5. [DOI] [PubMed] [Google Scholar]
- Farber JP. Laryngeal effects and respiration in the suckling opossum. Respir. Physiol. 1978;35:189–201. doi: 10.1016/0034-5687(78)90021-x. [DOI] [PubMed] [Google Scholar]
- Farber JP, Marlow TA. An obstructive apnea in the suckling opossum. Respir. Physiol. 1978;34:295–305. doi: 10.1016/0034-5687(78)90129-9. [DOI] [PubMed] [Google Scholar]
- Fenik V, Kubin L, Okabe S, Pack AI, Davies RO. Differential sensitivity of laryngeal and pharyngeal motonuerons to iontophoretic application of serotonin. Neuroscience. 1997;81:873–885. doi: 10.1016/s0306-4522(97)00215-7. [DOI] [PubMed] [Google Scholar]
- Fisher JT, Mortola JP, Smith JB, Fox GS, Weeks S. Respiration in newborns: development of the control of breathing. Am. Rev. Respir. Dis. 1982;125:650–657. doi: 10.1164/arrd.1982.125.6.650. [DOI] [PubMed] [Google Scholar]
- Garfinkel A, Chen PS, Walter DO, Karagueuzian HS, Kogan B, Evans SJ, Karpoukhin M, Hwang C, Uchida T, Gotoh M, Nwasokwa O, Sagar P, Weiss JN. Quasiperiodicity and chaos in cardiac fibrillation. J. Clin. Invest. 1997;99:305–314. doi: 10.1172/JCI119159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Smith JC, Feldman JL. Respiratory and locomotor patterns generated in the fetal rat brain stem-spinal cord in vitro. J. Neurophysiol. 1992;67:996–999. doi: 10.1152/jn.1992.67.4.996. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA, Mulkey DK. Retrotrapezoid nucleus: a litmus test for the identification of central chemoreceptors. Exp. Physiol. 2005;90:247–253. doi: 10.1113/expphysiol.2004.029637. [DOI] [PubMed] [Google Scholar]
- Harding R, England SJ, Stradling JR, Kozar LF, Phillipson EA. Respiratory activity of laryngeal muscles in awake and sleeping dogs. Respir. Physiol. 1986;66:315–326. doi: 10.1016/0034-5687(86)90083-6. [DOI] [PubMed] [Google Scholar]
- Hendricks T, Francis N, Fyodorov D, Deneris ES. The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J. Neurosci. 1999;19:10348–10356. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Hilaire G. Endogenous noradrenaline affects the maturation and function of the respiratory network: Possible implications for SIDS. Auton. Neurosci. 2006;126-127:320–331. doi: 10.1016/j.autneu.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Bou C, Monteau R. Serotonergic modulation of central respiratory activity in the neonatal mouse: an in vitro study. Eur. J. Pharmacol. 1997;329:115–120. [PubMed] [Google Scholar]
- Hilaire G, Duron B. Maturation of the mammalian respiratory system. Physiol. Rev. 1999;79:325–360. doi: 10.1152/physrev.1999.79.2.325. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Viemari JC, Coulon P, Simonneau M, Bévengut M. Modulation of the respiratory rhythm generator by the pontine noradrenergic A5 and A6 groups in rodents. Respir. Physiol. Neurobiol. 2004;143:187–197. doi: 10.1016/j.resp.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Holtman JR, Jr., Dick TE, Berger AJ. Serotonin-mediated excitation of recurrent laryngeal and phrenic motoneurons evoked by stimulation of the raphe obscurus. Brain Res. 1987;417:12–20. doi: 10.1016/0006-8993(87)90174-0. [DOI] [PubMed] [Google Scholar]
- Jansen AH, Chernick V. Fetal breathing and development of control of breathing. J. Appl. Physiol. 1991;70:1431–1446. doi: 10.1152/jappl.1991.70.4.1431. [DOI] [PubMed] [Google Scholar]
- Katz DM, Black IB. Expression and regulation of catecholaminergic traits in primary sensory neurons: relationship to target innervation in vivo. J. Neurosci. 1986;6:983–989. doi: 10.1523/JNEUROSCI.06-04-00983.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC. Abnormalities of the brainstem serotonergic system in the Sudden Infant Death Syndrome: a review. Pediatr. Dev. Pathol. 2005;8:507–524. doi: 10.1007/s10024-005-0067-y. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Myers MM, Belliveau RA, Randall LL, Trachtenberg FL, Ten Fingers S, Youngman M, Habbe D, Fifer WP. Subtle autonomic and respiratory dysfunction in sudden infant death syndrome associated with serotonergic brainstem abnormalities: a case report. J. Neuropathol. Exp. Neurol. 2005;64:689–694. doi: 10.1097/01.jnen.0000174334.27708.43. [DOI] [PubMed] [Google Scholar]
