Abstract
Estrogen receptor (ER) ligands can modulate innate and adaptive immunity and hematopoiesis, which may explain the clear sex differences in immune responses during autoimmunity, infection or trauma. Dendritic cells (DC) are antigen-presenting cells important for initiation of innate and adaptive immunity, as well as immune tolerance. DC progenitors and terminally differentiated DC express ER, indicating the ER ligands may regulate DC at multiple developmental and functional stages. Although there are profound differences in innate immunity between males and females or upon systemic imposition of sex hormones, studies are just beginning to link these differences to DC. Our and others studies demonstrate that estradiol and other ER ligands regulate the homeostasis of bone marrow myeloid and lymphoid progenitors of DC, as well as DC differentiation mediated by GM-CSF and Flt3 Ligand. Since DC have a brief lifespan, these data suggest that relatively short exposures to ER ligands in vivo will alter DC numbers and intrinsic functional capacity related to their developmental state. Studies in diverse experimental models also show that agonist and antagonist ER ligands modulate DC activation and production of inflammatory mediators. These findings have implications for human health and disease since they suggest that both DC development and functional capacity will be responsive to the physiological, pharmacological and environmental ER ligands to which an individual is exposed in vivo.
Keywords: Estrogen, Estrogen Receptor, Sex Hormone, SERM, Immunoendocrinology, Dendritic Cells, Antigen-presenting cells, cellular differentiation
1. Introduction
Clear sex biases in autoimmune disease and immunity against pathogens have been observed in humans and rodent models [1]. Progenitors and mature cells of the immune system express estrogen receptors (ER) and androgen receptors (AR), suggesting that steroid sex hormones directly modulate the development or function of immune cells, although the mechanisms by which this might occur are not well understood. Studies of immune responses in the normal state, during autoimmunity and after infection or trauma indicate that ER ligands can modulate innate and adaptive immunity and hematopoiesis [2–7]. Multiple studies have provided some evidence that normal or manipulated systemic levels of ER ligands alter antigen presenting cell (APC) numbers or functional capabilities in vivo or in vitro [6]. This review will discuss reports that ER ligands regulate dendritic cell (DC) differentiation and function, including our own work.
DC are antigen presenting cells important for both innate and adaptive immunity [8]. Multiple DC subsets in vivo have been distinguished on the basis of location, surface markers, function and migratory capacity, and whether they are present in steady-state conditions or develop as a consequence of inflammation or infection [9, 10]. DC begin innate immune responses via their ability to sense invariant pathogen molecules and secrete inflammatory cytokines. After activation and antigen exposure, DC display high levels of MHC-bound antigens and costimulatory molecules and produce cytokines, leading to the activation and differentiation of naïve T cells. DC also have a role in immunological tolerance [11]. Dysregulated DC function has been implicated in the pathogenesis of human autoimmunity and in murine autoimmune disease models, including diseases that preferentially affect women such as systemic lupus erythematosus (SLE) [11–13]. Thus an increased understanding of how sex hormones influence the development and function of DC will be of clinical interest.
2. Estrogen receptor expression, ligands and signaling pathways
Two subtypes of ER, α and β, form homodimers and heterodimers. Both ERα and ERβ are expressed in lymphoid organs and by hematopoietic progenitors in bone marrow (BM) [14, 15]. ERα, and in some cases ERβ, expression by professional APC including B lymphocytes, DC, macrophages, and monocytes has been reported [1, 16–19]. Murine splenic DC and peritoneal macrophages were reported to express ERα but not ERβ [20]. We have documented the expression of ERα and ERβ by murine DC generated during GM-CSF driven differentiation [21, 22]. However, we detected ERα, but not ERβ, mRNA in the BM myeloid progenitors that give rise to DC (EC and SK, manuscript submitted [23]). The relative expression and functional role of ERα and ERβ in immune cells, including DC, needs further study. Thus, DC progenitors and terminally differentiated DC express ER, indicating that ER ligands may regulate DC at multiple developmental and functional stages.
Ligand-bound nuclear ER are transcription factors and directly bind estrogen response sequences in regulatory regions of genes or form complexes with other transcription factors such as AP-1 [24]. Each structurally distinct ligand imparts a specific conformation to ER dimers, leading to recruitment of distinct profiles of coactivators or corepressors into multi protein transcription complexes [25]. Ligation of ER may lead to disparate patterns of gene expression in different cell types, depending on ligand form and concentration, the relative cellular expression of the two ER, and the availability of coactivators or corepressors [26, 27]. The genomic targets of ligated ER include genes involved in cellular differentiation, growth and survival [24]. Since tissue-specific responses to ER ligands result from control of discrete sets of genes, and depend on the relative expression of ER αα or ββ homodimers and αβ heterodimers, it will be important to define which receptor is involved in an immune response. ERα and ERβ have distinct and common target genes [28], and in some cell types, ERβ action modulates gene expression networks regulated by ERα [29].
Estrogens also elicit rapid (within minutes) “non-genomic” changes in cells such as alterations in signaling pathways due to stimulation of kinases or phosphatases, or Ca++ fluxes across membranes; currently there is no consensus as to whether these rapid signaling responses involve the classical ER [25, 30]. In ligand-independent pathways, ER function as transcription factors after being phosphorylated by kinases activated by growth factor receptor signaling [25].
Throughout life, the body is exposed to variable levels of both endogenous and exogenous ER ligands, which are likely to impact both DC differentiation and function during homeostasis and inflammation. Endogenous estrogens produced by the body include estrone (E1), 17-β-estradiol (E2) and estriol (E3). E2 is the major form present in adults and E3 is present at high levels only during pregnancy. Other ER ligands include pharmacological agents termed selective ER modulators (SERM) such as tamoxifen and raloxifene used for prevention or treatment of breast cancer and osteoporosis [31], dietary phytoestrogens such as the soy isoflavones [32], and environmental endocrine disruptors including organochlorine pesticides and the industrial chemical bisphenol A used in manufacture of plastics [2].
There are several important issues to consider when setting up experimental models in which ER expression or ER ligands are manipulated. A common approach to study the effects of ER signaling in immune cells has been to impose a constant level of E2 or other ER ligand in vivo or in culture models. The wide range of E2 concentrations used in these studies has made it difficult to discern a uniform effect of E2 on immune responses. The biological significance of reported data would best be interpreted in the context of the concentration and duration of E2 exposure, as well as other parameters such as the age and sex of the rodent, and whether the rodent was ovariectomized or placed on a phytoestrogen-free diet. The KD of the ER for E2 is reported as 0.1–1.0 nM (27–272 pg/ml). In humans, the peak serum level of E2 during the menstrual cycle is 200 – 500 pg/ml and at term of pregnancy is 16,000 – 30,000 pg/ml; estrogen replacement therapy results in serum levels of ~100 pg/ml [33]. Women receiving daily oral tamoxifen achieve plasma levels of 200 nM (Physicians’ Desk Reference). Reported serum levels of E2 in female mice cycle between ~25–35 pg/ml during diestrus and ~70–200 pg/ml during estrus; levels in male mice are ~8–15 pg/ml [34–36]. Differences in serum E2 levels between inbred murine strains also have been reported.
