Abstract
We prospectively examined the relationship between prenatal tobacco exposure (PTE) and child behavior in a birth cohort of 357 offspring of teenage mothers. PTE was defined as any exposure across pregnancy and, in separate analyses, exposure within each trimester. Outcomes included measures of behavior problems, activity, and attention. On average, the children were 6.4 years of age, 48% were females, and 69% were Black. Data on maternal tobacco and other substance use were collected prenatally and postnatally: 46% of the mothers smoked in the first trimester and 58% smoked 6 years later. Child urinary cotinine measured exposure to environmental tobacco smoke (ETS). Stepwise multiple regressions were run. PTE predicted significantly increased offspring activity; impulsivity; and aggression, externalizing, and total behavior problems in step 1. PTE remained a significant predictor of increased activity when maternal psychological characteristics, home environment, and ETS were added. The results were similar when PTE was examined by trimesters, although later pregnancy tobacco exposure predicted the most behavioral outcomes. In the final model, PTE (all three trimesters) and PTE (second trimester) were significant predictors of increased activity and attention problems, respectively. Other predictors of child behavior included maternal anxiety, depression, hostility, and home environment. ETS was not a significant predictor of child behavior when PTE was considered. Smoking during pregnancy among adolescents is a significant predictor of increased activity and attention problems in their offspring after controlling for covariates in the prenatal and current environments. Smoking cessation interventions are recommended for this population to avoid the effects of PTE on the offspring of pregnant adolescents. This is particularly important because these mothers will likely become pregnant again and many will increase their level of tobacco use as they mature.
Introduction
For both adolescent and adult women, tobacco is used during pregnancy more than any other substance (U.S. Department of Health and Human Services [USDHHS], 2005). Prenatal tobacco exposure (PTE) leads to growth deficits at birth (DiFranza & Lew, 1995; USDHHS, 2001) and behavioral problems as the offspring mature (Huizink & Mulder, 2006). Although the Centers for Disease Control and Prevention (2002) estimates that about 12% of women smoke during pregnancy, the rates of smoking among pregnant teenagers range from 19.5% to as high as 50% (Cornelius, Goldschmidt, Day, & Larkby, 2002; Gilchrist, Hussey, Gillmore, Lohr, & Morrison, 1996; Ventura, Martin, Curtin, Menacker, & Hamilton, 2001). Adolescent women are likely to smoke fewer cigarettes per day than adult women (Chassin, Presson, Rose, & Sherman, 1996; Cornelius, Day et al., 1999; Cornelius, Leech, & Goldschmidt, 2004). However, teenage mothers often become pregnant again (Meade & Ickovics, 2005), and as these girls transition from adolescence to young adulthood, their substance use increases (Cornelius, Goldschmidt, & Dempsey, 2003; Gilchrist et al., 1996), exposing future offspring to greater gestational tobacco exposures.
Children with PTE are at increased risk for a variety of problematic developmental outcomes (Huizink & Mulder, 2006; Matthews, 2001; Olds, 1997; Royal College of Physicians, 1992) including increased risk for externalizing behaviors (Batstra, Hadders-Algra, & Neeleman, 2003; Orlebeke, Knol, & Verhulst, 1997; Williams et al., 1998), oppositional and aggressive behaviors (Brook, Brook, & Whiteman, 2000; Day, Richardson, Goldschmidt, & Cornelius, 2000), and even clinical psychopathology such as conduct disorder and attention-deficit/hyperactivity disorder (Arnold et al., 2005; Knopik et al., 2006; Kotimaa et al., 2003; Langley, Rice, van den Bree, & Thapar, 2005; Mick, Biederman, Faraone, Sayer, & Kleinman, 2002; Milberger, Biederman, Faraone, Chen, & Jones, 1996; Wakschlag et al., 1997; Weissman, Warner, Wickramaratne, & Kandel, 1999). These negative behavioral effects of PTE persist into the adolescent and adult years (Brennan, Grekin, & Mednick, 1999; Cornelius & Day, 2000; Cornelius, Leech, Goldschmidt, & Day, 2005; Fergusson, Woodward, & Horwood, 1998; Kandel, Wu, & Davies, 1994; Rasanen et al., 1999).
Researchers also have reported associations between PTE and increased activity (Fried, Watkinson, & Gray, 1992; Kristjansson, Fried, & Watkinson, 1989; Linnet et al., 2005), and impulsivity and inattention levels (Cornelius, Ryan, Day, & Goldschmidt, 2001; Fried & Watkins, 2001; Fried et al., 1992; Leech, Richardson, Goldschmidt, & Day, 1999) in exposed offspring. Evidence from the animal literature underscores the findings in humans that prenatal nicotine exposure increases motor activity in laboratory animals (Ajarem & Ahmad, 1998; Fung & Lau, 1988; Johns, Louis, Becker, & Means, 1982; Richardson & Tizabi, 1994; Slotkin, Lappi, Tayyeb, & Seidler, 1991; Thomas, Garrison, Slawecki, Ehlers, & Riley, 2000; Tizabi, Popke, Rahman, Nespor, & Grunberg, 1997; Vaglenova, Birru, Pandiella, & Breese, 2004). Studies also have reported that children who grow up in homes with postnatal environmental tobacco smoke (ETS) exposure are at risk for negative behavioral outcomes (Eskenazi & Trupin, 1995; Fergusson, Horwood, & Lynskey, 1993; Weitzman, Gortmaker, & Sobol, 1992; Williams et al., 1998).
