Abstract
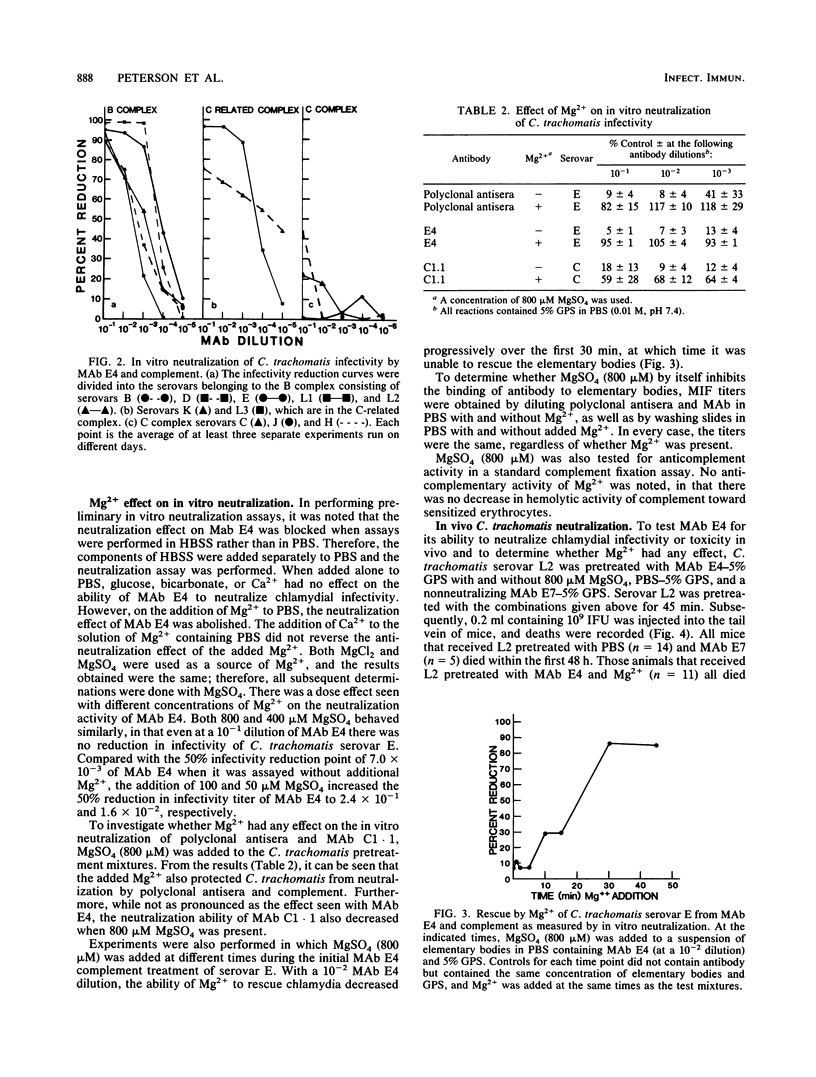
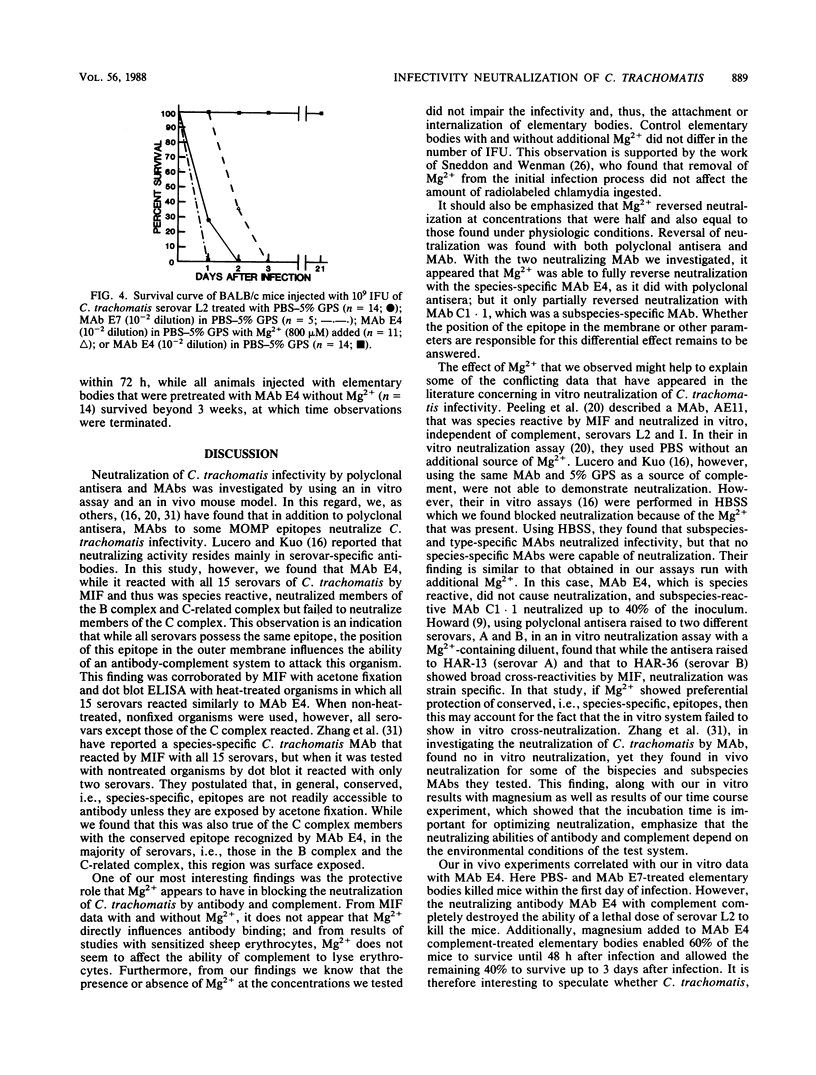
Neutralization of the infectivity of Chlamydia trachomatis was assessed by using polyclonal antisera and monoclonal antibodies (MAbs). Polyclonal antisera and a species-reactive MAb as well as a subspecies-specific MAb, both of which were directed toward the major outer membrane protein of C. trachomatis, reduced the number of chlamydial inclusion-forming units in an in vitro assay. Neutralization was dependent on the presence of complement. The species-specific MAb reacted with all 15 serovars by a microimmunofluorescence assay and a dot blot enzyme-linked immunosorbent assay with heat-treated elementary bodies. On the other hand, this same MAb reacted with all serovars, except those in the C complex, by the dot blot enzyme-linked immunosorbent assay with viable organisms and neutralized in vitro all 10 serovars tested, except those in the C complex. When neutralization assays were performed in a solution containing Mg2+, neutralization by both polyclonal antisera and MAbs was significantly reduced. A dose response to Mg2+ supplied as MgSO4 revealed that all concentrations tested from 50 to 800 microM had some effect. Concentrations of greater than or equal to 400 microM MgSO4 completely abolished neutralization at the lowest dilution of polyclonal antisera and species-reactive MAb tested. Although Mg2+ also blocked the neutralization effect of the subspecies-specific MAb, this neutralization was not as complete as that observed with the species-reactive MAb. Addition of Mg2+ to the assay over the initial 45 min of incubation of C. trachomatis with MAb and complement showed that the organisms could be rescued to some extent over the first 30 min of incubation, after which time neutralization of infectivity could not be reversed. C. trachomatis treated with Mg2+, the species-reactive MAb, and complement were lethal to mice in an in vivo toxicity and infectivity assay, whereas mice injected with organisms incubated with the same MAb and complement without Mg2+ survived.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brade L., Nurminen M., Mäkelä P. H., Brade H. Antigenic properties of Chlamydia trachomatis lipopolysaccharide. Infect Immun. 1985 May;48(2):569–572. doi: 10.1128/iai.48.2.569-572.