Thus, interpretation of future studies of the effects of E2 or other ER ligands on immune cell function would be helped by documentation of either physiological or pharmacological levels of the ER ligands achieved in vivo or in vitro, as well as ERα or ERβ expression by the specific cell type being studied. Studies in mice lacking ERα or ERβ should help to clarify the role of ER signaling in immune cell development and function, but these studies are complicated by the fact that ERα-deficient mice have high circulating levels of estradiol [34], which might alter the physiological role of ERβ. Furthermore, the absence of cross talk between the ERα and ERβ signaling pathways in single ER knockout mice might influence experimental outcome and conclusions regarding the relative roles of the two ER.
3. ER ligands regulate dendritic cell differentiation
3.1 Murine DC differentiation in cytokine-driven culture models
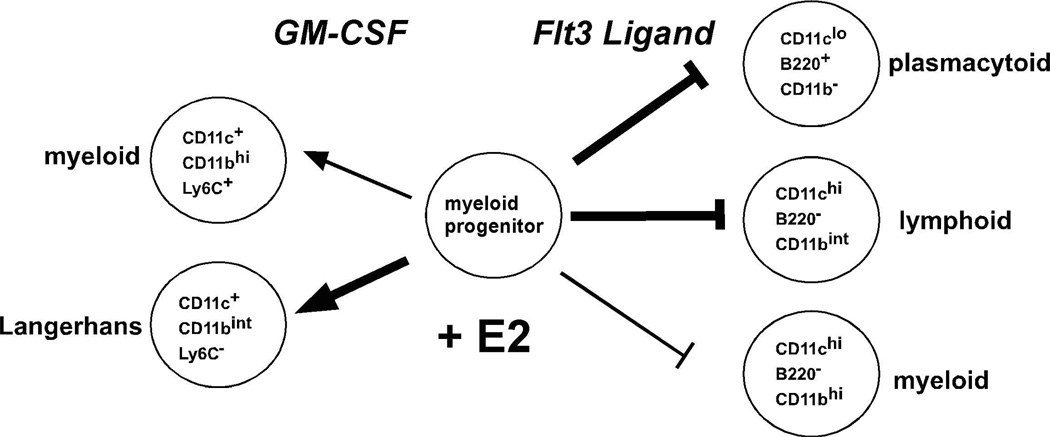
DC development from BM hematopoietic progenitors is instructed by granulocyte-macrophage colony-stimulating factor (GM-CSF) or FMS-like tyrosine kinase 3 ligand (Flt3L) in culture in vivo. Culture models in which DC differentiation is driven by Flt3L or GM-CSF lead to the development of DC subsets that are observed in lymphoid organs and skin (Fig. 1). BM cells cultured with Flt3L yield DC that are functionally and phenotypically equivalent to the conventional myeloid (CD11c+ B220− CD11bhi) and lymphoid (CD11c+ B220− CD11blo) DC, and plasmacytoid (CD11c+ CD11b− B220+) DC that are found in the spleen of mice in the steady state [37]. In BM cultures, GM-CSF promotes the differentiation of two CD11b+ myeloid DC types, one of which is the correlate of epidermal Langerhans cells (CD11c+ CD11bint Ly6C− langerin+) [22, 38]. As described below, we have found that estradiol acts in opposing manners on BM myeloid progenitors during murine GM-CSF and Flt3L mediated DC differentiation, suggesting that ER ligands are important regulators of cytokine receptor signaling pathways that instruct DC differentiation (Fig. 1).
Fig. 1. Estradiol acts on myeloid progenitors to differentially regulate DC differentiation mediated by GM-CSF and Flt3L.
The cytokines GM-CSF or Flt3L mediate DC differentiation from flt3+ myeloid progenitors present in murine BM. Estradiol (E2) levels are manipulated by addition of 0.1 nM E2 or vehicle to steroid hormone deficient and phenol red free culture medium. In the GM-CSF driven culture model, E2 promotes the differentiation of myeloid DC, including a subset with the characteristic features of Langerhans cells. In the Flt3L driven culture model, E2 inhibits cell viability and the differentiation of myeloid, lymphoid and plasmacytoid DC. The cell surface marker combinations used to define each DC subset are indicated. The thickness of the arrows or inhibition bars is related to the degree to which E2 influences the differentiation of each DC subset.
GM-CSF mediated DC differentiation. Differentiation of myeloid DC occurs upon culture of murine BM cells in GM-CSF for 7 days [38]. Since fetal calf serum (FCS) contains E2 and phenol red is a weak ER agonist [39], we used steroid depleted (charcoal dextran stripped) FCS and phenol red free RPMI to assess a requirement for E2 in BM DC cultures. Using this model system with total BM cells, we have found that differentiation of DC requires physiological amounts (near the KD of the ER, 0.1 nM) of 17-β-estradiol (E2) in the culture medium [21]. DC differentiation from both female and male BM cells was impaired in hormone deficient medium and was restored by inclusion of > 0.05–0.1 nM E2 during the 7 day culture period. The effect of E2 in hormone deficient medium was competitively inhibited by the steroidal ER antagonist ICI 182,780, demonstrating that E2 acts via ER. When included in BM cultures containing regular FCS, ICI 182,780 reduced DC numbers, indicating that DC differentiation depends upon the E2 in FCS. DC differentiation from ERα−/− BM cells also was inhibited, indicating an essential role for ERα. Testosterone or dihydrotestosterone did not restore DC differentiation in hormone deficient medium, indicating a specific effect of ER, but not AR, ligands [21].
The CD11c+ DC that develop in the GM-CSF driven BM cultures may be divided into two distinct populations, CD11bint Ly6C− and CD11bhi Ly6C+ [22]. E2 was preferentially required for the differentiation of the CD11bint Ly6C− DC, although it also promoted increased numbers of CD11bhi Ly6C+ DC. The estrogen-dependent CD11bint Ly6C− DC displayed higher intrinsic levels of MHCII and costimulatory molecules prior to activation than CD11bhi Ly6C+ DC and showed the greatest increase in MHCII and CD80, CD83, and CD86 after activation by Toll-like receptor (TLR) ligands, suggesting they were more potent APC. DC that differentiated in E2-supplemented medium were fully functional in their capacity to mediate presentation of self and foreign antigens and stimulate the proliferation of naïve CD4+ T cells. The E2-dependent CD11bint Ly6C− DC preferentially expressed langerin (CD207) and contained Birbeck granules characteristic of Langerhans cells (LC) [22]. In sum, our data in the GM-CSF model show that estradiol promotes the differentiation of a functional DC subset that most resembles epidermal Langerhans cells. Another study in a rat culture model also showed that estradiol promotes GM-CSF mediated DC differentiation [40].