Studies that consider covariates of maternal smoking provide more compelling evidence for an independent and unique contribution of PTE to childhood behavior problems. We examined the relationship between PTE among pregnant teenagers and child behavior problems in their offspring. Pregnancies among adolescent women, by themselves, have higher risks than those of adult women, including an increased risk of adverse obstetrical and perinatal outcomes, independent of sociodemographic factors (Fraser, Brockert, & Ward, 1995). In addition, adolescent mothers are less verbal (Furstenberg, Levine, & Brooks-Gunn, 1990; Hechtman, 1989; Osofsky, Culp, & Ware, 1988), are less warm, and have more negative attitudes toward parenting (Newberger, 1983) than matched samples of older mothers. These parenting characteristics are associated with less optimal child developmental outcomes (Slaughter, 1983). Further, a large developmental literature suggests that high levels of parental stress in the absence of adequate support are associated with less competent parenting and less optimal child outcomes (Crockenberg, 1987). Parenting might be compromised in mothers under stress or experiencing psychosocial difficulties, both of which covary with tobacco use (Ackerman, Kogos, Youngstrom, Schoff, & Izard, 1999; Campbell, Pierce, Moore, Marakovitz, & Newby, 1996; Greenberg, Lengua, Coie, & Pinderhughes, 1999; Shaw, Gilliom, Ingoldsby, & Nagin, 2003). Because of these differences, it is particularly important to control for maternal psychosocial characteristics as well as home environmental factors on the behavioral outcomes of children of adolescent mothers.
Few studies have investigated the timing of gestational tobacco exposure on child outcomes. In this prospective study, we collected trimester-specific maternal smoking information and assessed offspring behavior at age 6. We also measured maternal use of alcohol, marijuana, and illicit drugs pre- and postnatally, and assessed maternal psychosocial function, the quality of the home environment, and ETS exposure. We hypothesized that PTE would predict an increased rate of child behavior problems in these offspring and that this association would be significant after controlling for covariates of maternal smoking.
Method
Sample selection and study design
The data for this study came from the Maternal Health Practices and Child Development Project (MHPCD), a consortium of projects that evaluate the long-term effects of prenatal substance exposure. The recruitment, prenatal, and delivery phases of the study occurred between 1990 and 1994. These phases took place at the Magee-Womens Hospital, the teaching hospital for the departments of Obstetrics and Gynecology and Neonatology of the University of Pittsburgh Medical Center. The 6-year follow-up of the mothers and their offspring took place at the MHPCD offices between 1996 and 2000. The institutional review boards of the University of Pittsburgh and the Magee-Womens Hospital approved each phase of the study protocol. Participants were informed that the confidentiality of their data was protected by a Certificate of Confidentiality issued by the National Institute on Drug Abuse.
Pregnant teenagers were interviewed initially (Phase 1) when they came in for their fourth or fifth month prenatal visit. Interviews were conducted in a private setting in the prenatal clinic. Women were interviewed and their children were examined within 24-36 hr after delivery (Phase 2). Mothers and their children returned to MHPCD offices for their 6-year follow-up visit (Phase 3). The Phase 1 interview assessed maternal alcohol, tobacco, marijuana, cocaine, and other drug use for the year before pregnancy and during the first trimester. The Phase 2 interview obtained this information for the second and third trimesters. The Phase 3 interview collected this information for the 6 postpartum years. A core dataset gathered at all three phases included demographic measures, maternal psychological status, and medical and reproductive history. Earlier reports provided details on growth at the delivery and 6-year follow-up phases of this study (Cornelius et al., 2002; Cornelius, Taylor, & Geva, 1995). The present report focuses on the effects of PTE on the behavioral outcomes of the 357 six-year-old offspring.
Sample description
All pregnant adolescents (aged 12-18) who attended the prenatal clinic were eligible for the study. Only 3 of the 448 adolescents who were approached refused to participate, which represents an initial refusal rate of 0.7%. Of the remaining 445 women, 15 moved out of the area prior to delivery and 1 refused the delivery interview. Additional losses included six twin births, five spontaneous abortions, two stillborn infants, and three live-born premature infants who died. Thus, 413 live-born singletons were assessed at delivery.
At the 6-year postpartum phase, 10 mothers refused to participate, 25 were lost to follow-up, 9 had moved out of the state, and 5 children were in foster placement. In addition, there had been 6 child deaths and 1 child was adopted. A total of 357 assessments were completed at the 6-year phase. Prenatal substance exposure and demographic characteristics were not significantly different between the 56 children who were not assessed and the remaining children who were assessed.