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Perry L. J. Neutralization of Chlamydia trachomatis infectivity with antibodies to the major outer membrane protein. Infect Immun. 1982 Nov;38(2):745–754. doi: 10.1128/iai.38.2.745-754.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Schachter J. Antigenic analysis of the major outer membrane protein of Chlamydia spp. Infect Immun. 1982 Mar;35(3):1024–1031. doi: 10.1128/iai.35.3.1024-1031.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giugni T. D., James L. C., Haigler H. T. Epidermal growth factor stimulates tyrosine phosphorylation of specific proteins in permeabilized human fibroblasts. J Biol Chem. 1985 Dec 5;260(28):15081–15090. [PubMed] [Google Scholar]
- Goldman J. N., Ruddy S., Austen K. F., Feingold D. S. The serum bactericidal reaction. 3. Antibody and complement requirements for killing a rough Escherichia coli. J Immunol. 1969 Jun;102(6):1379–1387. [PubMed] [Google Scholar]
- Goldman R. C., Joiner K., Leive L. Serum-resistant mutants of Escherichia coli O111 contain increased lipopolysaccharide, lack an O antigen-containing capsule, and cover more of their lipid A core with O antigen. J Bacteriol. 1984 Sep;159(3):877–882. doi: 10.1128/jb.159.3.877-882.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard L. V. Neutralization of Chlamydia trachomatis in cell culture. Infect Immun. 1975 Apr;11(4):698–703. doi: 10.1128/iai.11.4.698-703.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Hammer C. H., Brown E. J., Cole R. J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. I. Terminal complement components are deposited and released from Salmonella minnesota S218 without causing bacterial death. J Exp Med. 1982 Mar 1;155(3):797–808. doi: 10.1084/jem.155.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Hammer C. H., Brown E. J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. II. C8 and C9 release C5b67 from the surface of Salmonella minnesota S218 because the terminal complex does not insert into the bacterial outer membrane. J Exp Med. 1982 Mar 1;155(3):809–819. doi: 10.1084/jem.155.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Schmetz M. A., Goldman R. C., Leive L., Frank M. M. Mechanism of bacterial resistance to complement-mediated killing: inserted C5b-9 correlates with killing for Escherichia coli O111B4 varying in O-antigen capsule and O-polysaccharide coverage of lipid A core oligosaccharide. Infect Immun. 