Flt3L mediated DC differentiation. Flt3L mediated DC differentiation from BM cells leads to B220+ CD11c+ plasmacytoid DC and B220− CD11c+ “conventional” myeloid and lymphoid DC [37]. To test the estrogen-dependence of this culture model, total BM cells were incubated in Flt3L-supplemented steroid deficient medium without or with E2. Relative to vehicle treated cultures, inclusion of physiological levels (0.1 nM) of E2 dramatically reduced the number of viable cells, leading to a decrease in development of all DC subsets (EC and SK, manuscript submitted [23]). Complementary results were observed in regular medium containing FCS; inclusion of the ER antagonist ICI 182,780 increased the numbers of viable cells and differentiated DC. Experiments with total BM cells from ERα−/− and ERβ−/− mice showed that E2 acted primarily via ERα to decrease Flt3L-mediated DC differentiation.
In sum, use of these culture models has allowed us to dissect the clearly distinct effects of ER ligands on the two cytokine driven pathways of differentiation (Fig. 1). Our data indicate that physiological levels of estradiol promote GM-CSF-mediated and decrease Flt3L-mediated murine DC differentiation, suggesting that ER signaling events can differentially regulate cytokine receptor signaling pathways. We currently are studying mechanisms by which ER signaling might regulate DC differentiation along these two pathways.
3.2 ER-mediated regulation of hematopoietic progenitors of DC
In the culture models of DC differentiation initiated from total BM cells, it was possible that E2 regulated DC differentiation by acting on one or more hematopoietic progenitors, or on mature cells in BM, which might produce factors necessary for DC differentiation. Thus we tested whether E2 acted directly on defined DC progenitors present in murine BM. Multiple BM progenitors give rise to the phenotypically and functionally diverse DC populations present in vivo. Based upon transfer of defined progenitor populations into mice, the flt3+ fractions of the common myeloid progenitor, CMP (Lin− c-kit+ Sca-1− CD34+ FcγRlo IL-7Rα−) and the common lymphoid progenitor, CLP (Lin− c-kit+ Sca-1lo IL-7Rα+) were each found to give rise to all DC subsets in vivo including splenic myeloid, lymphoid and plasmacytoid DC, and epidermal Langerhans cells [41–44]. In addition, some progenitor populations had more restricted DC subset potential. An early lymphoid progenitor (ELP), defined as Lin− c-kithi Sca-1hi IL-7Rα− RAG-1+/−, yielded two plasmacytoid DC populations in vivo [45]. A Lin− c-kit+ CD11b− CX3CR1+ clonogenic progenitor specific for macrophages, myeloid DC and monocytes also was defined [46].
We have found that ER ligands act directly on the same highly purified BM flt3+ myeloid progenitors to regulate the DC differentiation mediated by either Flt3L or GM-CSF (EC and SK, manuscript submitted [23]). These data indicate that ER signaling in DC progenitors regulates cellular differentiation by differential interaction with cytokine receptor signals. Our finding suggests a revised paradigm for understanding certain cell type specific effects of ER ligands. The current paradigm is that ER ligands have agonist or antagonist activity in a particular cell type depending on cell-intrinsic factors such as the complement of transcriptional coactivators and corepressors. In contrast, our data show that depending on the extracellular cytokine environment, the same highly purified cell type can respond differently to estradiol or an ER antagonist.
Some information is available about the sensitivity of DC progenitors to variable E2 levels in vivo. Plasmacytoid DC (pDC) were reported to arise with differential efficiency from flt3+ myeloid progenitors (CMP) and lymphoid progenitors, including the ELP and the CLP [42, 45, 47–50]. The sensitivity of pDC differentiation to the imposition of constant supra-physiological levels of E2 to ovary intact mice was used to determine the nature of pDC progenitors in vivo. Imposition of E2 for 1–2 weeks profoundly decreased numbers of BM lymphoid progenitors including the ELP and the CLP, while sparing myelopoiesis and reducing BM pDC numbers [5, 47]. These data suggested that pDC arise from E2-sensitive lymphoid progenitors. In another study, in vivo E2 treatment significantly depleted the ELP and CLP, yet also reduced numbers of CMP to a lesser extent, indicating that albeit less profound, E2 also negatively regulates numbers of myeloid progenitors in vivo [48]. Despite this reduction in numbers of myeloid and lymphoid progenitors, pDC numbers in BM and spleen were unaffected by E2 in the study, suggesting that pDC arise from myeloid progenitors that are relatively insensitive to E2 [48].
These studies of mice subjected to a constant supra-physiological level of E2 suggest differential sensitivity of myeloid and lymphoid progenitors to negative regulation by ER agonists. In sum, our and others data show that ER signaling regulates DC progenitor homeostasis, with agonist ER ligands such as E2 serving to limit the number of myeloid and lymphoid progenitors in the steady state.
3.3 Regulation of DC differentiation by selective ER modulators
Selective ER modulators (SERM) are a class of pharmacological compounds that are used to modulate ER-mediated responses in vivo. SERM in clinical use include drugs such as tamoxifen and raloxifene, which are administered for prevention or treatment of breast cancer and osteoporosis. SERM have cell-type specific agonist or antagonist effects, depending on the cellular complement of coactivators or corepressors [31]. We showed that both tamoxifen and raloxifene (10 – 500 nM) impaired GM-CSF mediated differentiation of the E2-dependent CD11c+ CD11bint Ly6C− DC subset, indicating that these SERM act as ER antagonists on DC BM progenitors, and when in excess, competitively inhibit E2 induced DC differentiation [51]. Although the numbers of DC in BM cultures exposed to SERM or the ER antagonist ICI 182,780 were reduced significantly, those DC that did develop displayed reduced resting levels of MHC class II and costimulatory molecules relative to untreated DC, perhaps indicative of an incompletely differentiated state (see section 4 below).
Human myeloid DC differentiation from monocytes is induced by GM-CSF and IL-4 in culture models. Addition of E2 to human monocyte cultures increased the number of DC. [52] The SERM toremifene and tamoxifen inhibited the differentiation of human ER+ monocytes or synovial MØ into DC [16, 53]. It is of note that SERM were used at 10 µM in these studies, a relatively high concentration (~2.8 µg/ml) since the steady state plasma concentration in humans taking oral tamoxifen at 20 mg/day is ~120 ng/ml (Physician’s Desk Reference). Thus ER ligands appear to have similar effects on GM-CSF mediated myeloid DC differentiation from human monocytes and murine BM myeloid progenitors.