Maternal demographic characteristics
The women, on average, were 16.3 years old (range=12-18) at study recruitment; 69% were Black and 31% were White. Five (1.4%) were married at delivery. At the 6-year follow-up, the mothers’ average monthly income was US$1,333 (range=$0-$8,000) and their mean education was 12.2 years (range=7-17). Some 80% had completed high school or received a GED, and 19.6% of the women were married. At delivery, 64.4% of the women were residing with a parent; 15.1% with the father of the baby or a male friend; and 20.5% with another relative or a friend, or in a group home. At the 6-year follow-up, 92.4% of the children were with their mothers; the remaining 7.6% of the children were with a custodian. If a child was not living with his or her mother, the current custodian was interviewed. Of the mothers who were living with their children, 18% were living with the child’s father, 26% lived with a husband or boyfriend who was not the child’s father, and 38% were living alone with their children.
Obstetrical and neonatal characteristics
The study pregnancy was the first for 77% of the teens (mean gravidity=1.3; range=1-4). The mean age of menarche was 11.9 years (range=8-16) and of first sexual intercourse was 14.2 years (range=6-17). A total of 14% of the mothers breast-fed their infants, and 84% had subsequent pregnancies after the index pregnancy. The average gravidity and parity at the 6-year follow-up were 3.0 (range=1-9) and 2.2 (range=1-6), respectively.
Among the infants, 52% were male. The mean gestational age by sonar was 38.9 weeks (range=27-43), and the mean birth weight was 3,155 g (range=996-4,863). Further, 8% of the infants were premature (<37 weeks), 9% were low birth weight (<2,500 g), and 9% were small-for-gestational age (SGA). These rates are comparable with those among a similar low-socioeconomic-status sample of offspring of adult mothers that was selected from the same site, where rates of prematurity, low birth weight, and SGA were 8%, 10%, and 9%, respectively (Day et al., 1992).
Measures of substance use
The women were interviewed in a private setting by interviewers who were comfortable discussing alcohol and drug use and who were trained to use the instrument reliably, accurately identify the drugs used, and assess the amount of use. For use during pregnancy, calendar landmarks were used to indicate time periods that corresponded with conception, recognition of pregnancy, and first, second, and third trimesters.
For current substance use at the 6-year phase, the women were asked about their average daily use of tobacco, alcohol, and marijuana over the past year. Tobacco use was measured by number of cigarettes smoked per day and brand of cigarette. Quantity and frequency of the usual, maximum, and minimum use of each alcoholic beverage were assessed. The average daily number of drinks was calculated from these data. Because average daily number of drinks was positively skewed, log linear transformation was used to reduce the skewness. Marijuana use was assessed during pregnancy and at the 6-year assessment as average number of joints smoked per day. Marijuana, hashish, and sensimilla use were transformed into average daily joints: A blunt of marijuana was converted to four joints, and a hashish cigarette or bowl was counted as three joints, based on the relative amount of delta-9-THC in each (Gold, 1989). The substance use measures used in this study were developed and extensively tested for studies of alcohol use during pregnancy in adult women. These questions were developed to reflect accurately both the pattern and level of use (Day & Robles, 1989). Use of cocaine, crack, and other illicit substances was ascertained. One teenager used crack prior to pregnancy and in the first trimester, but no further use was reported. No other illicit substances were reported.
Measures of the environment
Data from multiple domains were used to measure the current environment. Demographic status, socioeconomic status, church attendance, and the medical history of both the mother and child were assessed at each phase. Maternal and child characteristics considered in the analyses included age, race, and gender.
Current maternal tobacco, alcohol, and marijuana use were measured at the 6-year follow-up. These measures followed the same format as those used during pregnancy. Children provided a urine sample as a biological measure of their passive exposure to tobacco smoke. The urine sample was collected from the children with the help of their parent or the study nurse during the break in the assessment protocol. In general, this occurred approximately 1½-2 hr from the time the child arrived at the study office. The samples were sent to an independent laboratory and analyzed via a Varian 3600 gas chromatograph that incorporated nitrogen selective detection. The level of detection for cotinine at this laboratory was 1 ng/ml. Cotinine values below the level of detection are reported as zero. All samples were analyzed without knowledge of the parent’s report of the child’s exposure to tobacco smoke. More information on the measures of ETS exposure in this sample is reported in a related paper (Cornelius et al., 2003).
In addition, we measured the psychological environment, defined as maternal social support, life events (number of stressful events within the past year), and psychological status. Instruments to measure social support and life events were adapted for the study from instruments used in the Human Population Laboratory studies (Berkman & Syme, 1979) and the Psychiatric Epidemiology Research Interview (Dohrenwend & Dohrenwend, 1974), respectively. Maternal psychological status was measured with the Center for Epidemiological Studies-Depression Scale (Radloff, 1977) for depressive symptoms and the State-Trait Anxiety Inventory (Spielberger, Gorsuch, & Lushene, 1970) for anxiety and hostility.
The Home Screening Questionnaire (Frankenberg & Coons, 1986) was used to assess the quality of the home environment. This instrument measures environmental stimulation and characteristics of the home environment, such as disciplinary techniques, quality of environmental stimulation, and family interaction.