- Lindsay AD, Feldman JL. Modulation of respiratory activity of neonatal rat phrenic motoneurones by serotonin. J. Physiol. 1993;461:213–233. doi: 10.1113/jphysiol.1993.sp019510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortola JP. Breathing pattern in newborns. J. Appl. Physiol. 1984;56:1533–1540. doi: 10.1152/jappl.1984.56.6.1533. [DOI] [PubMed] [Google Scholar]
- Mortola JP. Dynamics of breathing in newborn mammals. Physiol. Rev. 1987;67:187–243. doi: 10.1152/physrev.1987.67.1.187. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Dotta A. Effects of hypoxia and ambient temperature on gaseous metabolism of newborn rats. Am. J. Physiol. 1992;263:R267–R272. doi: 10.1152/ajpregu.1992.263.2.R267. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Noworaj A. Two-sidearm tracheal cannula for respiratory airflow measurements in small animals. J. Appl. Physiol. 1983;55:250–253. doi: 10.1152/jappl.1983.55.1.250. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat. Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Murakoshi T, Suzue T, Tamai S. A pharmacological study on respiratory rhythm in the isolated brain stem-spinal cord preparation of the newborn rat. Br. J. Pharmacol. 1985;86:95–104. doi: 10.1111/j.1476-5381.1985.tb09439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayfeh AH, Balachandran B. Applied nonlinear dynamics: analytical, computational, and experimental methods. John Wiley & Sons; New York: 1995. [Google Scholar]
- Onimaru H, Shamoto A, Homma I. Modulation of respiratory rhythm by 5-HT in the brainstem-spinal cord preparation from newborn rat. Pflugers Arch. 1998;435:485–494. doi: 10.1007/s004240050543. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall RD, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. J. Amer. Med. Ass. 2006;296:2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- Peña F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J. Neurosci. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel M, Joh TH, Chan J, Beaudet A. Serotoninergic terminals: Ultrastructural and synaptic interaction with catecholamine-containing neurons in the medial nuclei of the solitary tracts. J. Comp. Neurol. 1984;225:291–301. doi: 10.1002/cne.902250212. [DOI] [PubMed] [Google Scholar]
- Radvanyi-Bouvet MF, Monset-Couchard M, Morel-Kahn F, Vicente G, Dreyfus-Brisac C. Expiratory patterns during sleep in normal full-term and premature neonates. Biol. Neonate. 1982;41:74–84. doi: 10.1159/000241520. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Viemari JC. Determinants of inspiratory activity. Respir. Physiol. Neurobiol. 2005;147:145–157. doi: 10.1016/j.resp.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Reeke GN, Coop AD. Estimating the temporal interval entropy of neuronal discharge. Neural. Comput. 2004;16:941–970. doi: 10.1162/089976604773135050. [DOI] [PubMed] [Google Scholar]
- Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat. Rev. Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wang W, Hodges MR, Dohle CI, Diez-Sampedro A. Homing in on the specific phenotype(s) of central respiratory chemoreceptors. Exp. Physiol. 2005;90:259–266. doi: 10.1113/expphysiol.2005.029843. [DOI] [PubMed] [Google Scholar]
- Ritter B, Zhang W. Early postnatal maturation of GABAA-mediated inhibition in the brainstem respiratory rhythm-generating network of the mouse. Eur. J. Neurosci. 2000;12:2975–2984. doi: 10.1046/j.1460-9568.2000.00152.x. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Pestean A, Günther S, Ballanyi K. Serotonergic modulation of respiratory motoneurons and interneurons in brainstem slices of perinatal rats. Neuroscience. 2002;115:1247–1259. doi: 10.1016/s0306-4522(02)00540-7. [DOI] [PubMed] [Google Scholar]