3.4 Regulation of DC differentiation by ER ligands: Potential biological significance
Our data support the hypothesis that ER ligands in combination with cytokines present in the local extracellular environment will modulate pathways of DC differentiation and ultimately regulate numbers of DC in tissues. The finding that ER signaling differentially regulates GM-CSF and Flt3L mediated DC differentiation raises the question of how and when ER ligands modulate these two pathways to regulate numbers of DC in vivo. Studies of mice lacking these cytokines or their receptors, or mice subjected to supra-physiological levels of GM-CSF or Flt3L, have shown that both GM-CSF and Flt3L contribute to steady state DC differentiation in vivo [54–59]. Flt3L deficiency most significantly reduced all DC populations in lymphoid organs, and decreased numbers of BM myeloid and lymphoid progenitors [54]. GM-CSF and GM-CSFRβc deficient mice had a minor decrease in numbers of DC populations, with the most significant impact on the steady state development of CD11bhi CD8α− DC, and exhibited deficient T-cell function mediated by DC [60]. Studies of Langerhans cell (LC) development in GM-CSFRβc deficient, GM-CSF transgenic and Flt3L-injected mice provided evidence that the development of epidermal LC is dependent upon GM-CSF, while independent of Flt3L [61]. These data are consistent with the differentiation of LC in GM-CSF but not Flt3L driven culture models.
The role of GM-CSF as a regulator of myeloid cell survival, proliferation, activation or differentiation in vivo is most prominent during inflammation, during which the production and action of GM-CSF occurs locally [62]. In response to TNFα or IL-1, GM-CSF is produced by multiple cell types and acts locally upon macrophages, neutrophils and GM-CSF receptor bearing myeloid progenitors. While GM-CSF has a minor but normal role in DC (including LC) differentiation in the steady state, GM-CSF mediated DC differentiation is likely to be most important in vivo as GM-CSF levels rise during inflammation resulting from infection, injury or autoimmunity. Indeed, LC turn over very slowly in the steady state, and are replaced by newly differentiated cells only after skin inflammation leads to LC migration from the skin, suggesting that GM-CSF induced by inflammation may promote LC differentiation [43, 63]. Thus, we hypothesize that agonist ER ligands in synergy with GM-CSF receptor signaling will have the most impact on LC differentiation and on new DC differentiation that might occur during systemic or local inflammation.
In contrast, the negative regulation of DC progenitors and Flt3L mediated DC differentiation by ER agonists including estradiol may lead to a homeostatic level of DC numbers in lymphoid organs that are optimal for maintaining immunity in adults. The number of newly differentiated DC during pregnancy also might be expected to decrease due to this negative regulation of DC progenitors in BM.
In sum, our and others data suggest that due to the action of ER ligands on myeloid progenitors, DC differentiation in vivo will be responsive to physiological, pharmacological and environmental ER ligands present in the individual. These effects of ER ligands are likely to be dependent on the DC-promoting cytokine pathways that might be operative in the steady state or during inflammation and disease.
4. Regulation of DC activation or function by ER ligands
In our experiments with the GM-CSF driven culture model, immature (resting) DC that differentiated in the presence of the ER antagonist ICI 182,780 or the SERM tamoxifen or raloxifene showed decreased MHCII and CD86 surface expression relative to vehicle treated cells [51]. Upon incubation with activating TLR ligands such as bacterial LPS, SERM-exposed DC were hyporesponsive; these DC showed a significant impairment in upregulation of MHCII and CD86, as well as IL-12 production, despite normal levels of TLR4. Thus the DC that differentiate in the context of altered or deficient ER signaling have a developmental defect that makes them less capable of initiating an activation program in reponse to TLR ligands. Consistent with this observation, addition of SERM to differentiated DC only during LPS activation did not impair the increase in surface expression of MHCII nor CD86, suggesting that SERM do not directly modulate signals resulting from TLR signaling in myeloid DC. Thus the influence of ER ligands on DC development may ultimately affect their subsequent functionality.
However, work in other culture models has provided evidence that ER ligands may directly activate or inhibit antigen presenting cell function of DC. Splenic DC cultured in vitro for 48 hr with E2 (500–2000 pg/ml) showed reduced capacity to present antigen to T cells [64]. In contrast, culture of splenic CD11c+ DC for 5 days with comparable amounts of E2 (2500 pg/ml) increased MHCII gene transcription, leading to DC with increased surface MHCII and capacity for stimulation of antigen-specific T cells [65]; E2 also increased viability, MHCII surface expression and T cell stimulatory capacity of splenic CD11c+ DC in another study [66].
A few studies have addressed the effects of ER ligands on human DC function in vitro, but the significance of some of these studies is limited by the very high concentrations of ER ligands used. A short term exposure of monocyte-derived DC to supra-physiological (above those of pregnancy) levels of E2 (1–20 µg/ml) led to increased production of pro-inflammatory cytokines and chemokines [67]. In another study, DC generated from human monocytes produced more IL-10 and less IL-18 in response to E2, without changes in surface MHCII or costimulatory molecules [68]. Human DC incubated with levels of SERM (5–10 µM) above typical pharmacological concentrations showed impaired activation and function [16]. In response to maturation stimuli, SERM treated human DC failed to increase expression of CD1a, CD83 and HLA-DR, produced little IL-12p70, and were unable to stimulate mixed lymphocyte reactions.
Compared to the paucity of information regarding effects of ER ligands on DC activation and function, there is a significant body of work describing effects of ER ligands on monocytes and macrophages, which was compiled in a recent review article [17]. Estradiol is considered to have anti-inflammatory effects in vivo, and the primary effects of estrogens on monocytes and macrophages are repressive. An important consequence of TLR signaling is the activation of NF-κB, which leads to production of pro-inflammatory cytokines. ER signaling was reported to regulate NF-κB in several studies [69]. For example, upon activation of macrophages with LPS in the presence of physiological amounts of E2, NF-κB was functionally inactivated, providing an explanation for the anti-inflammatory effects of E2 [70].
ER signaling also regulates apoptosis and survival of antigen presenting cells. Estrogen was reported to increase FasL on monocytes, and depending on the differentiation state of the monocyte/macrophage, estrogen induced apoptosis [18]. Estrogen acting via ERα also induced FasL and apoptosis in BM-derived osteoclasts, providing an explanation for the prevention of bone loss by estrogen [71]. E2 administration immediately after trauma-hemorrhage prevented splenic DC apoptosis and the decrease in other DC functions that normally occur in this model, suggesting that E2 promotes DC survival [72]. Exposure to E2 in vivo was shown to regulate survival pathways in B lymphocytes and early B cell precursors by modulating levels of Bcl-2 [19, 73]. In sum, the molecular mechanisms by which ER signaling regulates DC activation and function need further study, but it is probable that some of the ER-regulated mechanisms that operate in macrophages and monocytes, or B cells, will operate in DC as well.
5. Effects of ER ligands on DC function during innate and adaptive immunity in vivo
Although there are profound differences in innate immunity between males and females or upon systemic imposition of sex hormones, very few studies have directly linked these differences to DC [4, 7]. Since DC in lymphoid organs have a lifespan of 3–12 days [74], relatively short in vivo exposures to agonist or antagonist ER ligands might alter de novo DC differentiation mediated by GM-CSF or Flt3L, with a consequent impact on DC numbers and intrinsic functional capacity related to their developmental state. Thus in studies of DC function after modulation of ER ligands or ER expression in vivo, it will be important to distinguish between the potentially distinct effects of ER ligands on DC differentiation or pathways of DC activation operative in completely differentiated DC.