Outcome measures
Outcome measures included the Child Behavior Checklist (CBCL; Achenbach, 1991), Routh Activity Scale (RAS; Routh, Schroeder, & O’Tuama, 1974), and the SNAP (Pelham & Bender, 1982). The CBCL has 118 problem items and 20 social competence items. The total profile includes eight problem scales (anxious/depressed, withdrawn, somatic problems, aggressive behavior, delinquent behavior, attention problems, thought problems, and social problems), internalizing and externalizing scales, and a total problems score. The CBCL has adequate reliability; test-retest scores for all of the problem scales were between .8 and .9. The CBCL scales used for this study were the total score; the internalizing and externalizing scales; and the aggression, delinquency, and attention problem scales.
The RAS (Routh et al., 1974) is a reliable and valid measure of child activity. It assesses children’s activity levels in daily routines such as mealtime, bedtime, and playtime. This instrument has been shown to discriminate between problem children and control subjects, and the score correlates with independent measures of activity. It had convergent validity with other parent ratings of activity in a large nonreferred sample (Campbell & Breaux, 1983).
The SNAP is a 25-item rating scale completed by mothers (Pelham & Bender, 1982) to assess their child’s activity level, attention span, impulsivity, and peer interactions. Pelham and Bender (1982) reported that 92% of the children defined as hyperactive on the SNAP also were termed hyperactive on the Conner’s Teacher Rating Scale.
Data analyses
The outcome variables used in the analyses were the RAS, the raw scores of the CBCL total score and attention, delinquency, aggression, internalizing, and externalizing subscales. The CBCL’s author recommends that the raw scores be used for the scales (Achenbach, 1991). The raw scores are not adjusted for age or gender; therefore, child age and gender were considered as covariates in the analyses. The SNAP activity, attention, impulsivity, and peer problem interaction scales were each used as a continuous variable.
Initially bivariate associations were examined between PTE and the other independent variables. Bivariate analyses were then conducted with PTE and each of the behavioral outcome measures. Neither the scatterplots of PTE versus behavioral outcomes nor the average behavior problems at different levels of tobacco exposure indicated a linear or dose-response relationship. Therefore, PTE was dichotomized as exposure versus no exposure to maximize the statistical power comparing exposed children with nonexposed children. In the first step, a t test was used to compare the behavioral outcomes between the two groups without adjusting for any covariates, followed by a multivariate analysis including significant covariates. In addition, correlations between birth weight, gestational age, pregnancy and labor complications, and behavior problem outcomes were calculated. None of these correlations was significant. Therefore, these measures could not be confounds and were excluded from the current analyses.
Stepwise multivariate linear regressions were used to examine each of these continuous outcomes with PTE at any time during pregnancy, and then for first, second, and third trimesters separately. These analyses were performed hierarchically. In the first step, PTE was entered, along with demographic factors: maternal age (years), education (years), income ($/month), presence of man in the household (present/not present), child age (years), race (White/Black), gender (male/female), prenatal alcohol (average drinks/day, log transformed), and prenatal marijuana exposure (average joints/day, log transformed). Other illicit drug use during pregnancy was rare and therefore was not considered in these analyses. In the second step, quality of the home environment, current maternal use of alcohol and marijuana, maternal life events, and maternal psychological variables (i.e., anxiety, depression, and hostility) were added to the model. In the third step, ETS was included in the model. Only significant variables from previous steps were retained in the next step. The modified Cook’s distance (Cook & Weisberg, 1982) was used to identify influential cases, and the standardized residuals were used to identify extreme outliers (four for CBCL internalizing, two for CBCL delinquency, one for CBCL aggression, three for CBCL total, one for CBCL externalizing, two for SNAP impulsivity, and four for SNAP peer problems). The results reported here excluded influential and outlier cases. One-sided p-values were used because we hypothesized that PTE was associated with increased behavior problems.
Results
Maternal tobacco and other substance use
In the year prior to pregnancy, 52% of the teenage mothers smoked cigarettes. This rate dropped to 46% in the first trimester and rose to 58% by the third trimester (Figure 1). By contrast, marijuana and alcohol use decreased across pregnancy (Cornelius, Geva, & Day, 1994; Cornelius, Goldschmidt, Taylor, & Day, 1999). Of the girls who smoked in the first trimester, 98% were still smoking in the third trimester. A total of 44 girls (21.2%) smoked in the third trimester but not in the first trimester. The average number of cigarettes per day among the smokers was 7.7 prepregnancy (range=0.5-50, median=6, mode=10), 9.7 in the first trimester (range=0.5-50, median=8.5, mode=10), 8.0 in the second trimester (range=0.50-40, median=5.0, mode=10), and 7.7 in the third trimester (range= 0.33-40, median=5.0, mode=10). At the sixth postpartum year, 58% of the women smoked. The smokers smoked more heavily at the 6-year phase than they did during adolescence. At the 6-year follow-up, the average number of cigarettes per day among smokers was 11.7 (range=0.5-50, median=10.0, mode=10.0).
Figure 1.
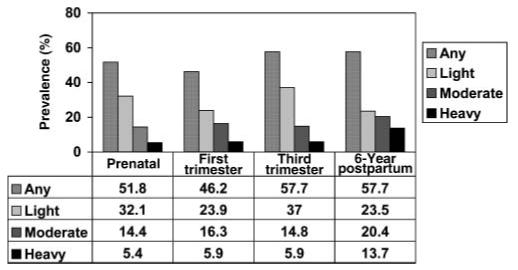
Prevalence of tobacco use at all study phases. Light=more than 0 to 9 cigarettes/day; moderate=10-19 cigarettes/day; heavy=20+ cigarettes/day.