- Shirasawa S, Arata A, Onimaru H, Roth KA, Brown GA, Horning S, Arata S, Okumura K, Sasazuki T, Korsmeyer SJ. Rnx deficiency results in congenital central hypoventilation. Nat. Gen. 2000;24:287–290. doi: 10.1038/73516. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. PreBötzinger complex: a brain stem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Berkowitz RG, Goodchild AK, Pilowsky PM. Serotonin inputs to inspiratory laryngeal motoneurons in the rat. J. Comp. Neurol. 2002;451:91–98. doi: 10.1002/cne.10329. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Trinh JB, Champagnat J, Fortin G. Emergence of the preBötzinger respiratory rhythm generator in the mouse embryo. J. Neurosci. 2005;25:4307–4318. doi: 10.1523/JNEUROSCI.0551-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari JC, Bévengut M, Burnet H, Coulon P, Pequignot JM, Tiveron MC, Hilaire G. Phox2a gene, A6 neurons, and noradrenaline are essential for development of normal respiratory rhythm in mice. J. Neurosci. 2004;24:928–937. doi: 10.1523/JNEUROSCI.3065-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari JC, Burnet H, Bévengut M, Hilaire G. Perinatal maturation of the mouse respiratory rhythm-generator: in vivo and in vitro studies. Eur. J. Neurosci. 2003;17:1233–1244. doi: 10.1046/j.1460-9568.2003.02561.x. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Hilaire G. Monoamine oxidase A-deficiency and noradrenergic respiratory regulations in neonatal mice. Neurosci. Lett. 2003;340:221–224. doi: 10.1016/s0304-3940(03)00128-9. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Maussion G, Bévengut M, Burnet H, Pequignot JM, Népote V, Pachnis V, Simonneau M, Hilaire G. Ret deficiency in mice impairs the development of A5 and A6 neurons and the functional maturation of the respiratory rhythm. Eur. J. Neurosci. 2005a;22:2403–2412. doi: 10.1111/j.1460-9568.2005.04441.x. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Roux JC, Tryba AK, Saywell V, Burnet H, Peña F, Zanella S, Bévengut M, Barthelemy-Requin M, Herzing LBK, Moncla A, Mancini J, Ramirez JM, Villard L, Hilaire G. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J. Neurosci. 2005b;25:11521–11530. doi: 10.1523/JNEUROSCI.4373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zaykin AV, Tiwari JK, Bradley SR, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J. Neurophysiol. 2001;85:2224–2235. doi: 10.1152/jn.2001.85.5.2224. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gunderson HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 1991;231(4):482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Zanella S, Roux JC, Viemari JC, Hilaire G. Possible modulation of the mouse respiratory rhythm generator by A1/C1 neurons. Respir. Physiol. Neurobiol. 2006;153:126–138. doi: 10.1016/j.resp.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Zhang W, Barnbrock A, Gajic S, Pfeiffer A, Ritter B. Differential ontogeny of GABAB-receptor-mediated pre- and postsynaptic modulation of GABA and glycine transmission in respiratory rhythm-generating network in mouse. J. Physiol. 2002;540:435–446. doi: 10.1113/jphysiol.2001.013225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mean body weights (± SD) of wild type (+/+), Pet-1 heterozygous (+/−) and Pet-1 homozygous (−/−) mice from the day of birth (P0.5) through postnatal day 10 (P10.5). (a) Mean body weights (± SD) of mice in the pathogen-exposed (PE) colony. (b) Mean body weights (± SD) of mice in the pathogen-free (PF) colony. (c) Results of post-hoc tests (Bonferroni (LSD) method) comparing body weights between genotypes within the PE colony following one-way ANOVA (α=0.05) at each age. (d) Results of post-hoc tests comparing body weights between genotypes within the PF colony following one-way ANOVA at each age. (e) Body weight comparisons between wild type or Pet-1−/− mice across colonies at each age (two-tailed t-test). *P<0.01. n=sample size.