Contrasting results from DC studies in murine autoimmune disease models, in which a constant amount of E2 was imposed, suggest that it will be difficult to define a single effect of estrogen on regulation of DC numbers and function in vivo. E2 levels higher than those in female estrus led to decreased DC numbers in lymphoid organs during experimental autoimmune encephalomyelitis, and DC showed reduced inflammatory cytokine levels after LPS stimulation. This correlated with a dominant Th2 response and disease amelioration [64]. Splenic DC isolated from the E2 treated mice (500–2000 pg/ml) showed reduced production of TNFα, IFNγ and IL-12 upon LPS activation. E2 also was reported to increase the costimulatory molecule PD-1 in this model [75]. In contrast, elevation of systemic E2 levels (~700 pg/ml) prior to induction of disease led to increased splenic CD8α+ lymphoid DC numbers in experimental autoimmune myasthenia gravis. These DC showed enhanced IL-12 production after TLR ligand stimulation, correlating with a dominant Th1 response [76].
In addition, some experiments using ER-deficient mice indirectly implicate a role for ER signaling in DC development or function. E2 acting via ERα was required for an appropriate innate immune response to bacterial LPS and viruses in the female brain [77]. E2 acting via ERα enhanced primary antigen-specific CD4+ T cell responses and Th1 development in vivo [78]. Aged ERβ−/− mice developed a myeloproliferative disease [79], suggesting a role for ERβ in regulating myelopoiesis.
An interesting example of regulation of myeloid cell numbers by estrogens and GM-CSF occurs in the female reproductive tract. Uterine epithelial cells produce more GM-CSF during the estrus phase of the cycle when E2 levels are highest [80]. Studies of myeloid cells in the female mouse or rat uterus showed that numbers of macrophages and DC were highest during estrus phase, and were reduced during diestrus phase when E2 levels wane [81, 82]. GM-CSF deficient mice showed changes in the numbers and activation status of macrophages and DC in the female reproductive tract [83]. These studies suggest that normal hormonal cycle changes in estradiol concentration modulate the levels of GM-CSF and GM-CSF mediated pathways of DC differentiation, with a consequent effect on the number of myeloid cells in the female reproductive tract.
Thus, the role of ER expression or impact of alterations in levels of ER ligands on DC biology in vivo has been studied in diverse experimental models, encompassing autoimmunity, infection, and tissue specific sites such as the female reproductive tract. The disparate experimental models and technical approaches used in these few studies make it difficult to reach firm conclusions about the role of ER in DC differentiation or function in vivo, and in fact suggest that ER regulation of DC-mediated immunity may vary with the nature of the disease model.
6. Male-female differences in immunity correlate with differential effects of hormones on innate immune cells
Evidence shows that relative to males, females have increased immunity to bacterial and viral pathogens and parasites [1, 4, 7]. Females generate stronger adaptive immune responses, particularly B cell mediated responses, after vaccination or infection. Interestingly, females tend to have more controlled innate immune responses, while males suffer more pathology due to excessive inflammation [4]. Females are less prone to bacterial sepsis and complications of traumatic injury, correlating with lower production of proinflammatory mediators and attenuation of IL-6 production by estrogen [3, 4]. Consistent with more robust immunity, females show a significantly higher incidence of autoimmune disease, and modulation of innate or adaptive immune function by sex hormones has been demonstrated in rodent models of autoimmunity [84].
Although manipulation of E2 levels alters DC function in vivo as discussed above, divergent function of DC in males and females during infection or autoimmunity has not been reported. However, results in some infection models indirectly implicate differences in DC function in males and females, due to sex based expression differences in immune response genes or proteins normally expressed by DC [85–87]. A comprehensive study of potential differences in numbers or composition of splenic DC subsets in male and female mice has not been reported; however, differences in epidermal Langerhans cells in male and female C57BL/6 and Balb/c mice were found [88], suggesting that sex hormones might control GM-CSF mediated Langerhans cell differentiation during homeostasis.
7. Summary
Murine and human DC progenitors and differentiated DC express ER, indicating that ER ligands are likely to regulate DC development, lifespan, activation and function during innate and adaptive immunity. Our lab has shown that estradiol acting via ERα differentially regulates the differentiation of DC mediated by GM-CSF and Flt3L, two cytokines that mediate DC differentiation in vivo. Other studies have found that ER signaling in response to estradiol or SERM regulates DC activation, production of inflammatory mediators and T cell stimulatory capacity. Since DC in lymphoid organs have a brief lifespan, relatively short in vivo exposures to ER ligands might alter DC progenitor homeostasis and new DC differentiation, with a consequent impact on DC numbers and intrinsic functional capacity related to their developmental state. Thus in studies of DC function after manipulation of ER ligands or ER expression in vivo, it will be important to distinguish between the potentially distinct effects of ER ligands on DC differentiation or pathways of DC activation.
An understanding of how diverse physiological, pharmacological and environmental ER ligands regulate DC differentiation and function will be relevant to human disease. Humans are being treated with ER ligands, including the SERM tamoxifen and raloxifene, and the pregnancy hormone estriol, for prevention or treatment of breast cancer, osteoporosis and multiple sclerosis. Thus it is of clinical importance to understand how ER ligands modulate the immune system. Estradiol promotes DC differentiation mediated by GM-CSF, a cytokine that is produced locally upon inflammation, and is elevated in autoimmune diseases such as SLE, which is associated with abnormal DC function. Thus, we hypothesize that estradiol and other ER agonists will promote DC differentiation during inflammation, perhaps explaining in part the increased autoimmunity observed in women.