Bivariate relationships between PTE and independent variables
Lower maternal education and White race were significant correlates of smoking during pregnancy (Table 1). Smoking during pregnancy also was associated with drinking more alcohol and using more marijuana during pregnancy, more life events, and higher rates of hostility and anxiety. PTE also was significantly associated with the children’s urinary cotinine levels 6 years later.
Table 1.
Bivariate associations among prenatal tobacco exposure (PTE) and demographic, maternal psychosocial, and environmental covariates
| Covariate | No PTE (n=147) | PTE (n=210) | p value |
|---|---|---|---|
| Demographics | |||
| Maternal age (years) | 24.2 | 24.7 | ns |
| Child age (years) | 6.3 | 6.4 | ns |
| Child gender (% boys) | 53.0 | 51.0 | ns |
| Maternal education (years) | 12.5 | 12.0 | .001 |
| Family monthly income (US$) | 1376 | 1304 | ns |
| Race (% White) | 14 | 42 | <.001 |
| Other prenatal exposures | |||
| Alcohol use % | 31.0 | 58.0 | <.001 |
| Average prenatal alcohol (drinks/day) | 0.17 | 0.46 | <.010 |
| Marijuana use % | 4.0 | 25.0 | <.001 |
| Average prenatal marijuana (joints/day) | 0.01 | 0.19 | <.010 |
| Maternal psychosocial measures | |||
| Life events | 4.9 | 5.8 | <.01 |
| Depressive symptoms | 37.2 | 38.4 | ns |
| Hostility symptoms | 15.0 | 16.3 | <.01 |
| Anxiety symptoms | 15.3 | 16.6 | <.01 |
| Environmental characteristics | |||
| Environmental tobacco smoke (ng/ml cotinine) | 12.2 | 18.3 | <.001 |
| Home environment | 11.5 | 11.9 | ns |
| Man in household (%) | 43 | 46 | ns |
Note. Based on t tests for the continuous variables; Mann-Whitney test for non-normal variables, and chi-square test for dichotomous variables. ns, not significant.
Bivariate relationships between PTE and dependent behavioral variables
The total scale and externalizing, internalizing, attention, delinquency, and aggression subscales of the CBCL were significantly higher in the children with PTE than in those with no PTE. In addition, the scores on the RAS and the SNAP impulsivity subscale were significantly related to PTE (Table 2). Second- and third-trimester PTE yielded more significant associations in many of these measures than did first-trimester PTE.
Table 2.
Bivariate associations among prenatal tobacco exposure (PTE; any use during pregnancy and by trimester) and child behavioral outcomes at age 6 years
| Child outcome | No PTE | PTE | t |
|---|---|---|---|
| Any use during pregnancy | (n=197) | (n=210) | |
| CBCL total score | 22.0 | 26.4 | 2.50** |
| CBCL externalizing score | 9.3 | 11.4 | 2.50** |
| CBCL internalizing score | 4.1 | 5.2 | 2.39** |
| CBCL attention problem score | 2.8 | 3.6 | 2.01* |
| CBCL aggression problem score | 7.3 | 9.0 | 2.58** |
| CBCL delinquency problem score | 2.0 | 2.4 | 1.72# |
| Routh Activity Scale | 38.0 | 41.1 | 2.63** |
| SNAP activity problem score | 11.2 | 11.4 | 0.23 |
| SNAP attention problem score | 8.7 | 9.4 | 1.38 |
| SNAP impulsivity problem score | 10.6 | 11.6 | 2.30* |
| SNAP peer problem score | 11.4 | 12.2 | 1.68# |
| First trimester | (n=191) | (n=164) | |
| CBCL total score | 22.8 | 26.4 | 1.96* |
| CBCL externalizing score | 9.8 | 11.3 | 1.70# |
| CBCL internalizing score | 4.3 | 5.3 | 2.06* |
| CBCL attention problem score | 3.0 | 3.5 | 1.54 |
| CBCL aggression problem score | 7.7 | 8.9 | 1.79# |
| CBCL delinquency problem score | 2.1 | 2.3 | 1.06 |
| Routh Activity Scale | 38.8 | 40.9 | 1.86# |
| SNAP activity problem score | 11.3 | 11.3 | 0 |
| SNAP attention problem score | 9.0 | 9.1 | 0.32 |
| SNAP impulsivity problem score | 10.7 | 11.6 | 2.14* |
| SNAP peer problem score | 11.7 | 12.0 | 0.72 |
| Second trimester | (n=154) | (n=202) | |
| CBCL total score | 22.0 | 26.4 | 2.50** |
| CBCL externalizing score | 9.3 | 11.4 | 2.50** |
| CBCL internalizing score | 4.1 | 5.2 | 2.39** |
| CBCL attention problem score | 2.8 | 3.6 | 2.01* |
| CBCL aggression problem score | 7.3 | 9.0 | 2.58** |
| CBCL delinquency problem score | 2.0 | 2.4 | 1.72# |
| Routh Activity Scale | 38.0 | 41.1 | 2.63** |
| SNAP activity problem score | 11.2 | 11.4 | 0.23 |
| SNAP attention problem score | 8.7 | 9.4 | 1.38 |
| SNAP impulsivity problem score | 10.6 | 11.6 | 2.30* |
| SNAP peer problem score | 11.4 | 12.2 | 1.68# |
| Third trimester | (n=151) | (n=206) | |
| CBCL total score | 21.8 | 26.5 | 2.54** |
| CBCL externalizing score | 9.3 | 11.4 | 2.58** |
| CBCL internalizing score | 4.1 | 5.2 | 2.38** |
| CBCL attention problem score | 2.8 | 3.5 | 2.11* |
| CBCL aggression problem score | 7.3 | 9.1 | 2.66** |
| CBCL delinquency problem score | 2.0 | 2.4 | 1.76# |
| Routh Activity Scale | 37.9 | 41.1 | 2.73** |
| SNAP activity problem score | 11.2 | 11.4 | 0.27 |
| SNAP attention problem score | 8.8 | 9.3 | 1.56 |
| SNAP impulsivity problem score | 10.6 | 11.6 | 2.43** |
| SNAP peer problem score | 11.4 | 12.1 | 1.80# |
Note. CBCL, Child Behavior Checklist. One-tailed
p-value: p<.050
p-value: p<.025
p-value: p<.010.