1984 Jul;45(1):113–117. doi: 10.1128/iai.45.1.113-117.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lucero M. E., Kuo C. C. Neutralization of Chlamydia trachomatis cell culture infection by serovar-specific monoclonal antibodies. Infect Immun. 1985 Nov;50(2):595–597. doi: 10.1128/iai.50.2.595-597.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurminen M., Leinonen M., Saikku P., Mäkelä P. H. The genus-specific antigen of Chlamydia: resemblance to the lipopolysaccharide of enteric bacteria. Science. 1983 Jun 17;220(4603):1279–1281. doi: 10.1126/science.6344216. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Peeling R., Maclean I. W., Brunham R. C. In vitro neutralization of Chlamydia trachomatis with monoclonal antibody to an epitope on the major outer membrane protein. Infect Immun. 1984 Nov;46(2):484–488. doi: 10.1128/iai.46.2.484-488.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. L., Pruul H. Protective role of smooth lipopolysaccharide in the serum bactericidal reaction. Infect Immun. 1971 Dec;4(6):764–771. doi: 10.1128/iai.4.6.764-771.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. L., Pruul H. Sensitization of complement-resistant smooth gram-negative bacterial strains. Infect Immun. 1971 Mar;3(3):365–372. doi: 10.1128/iai.3.3.365-372.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. L., Rowley D. Sensitization of complement resistant bacterial strains. Nature. 1969 Mar 29;221(5187):1259–1261. doi: 10.1038/2211259a0. [DOI] [PubMed] [Google Scholar]
- Schiller N. L., Joiner K. A. Interaction of complement with serum-sensitive and serum-resistant strains of Pseudomonas aeruginosa. Infect Immun. 1986 Dec;54(3):689–694. doi: 10.1128/iai.54.3.689-694.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon J. M., Wenman W. M. The effect of ions on the adhesion and internalization of Chlamydia trachomatis by HeLa cells. Can J Microbiol. 1985 Apr;31(4):371–374. doi: 10.1139/m85-071. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S., Joseph T., Taylor H. R., Caldwell H. D. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987 Jan 15;138(2):575–581. [PubMed] [Google Scholar]
- de la Maza L. M., Peterson E. M. Genital infections. Med Clin North Am. 1983 Sep;67(5):1059–1073. doi: 10.1016/s0025-7125(16)31166-x. [DOI] [PubMed] [Google Scholar]