Acknowledgements
S.K. was supported by grants from the NIH NIAID and the Oklahoma Center for the Advancement of Science and Technology. We thank our colleagues at OMRF and elsewhere for helpful discussions about this topic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whitacre CC, Reingold SC, O'Looney PA. A gender gap in autoimmunity. Science. 1999;283:1277–1278. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed SA, Hissong BD, Verthelyi D, Donner K, Becker K, Karpuzoglu-Sahin E. Gender and risk of autoimmune diseases: possible role of estrogenic compounds. Environ Health Perspect. 1999;107 Suppl 5:681–686. doi: 10.1289/ehp.99107s5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovacs EJ. Aging, traumatic injury, and estrogen treatment. Exp Gerontol. 2005;40:549–555. doi: 10.1016/j.exger.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Marriott I, Huet-Hudson YM. Sexual dimorphism in innate immune responses to infectious organisms. Immunol Res. 2006;34:177–192. doi: 10.1385/IR:34:3:177. [DOI] [PubMed] [Google Scholar]
- 5.Medina KL, Garrett KP, Thompson LF, Rossi MI, Payne KJ, Kincade PW. Identification of very early lymphoid precursors in bone marrow and their regulation by estrogen. Nat Immunol. 2001;2:718–724. doi: 10.1038/90659. [DOI] [PubMed] [Google Scholar]
- 6.Nalbandian G, Kovats S. Understanding sex biases in immunity: Effects of estrogen on the differentiation and function of antigen presenting cells. Immunol Res. 2005;31:91–106. doi: 10.1385/IR:31:2:091. [DOI] [PubMed] [Google Scholar]
- 7.Klein SL. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol. 2004;26:247–264. doi: 10.1111/j.0141-9838.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- 8.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 9.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 10.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 11.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 13.Banchereau J, Pascual V, Palucka AK. Autoimmunity through cytokine-induced dendritic cell activation. Immunity. 2004;20:539–550. doi: 10.1016/s1074-7613(04)00108-6. [DOI] [PubMed] [Google Scholar]
- 14.Phiel KL, Henderson RA, Adelman SJ, Elloso MM. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett. 2005;97:107–113. doi: 10.1016/j.imlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Igarashi H, Kouro T, Yokota T, Comp PC, Kincade PW. Age and stage dependency of estrogen receptor expression by lymphocyte precursors. Proc Natl Acad Sci U S A. 2001;98:15131–15136. doi: 10.1073/pnas.011513098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komi J, Lassila O. Nonsteroidal anti-estrogens inhibit the functional differentiation of human monocyte-derived dendritic cells. Blood. 2000;95:2875–2882. [PubMed] [Google Scholar]
- 17.Harkonen PL, Vaananen HK. Monocyte-macrophage system as a target for estrogen and selective estrogen receptor modulators. Ann N Y Acad Sci. 2006;1089:218–227. doi: 10.1196/annals.1386.045. [DOI] [PubMed] [Google Scholar]
- 18.Mor G, Sapi E, Abrahams VM, Rutherford T, Song J, Hao XY, Muzaffar S, Kohen F. Interaction of the estrogen receptors with the Fas ligand promoter in human monocytes. J Immunol. 2003;170:114–122. doi: 10.4049/jimmunol.170.1.114. [DOI] [PubMed] [Google Scholar]
- 19.Grimaldi CM, Cleary J, Dagtas AS, Moussai D, Diamond B. Estrogen alters thresholds for B cell apoptosis and activation. J Clin Invest. 2002;109:1625–1633. doi: 10.1172/JCI14873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lambert KC, Curran EM, Judy BM, Lubahn DB, Estes DM. Estrogen receptor-alpha deficiency promotes increased TNF-alpha secretion and bacterial killing by murine macrophages in response to microbial stimuli in vitro. J Leukoc Biol. 2004;75:1166–1172. doi: 10.1189/jlb.1103589. [DOI] [PubMed] [Google Scholar]
- 21.Paharkova-Vatchkova V, Maldonado R, Kovats S. Estrogen preferentially promotes the differentiation of CD11c+ CD11b (intermediate) dendritic cells from bone marrow precursors. J Immunol. 2004;172:1426–1436. doi: 10.4049/jimmunol.172.3.1426. [DOI] [PubMed] [Google Scholar]
- 22.Mao A, Paharkova-Vatchkova V, Hardy J, Miller MM, Kovats S. Estrogen selectively promotes the differentiation of dendritic cells with characteristics of Langerhans cells. J Immunol. 2005;175:5146–5151. doi: 10.4049/jimmunol.175.8.5146. [DOI] [PubMed] [Google Scholar]
- 23.Carreras E, Turner S, Paharkova-Vatchkova V, Mao A, Dascher C, Kovats S. Estradiol acts directly on bone marrow myeloid progenitors to differentially regulate GM-CSF or Flt3 Ligand mediated dendritic cell differentiation. 2007 doi: 10.4049/jimmunol.180.2.727. Submitted. [DOI] [PubMed] [Google Scholar]
- 24.O'Lone R, Frith MC, Karlsson EK, Hansen U. Genomic targets of nuclear estrogen receptors. Mol Endocrinol. 2004;18:1859–1875. doi: 10.1210/me.2003-0044. [DOI] [PubMed] [Google Scholar]
- 25.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 26.McDonnell DP. The molecular determinants of estrogen receptor pharmacology. Maturitas. 2004;48 Suppl 1:S7–S12. doi: 10.1016/j.maturitas.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS. Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology. 2003;144:4562–4574. doi: 10.1210/en.2003-0567. [DOI] [PubMed] [Google Scholar]
- 28.Stossi F, Barnett DH, Frasor J, Komm B, Lyttle CR, Katzenellenbogen BS. Transcriptional profiling of estrogen-regulated gene expression via estrogen receptor (ER) alpha or ERbeta in human osteosarcoma cells: distinct and common target genes for these receptors. Endocrinology. 2004;145:3473–3486. doi: 10.1210/en.2003-1682. [DOI] [PubMed] [Google Scholar]
- 29.Chang EC, Frasor J, Komm B, Katzenellenbogen BS. Impact of estrogen receptor beta on gene networks regulated by estrogen receptor alpha in breast cancer cells. Endocrinology. 2006;147:4831–4842. doi: 10.1210/en.2006-0563. [DOI] [PubMed] [Google Scholar]
- 30.Manavathi B, Kumar R. Steering estrogen signals from the plasma membrane to the nucleus: Two sides of the coin. J Cell Physiol. 2006;207:594–604. doi: 10.1002/jcp.20551. [DOI] [PubMed] [Google Scholar]
- 31.Dutertre M, Smith CL. Molecular mechanisms of selective estrogen receptor modulator (SERM) action. J Pharmacol Exp Ther. 2000;295:431–437. [PubMed] [Google Scholar]
- 32.Ren MQ, Kuhn G, Wegner J, Chen J. Isoflavones, substances with multi-biological and clinical properties. Eur J Nutr. 2001;40:135–146. doi: 10.1007/pl00007388. [DOI] [PubMed] [Google Scholar]
- 33.Askanase AD, Buyon JP. Reproductive health in SLE. Best Pract Res Clin Rheumatol. 2002;16:265–280. doi: 10.1053/berh.2002.0225. [DOI] [PubMed] [Google Scholar]