Multivariate relationships between PTE and dependent behavioral variables
Hierarchical regressions included the variables that were significantly related to PTE in the bivariate analyses. In step 1, PTE significantly predicted higher activity levels on the RAS and the SNAP impulsivity subscale, and more aggression, externalizing, and total problems on the CBCL when other prenatal substance exposures and demographic characteristics were controlled. However, after controlling for maternal psychological symptoms and the home environment (step 2), we found that PTE remained a significant predictor only of the RAS (Table 3). Children whose mothers smoked during pregnancy had higher levels of activity when all of the above covariates were controlled in the model. ETS, added in the third step, was not significant for any of the outcomes. Prenatal marijuana exposure significantly predicted higher activity on the RAS and total behavior problems on the CBCL in the final model.
Table 3.
Hierarchical analysis of the relationship between prenatal tobacco exposure (PTE) and child behavioral outcomes
| Behavioral measure |
Behavioral measure |
||||
|---|---|---|---|---|---|
|
Beta |
t |
Beta |
t |
||
| Significant covariates | Routh Activity Scale | Significant covariates | SNAP impulsivity | ||
| Step 1: Includes prenatal substance exposure and demographic characteristics | |||||
| PTE | 2.85 | 2.68** | PTE | 0.81 | 1.98* |
| Child age (younger) | -1.89 | 2.67** | Child age (younger) | -0.58 | 2.09* |
| Child gender (male) | 1.75 | 1.73# | Maternal education (less) | -0.33 | 2.26* |
| Maternal education (less) | -0.65 | 1.71# | |||
| Prenatal marijuana (more) | 5.02 | 2.38** | |||
| Step 2: Adding maternal psychological characteristics and home environment | |||||
| PTE | 2.90 | 2.82** | Child gender (male) | 0.64 | 1.72# |
| Maternal depression (more) | 0.21 | 3.64** | Maternal hostility (more) | 0.12 | 2.30* |
| Child age (younger) | -1.32 | 1.87# | Maternal anxiety (more) | 0.17 | 3.40** |
| Home environment (poorer) | -0.32 | 1.73# | Home environment (more) | -0.20 | 2.99** |
| Prenatal marijuana (more) | 4.32 | 2.09* | |||
| Step 3: Adding environmental tobacco exposure | |||||
| PTE | 2.90 | 2.82** | Child gender (male) | 0.64 | 1.72# |
| Maternal depression (more) | 0.21 | 3.64** | Maternal hostility (more) | 0.12 | 2.30* |
| Child age (younger) | -1.32 | 1.87# | Maternal anxiety (more) | 0.17 | 3.40** |
| Home environment (poorer) | -0.32 | 1.73# | Home environment (more) | -0.20 | 2.99** |
| Prenatal marijuana (more) | 4.32 | 2.09* | |||
| CBCL aggression | CBCL externalizing | ||||
|---|---|---|---|---|---|
| Step 1: Includes prenatal substance exposure and demographic characteristics | |||||
| PTE | 1.39 | 2.19* | PTE | 1.65 | 2.13# |
| Household Income (lower) | -0.001 | 2.99** | Household Income (lower) | 20.02 | 3.12** |
| Step 2: Adding maternal psychological characteristics and home environment | |||||
| Maternal hostility (more) | 0.16 | 2.01* | Maternal hostility (more) | 0.23 | 2.28* |
| Maternal anxiety (more) | 0.34 | 4.51** | Maternal anxiety (more) | 0.36 | 3.92** |
| Home environment (poorer) | -0.39 | 3.71** | Home environment (poorer) | -0.54 | -4.28** |
| Maternal age (older) | 0.08 | 1.84 | Life events (more) | 0.20 | 1.72# |
| Maternal age (older) | 0.12 | 2.09* | |||
| Step 3: Adding environmental tobacco exposure | |||||
| Maternal hostility (more) | 0.16 | 2.01* | Maternal hostility (more) | 0.23 | 2.28* |
| Maternal anxiety (more) | 0.34 | 4.51** | Maternal anxiety (more) | 0.36 | 3.92** |
| Home environment (poorer) | -0.39 | 3.71** | Home environment (poorer) | -0.54 | -4.28** |
| Maternal age (older) | 0.08 | 1.84 | Life events (more) | 0.20 | 1.72# |
| Maternal age (older) | 0.12 | 2.09* | |||
| Behavioral measure |
||
|---|---|---|
| Beta |
t |
|
| Significant covariates | CBCL total behavioral problems | |