- 34.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 35.Foster HL, Small JD, Fox JG. Normative biology, immunology and husbandry. Orlando, FL: Academic Press Inc; 1983. p. 215. [Google Scholar]
- 36.Walmer DK, Wrona MA, Hughes CL, Nelson KG. Lactoferrin expression in the mouse reproductive tract during the natural estrous cycle: correlation with circulating estradiol and progesterone. Endocrinology. 1992;131:1458–1466. doi: 10.1210/endo.131.3.1505477. [DOI] [PubMed] [Google Scholar]
- 37.Naik SH, Proietto AI, Wilson NS, Dakic A, Schnorrer P, Fuchsberger M, Lahoud MH, O'Keeffe M, Shao QX, Chen WF, Villadangos JA, Shortman K, Wu L. Cutting edge: generation of splenic CD8+ and CD8− dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J Immunol. 2005;174:6592–6597. doi: 10.4049/jimmunol.174.11.6592. [DOI] [PubMed] [Google Scholar]
- 38.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986;83:2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang QH, Hu YZ, Cao J, Zhong YQ, Zhao YF, Mei QB. Estrogen influences the differentiation, maturation and function of dendritic cells in rats with experimental autoimmune encephalomyelitis. Acta Pharmacol Sin. 2004;25:508–513. [PubMed] [Google Scholar]
- 41.Karsunky H, Merad M, Cozzio A, Weissman IL, Manz MG. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J Exp Med. 2003;198:305–313. doi: 10.1084/jem.20030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D'Amico A, Wu L. The early progenitors of mouse dendritic cells and plasmacytoid predendritic cells are within the bone marrow hemopoietic precursors expressing Flt3. J Exp Med. 2003;198:293–303. doi: 10.1084/jem.20030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mende I, Karsunky H, Weissman IL, Engleman EG, Merad M. Flk2+ myeloid progenitors are the main source of Langerhans cells. Blood. 2006;107:1383–1390. doi: 10.1182/blood-2005-05-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manz MG, Traver D, Miyamoto T, Weissman IL, Akashi K. Dendritic cell potentials of early lymphoid and myeloid progenitors. Blood. 2001;97:3333–3341. doi: 10.1182/blood.v97.11.3333. [DOI] [PubMed] [Google Scholar]
- 45.Pelayo R, Hirose J, Huang J, Garrett KP, Delogu A, Busslinger M, Kincade PW. Derivation of 2 categories of plasmacytoid dendritic cells in murine bone marrow. Blood. 2005;105:4407–4415. doi: 10.1182/blood-2004-07-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 47.Welner RS, Pelayo R, Garrett KP, Chen X, Perry SS, Sun XH, Kee BL, Kincade PW. Interferon-producing killer dendritic cells (IKDCs) arise via a unique differentiation pathway from primitive c-kitHiCD62L+ lymphoid progenitors. Blood. 2007;109:4825–4931. doi: 10.1182/blood-2006-08-043810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harman BC, Miller JP, Nikbakht N, Gerstein R, Allman D. Mouse plasmacytoid dendritic cells derive exclusively from estrogen-resistant myeloid progenitors. Blood. 2006:878–885. doi: 10.1182/blood-2005-11-4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karsunky H, Merad M, Mende I, Manz MG, Engleman EG, Weissman IL. Developmental origin of interferon-alpha-producing dendritic cells from hematopoietic precursors. Exp Hematol. 2005;33:173–181. doi: 10.1016/j.exphem.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 50.Yang GX, Lian ZX, Kikuchi K, Moritoki Y, Ansari AA, Liu YJ, Ikehara S, Gershwin ME. Plasmacytoid dendritic cells of different origins have distinct characteristics and function: studies of lymphoid progenitors versus myeloid progenitors. J Immunol. 2005;175:7281–7287. doi: 10.4049/jimmunol.175.11.7281. [DOI] [PubMed] [Google Scholar]
- 51.Nalbandian G, Paharkova-Vatchkova V, Mao A, Nale S, Kovats S. The selective estrogen receptor modulators, tamoxifen and raloxifene, impair dendritic cell differentiation and activation. Journal of Immunology. 2005;175:2666–2675. doi: 10.4049/jimmunol.175.4.2666. [DOI] [PubMed] [Google Scholar]
- 52.Hoek A, Allaerts W, Leenen PJ, Schoemaker J, Drexhage HA. Dendritic cells and macrophages in the pituitary and the gonads. Evidence for their role in the fine regulation of the reproductive endocrine response. Eur J Endocrinol. 1997;136:8–24. doi: 10.1530/eje.0.1360008. [DOI] [PubMed] [Google Scholar]
- 53.Komi J, Mottonen M, Luukkainen R, Lassila O. Non-steroidal anti-oestrogens inhibit the differentiation of synovial macrophages into dendritic cells. Rheumatology. 2001:185–191. doi: 10.1093/rheumatology/40.2.185. [DOI] [PubMed] [Google Scholar]
- 54.McKenna HJ, Stocking KL, Miller RE, Brasel K, De Smedt T, Maraskovsky E, Maliszewski CR, Lynch DH, Smith J, Pulendran B, Roux ER, Teepe M, Lyman SD, Peschon JJ. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489–3497. [PubMed] [Google Scholar]
- 55.Vremec D, Lieschke GJ, Dunn AR, Robb L, Metcalf D, Shortman K. The influence of granulocyte/macrophage colony-stimulating factor on dendritic cell levels in mouse lymphoid organs. Eur J Immunol. 1997;27:40–44. doi: 10.1002/eji.1830270107. [DOI] [PubMed] [Google Scholar]
- 56.O'Keeffe M, Hochrein H, Vremec D, Pooley J, Evans R, Woulfe S, Shortman K. Effects of administration of progenipoietin 1, Flt-3 ligand, granulocyte colony-stimulating factor, and pegylated granulocyte-macrophage colony-stimulating factor on dendritic cell subsets in mice. Blood. 2002;99:2122–2130. doi: 10.1182/blood.v99.6.2122. [DOI] [PubMed] [Google Scholar]
- 57.Daro E, Pulendran B, Brasel K, Teepe M, Pettit D, Lynch DH, Vremec D, Robb L, Shortman K, McKenna HJ, Maliszewski CR, Maraskovsky E. Polyethylene glycol-modified GM-CSF expands CD11b(high)CD11c(high) but notCD11b(low)CD11c(high) murine dendritic cells in vivo: a comparative analysis with Flt3 ligand. J Immunol. 2000;165:49–58. doi: 10.4049/jimmunol.165.1.49. [DOI] [PubMed] [Google Scholar]
- 58.Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, McKenna HJ. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mach N, Gillessen S, Wilson SB, Sheehan C, Mihm M, Dranoff G. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000;60:3239–3246. [PubMed] [Google Scholar]
- 60.Wada H, Noguchi Y, Marino MW, Dunn AR, Old LJ. T cell functions in granulocyte/macrophage colony-stimulating factor deficient mice. Proc Natl Acad Sci U S A. 1997;94:12557–12561. doi: 10.1073/pnas.94.23.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burnham K, Robb L, Scott CL, O'Keeffe M, Shortman K. Effect of granulocyte-macrophage colony-stimulating factor on the generation of epidermal Langerhans cells. J Interferon Cytokine Res. 2000;20:1071–1076. doi: 10.1089/107999000750053735. [DOI] [PubMed] [Google Scholar]