| Step 1: Includes prenatal substance exposure and demographic characteristics | ||
| PTE | 3.12 | 1.90# |
| Household income (lower) | -0.002 | -3.04** |
| Prenatal marijuana (more) | 5.88 | 1.76# |
| Step 2: Adding maternal psychological characteristics and home environment | ||
| Maternal depression (more) | 0.31 | 2.69** |
| Maternal hostility (more) | 0.38 | 1.85# |
| Maternal anxiety (more) | 0.58 | 2.57** |
| Home environment (poorer) | -0.85 | -3.23** |
| Prenatal marijuana (more) | 5.37 | 1.83# |
| Step 3: Adding environmental tobacco exposure | ||
| Maternal depression (more) | 0.31 | 2.69** |
| Maternal hostility (more) | 0.38 | 1.85# |
| Maternal anxiety (more) | 0.58 | 2.57** |
| Home environment (poorer) | -0.85 | -3.23** |
| Prenatal marijuana (more) | 5.37 | 1.83# |
Note. CBCL: Child Behavior Checklist. One-tailed
p-value: p<.050
p-value: p<.025
p-value: p<.010.
The effect of PTE on child behavior problems was analyzed next as a function of trimester-specific cigarette exposure. After controlling for other prenatal substances and demographic characteristics (step 1), we found that first-trimester cigarette use was significantly related only to the RAS (Table 4). PTE in the second and third trimesters was a significant predictor of many of the child behavioral measures including activity; CBCL subscale values on the aggression, attention problems, delinquency, internalizing, externalizing, and total behavior problems; and the SNAP attention and impulsivity scores. When current maternal psychosocial status, home environment, and ETS were entered into the model (steps 2 and 3), second- and third-trimester PTE remained significant predictors of the RAS and second-trimester PTE remained a significant predictor of CBCL attention problems (Table 4).
Table 4.
Prenatal tobacco exposure (PTE) and child behavior at age 6 years by trimester of exposure
| Behavioral measure | Beta | t | |
|---|---|---|---|
| Step 1: Controlling for other prenatal substances and demographic characteristics | |||
| Trimester | |||
| First-trimester PTE | |||
| Routh Activity Scale | Activity score | 2.42 | 2.34** |
| Second-trimester PTE | |||
| Routh Activity Scale | Activity score | 3.05 | 2.92** |
| CBCL | Aggression problem score | 1.29 | 2.05* |
| Attention problem score | 0.53 | 1.66# | |
| Internalizing score | 0.83 | 1.94# | |
| Total score | 2.79 | 1.71# | |
| SNAP | Attention score | 0.76 | 2.10* |
| Impulsivity score | 0.77 | 1.89# | |
| Third-trimester PTE | |||
| Routh Activity Scale | Activity score | 2.96 | 2.80** |
| CBCL | Aggression problem score | 1.43 | 2.28* |
| Delinquency problem score | 0.44 | 2.20* | |
| Externalizing problem score | 1.70 | 2.21* | |
| Total score | 2.75 | 1.66# | |
| SNAP | Attention score | 0.69 | 1.89# |
| Impulsivity score | 0.87 | 2.15* | |
| Steps 2 and 3: Controlling for maternal psychosocial variables and environmental tobacco smoke | |||
| First-trimester PTE | |||
| Routh Activity Scale | Activity score | 2.37 | 2.32** |
| Second Trimester PTE | |||
| Routh Activity Scale | Activity score | 2.97 | 2.94** |
| CBCL | Attention problem score | 0.49 | 1.66# |
| Third-trimester PTE | |||
| Routh Activity Scale | Activity score | 2.89 | 2.83** |
Note. CBCL, Child Behavior Checklist. One-tailed
p-value: p<.050
p-value: p<.025
p-value: p<.010.
Discussion
PTE predicts higher activity levels in exposed children, after controlling for significant covariates. These agree with data from both human and animal research (Ajarem & Ahmad, 1998; Fried et al., 1992; Fung & Lau, 1988; Johns et al., 1982; Kristjansson et al., 1989; Richardson & Tizabi, 1994; Thomas et al., 2000; Tizabi et al., 1997). However, many earlier human studies did not systematically control for significant covariates, particularly other prenatal exposures, maternal psychological status, home environment, and ETS. Therefore, the present study provides new evidence of the strength of this association.