- 62.Hamilton JA. GM-CSF in inflammation and autoimmunity. Trends Immunol. 2002;23:403–408. doi: 10.1016/s1471-4906(02)02260-3. [DOI] [PubMed] [Google Scholar]
- 63.Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, Weissman IL, Cyster JG, Engleman EG. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu HY, Buenafe AC, Matejuk A, Ito A, Zamora A, Dwyer J, Vandenbark AA, Offner H. Estrogen inhibition of EAE involves effects on dendritic cell function. J Neurosci Res. 2002;70:238–248. doi: 10.1002/jnr.10409. [DOI] [PubMed] [Google Scholar]
- 65.Murphy HS, Sun Q, Murphy BA, Ray D, Richardson BC. Estradiol upregulates MHCII and enhances antigen presentation by dendritic cells. Dendritic Cells: Interfaces with Immunobiology and Medicine. 2003 [Google Scholar]
- 66.Yang L, Hu Y, Hou Y. Effects of 17beta-estradiol on the maturation, nuclear factor kappa B p65 and functions of murine spleen CD11c-positive dendritic cells. Mol Immunol. 2006;43:357–366. doi: 10.1016/j.molimm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 67.Bengtsson AK, Ryan EJ, Giordano D, Magaletti DM, Clark EA. 17beta-estradiol (E2) modulates cytokine and chemokine expression in human monocyte-derived dendritic cells. Blood. 2004;104:1404–1410. doi: 10.1182/blood-2003-10-3380. [DOI] [PubMed] [Google Scholar]
- 68.Huck B, Steck T, Habersack M, Dietl J, Kammerer U. Pregnancy associated hormones modulate the cytokine production but not the phenotype of PBMC-derived human dendritic cells. Eur J Obstet Gynecol Reprod Biol. 2005;122:85–94. doi: 10.1016/j.ejogrb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 69.McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev. 1999;20:435–459. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- 70.Ghisletti S, Meda C, Maggi A, Vegeto E. 17beta-estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Mol Cell Biol. 2005;25:2957–2968. doi: 10.1128/MCB.25.8.2957-2968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S. Estrogen Prevents Bone Loss via Estrogen Receptor alpha and Induction of Fas Ligand in Osteoclasts. Cell. 2007;130:811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 72.Kawasaki T, Choudhry MA, Suzuki T, Schwacha MG, Bland KI, Chaudry IH. 17beta-Estradiol's salutary effects on splenic dendritic cell functions following trauma-hemorrhage are mediated via estrogen receptor-alpha. Mol Immunol. 2008;45:376–385. doi: 10.1016/j.molimm.2007.06.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Medina KL, Strasser A, Kincade PW. Estrogen influences the differentiation, proliferation, and survival of early B-lineage precursors. Blood. 2000;95:2059–2067. [PubMed] [Google Scholar]
- 74.Kamath AT, Henri S, Battye F, Tough DF, Shortman K. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 2002;100:1734–1741. [PubMed] [Google Scholar]
- 75.Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Estrogen-mediated immunomodulation involves reduced activation of effector T cells, potentiation of Treg cells, and enhanced expression of the PD-1 costimulatory pathway. J Neurosci Res. 2006;84:370–378. doi: 10.1002/jnr.20881. [DOI] [PubMed] [Google Scholar]
- 76.Delpy L, Douin-Echinard V, Garidou L, Bruand C, Saoudi A, Guery JC. Estrogen enhances susceptibility to experimental autoimmune myasthenia gravis by promoting type 1-polarized immune responses. J Immunol. 2005;175:5050–5057. doi: 10.4049/jimmunol.175.8.5050. [DOI] [PubMed] [Google Scholar]
- 77.Soucy G, Boivin G, Labrie F, Rivest S. Estradiol is required for a proper immune response to bacterial and viral pathogens in the female brain. J Immunol. 2005;174:6391–6398. doi: 10.4049/jimmunol.174.10.6391. [DOI] [PubMed] [Google Scholar]
- 78.Maret A, Coudert JD, Garidou L, Foucras G, Gourdy P, Krust A, Dupont S, Chambon P, Druet P, Bayard F, Guery JC. Estradiol enhances primary antigen-specific CD4 T cell responses and Th1 development in vivo. Essential role of estrogen receptor alpha expression in hematopoietic cells. Eur J Immunol. 2003;33:512–521. doi: 10.1002/immu.200310027. [DOI] [PubMed] [Google Scholar]
- 79.Shim GJ, Wang L, Andersson S, Nagy N, Kis LL, Zhang Q, Makela S, Warner M, Gustafsson JA. Disruption of the estrogen receptor beta gene in mice causes myeloproliferative disease resembling chronic myeloid leukemia with lymphoid blast crisis. Proc Natl Acad Sci U S A. 2003;100:6694–6699. doi: 10.1073/pnas.0731830100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robertson SA, Mayrhofer G, Seamark RF. Ovarian steroid hormones regulate granulocyte-macrophage colony-stimulating factor synthesis by uterine epithelial cells in the mouse. Biol Reprod. 1996;54:183–196. doi: 10.1095/biolreprod54.1.183. [DOI] [PubMed] [Google Scholar]
- 81.Kaushic C, Frauendorf E, Rossoll RM, Richardson JM, Wira CR. Influence of the estrous cycle on the presence and distribution of immune cells in the rat reproductive tract. Am J Reprod Immunol. 1998;39:209–216. doi: 10.1111/j.1600-0897.1998.tb00355.x. [DOI] [PubMed] [Google Scholar]
- 82.Hunt JS, Miller L, Platt JS. Hormonal regulation of uterine macrophages. Dev Immunol. 1998;6:105–110. doi: 10.1155/1998/87527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jasper MJ, Robertson SA, Van der Hoek KH, Bonello N, Brannstrom M, Norman RJ. Characterization of ovarian function in granulocyte-macrophage colony-stimulating factor-deficient mice. Biol Reprod. 2000;62:704–713. doi: 10.1095/biolreprod62.3.704. [DOI] [PubMed] [Google Scholar]
- 84.Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 85.Cernetich A, Garver LS, Jedlicka AE, Klein PW, Kumar N, Scott AL, Klein SL. Involvement of gonadal steroids and gamma interferon in sex differences in response to blood-stage malaria infection. Infect Immun. 2006;74:3190–3203. doi: 10.1128/IAI.00008-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barna M, Komatsu T, Bi Z, Reiss CS. Sex differences in susceptibility to viral infection of the central nervous system. J Neuroimmunol. 1996;67:31–39. doi: 10.1016/0165-5728(96)00022-7. [DOI] [PubMed] [Google Scholar]
- 87.Frisancho-Kiss S, Davis SE, Nyland JF, Frisancho JA, Cihakova D, Barrett MA, Rose NR, Fairweather D. Cutting edge: cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J Immunol. 2007;178:6710–6714. doi: 10.4049/jimmunol.178.11.6710. [DOI] [PubMed] [Google Scholar]
- 88.Koyama Y, Nagao S, Ohashi K, Takahashi H, Marunouchi T. Sex differences in the densities of epidermal Langerhans cells of the mouse. J Invest Dermatol. 1987;88:541–544. doi: 10.1111/1523-1747.ep12470104. [DOI] [PubMed] [Google Scholar]