Bivariate analyses resulted in a larger number of significant associations between PTE and the outcome measures of behavior. Many of these associations were not significant after controlling for the significant covariates in the multivariate model. Thus, many of the long-term effects of PTE that have been reported likely would not have been significant if the research had controlled for more measures of the prenatal and postnatal environment. Also, ETS was not a significant factor in determining behavioral outcomes after prenatal and other covariates were considered. There are alternative explanations for the findings that PTE affects offspring outcomes. The higher rates of behavior problems in exposed offspring could result from genetic transmission of these traits or from the effects of parental traits on the pre- and postnatal environment (Wakschlag & Hans, 2002). Problem behaviors cluster within families (Baillargeon, Tremblay, & Willms, 2002; Martin & Burchinal, 1992; Serbin et al., 1998; Serbin et al., 2004); parental characteristics may lead to child behavior problems (Conger & Simons, 1997; Keenan & Shaw, 1995; Scaramella & Leve, 2004). Women who smoke during pregnancy also have higher rates of antisocial and externalizing behavior, mates that have more antisocial behaviors, higher rates of depression, and lower socioeconomic status (Cornelius, Day et al., 1999; Maughan, Taylor, Caspi, & Moffitt, 2004). In one study, an association between maternal smoking during pregnancy and conduct disorder in adolescent boys was no longer significant after maternal conduct symptoms were considered in the analyses (Silberg et al., 2003). However, PTE had a direct effect on behavior problems in twin studies, even after controlling for heritability (Maughan et al., 2004; Thapar et al., 2003).
The present study is the first to examine the gestational timing of PTE on behavioral outcomes in offspring of adolescents. Exposure in later pregnancy had a greater impact on children’s behavioral problems than did exposure early in the pregnancy. This finding is consistent with Day and colleagues’ (1992) study of adult women, in which third-trimester cigarette smoking predicted more externalizing behavior in the women’s 3-year-old offspring. Laboratory studies demonstrate that nicotinic acetylcholine receptors (nAChRs) are detectable early in embryonic development (Atluri et al., 2001; Schneider et al., 2002) and are widely distributed in the human adult brain. Exogenous nicotine dysregulates the timing of trophic events linked to nAChRs (Levin & Slotkin, 1998; Slotkin, 1992, 1998, 1999). Changes in nAChR density in several brain regions occur during a critical period of brain development, equivalent to the third trimester of pregnancy in humans, and are associated with a temporal vulnerability to the actions of nicotine exposure (Zhang, Liu, Miao, Gong, & Nordberg, 1998). Nicotine exposure during this period of brain development results in decreased cell numbers, including Purkinje cells in the developing cerebellum (Chen, Parnell, & West, 1998), and a transient increase in nicotinebinding sites in cortex, hippocampus, striatum, thalamus, and brain stem (Miao et al., 1998). These studies demonstrate the association between prenatal nicotine exposure during the third trimester and long-lasting alterations in neuronal maturation in brain regions known to contribute to neurobehavioral effects. This finding is particularly relevant to smoking among pregnant teenagers who are less likely than older women to quit during pregnancy. Thus the present study shows that PTE effects on offspring activity levels and timing of exposure in later pregnancy converge with laboratory studies.
Another strength of the study design was that other prenatal substance exposures were measured prospectively and considered in the statistical analyses. Prenatal alcohol exposure did not predict any of the child behavioral outcomes in our analyses. This finding is not consistent with studies that have found a relationship with fetal alcohol exposure and decreased attention and more aggression in exposed offspring (Coles, Platzman, & Raskind-Hood, 1997; Jacobson & Jacobson, 2002). However, the levels of drinking in our sample were very low and were mostly confined to the first trimester (Cornelius, Goldschmidt et al., 1999). The present study found that prenatal marijuana exposure significantly predicted higher activity levels on the RAS and higher CBCL total behavioral problems even after controlling for covariates. Prenatal marijuana effects also have been linked to behavior outcomes in offspring of adult samples (Fried, 2002; Fried et al., 1992; Goldschmidt, Day, & Richardson, 2000).
The present study had some limitations. The outcome measures were child behavior ratings completed by the mother, and the ratings may have been influenced by the mother’s own psychosocial status. Although current maternal symptoms and stress were statistically controlled in the regression models, it would be useful to include observational methods for gathering data on the children’s behavior in future studies. Further, gestational use was not verified biologically, and maternal drug use was based on self-report. To increase the accuracy of the reported data, we constructed detailed questions, carefully selected interviewers, and extensively trained our staff in interviewing techniques. The correlations between reports from each trimester of pregnancy were high, indicating a consistency in reporting and indicating that the maternal reports were accurate. Biological measures also have disadvantages, in that they can measure use for only a short window of time, whereas questionnaire data can elicit patterns of use over time.
Although smoking prevalence rates among these pregnant teenagers were similar to those of comparable adult samples, the number of cigarettes per day was lower in the teenaged sample (Cornelius & Day, 2000; Willford, Day, & Cornelius, 2006), resulting in lower daily doses of tobacco exposure. Even in this population with less daily exposure, PTE had a significant effect on children’s activity and attention levels at 6 years of age, after controlling for the appropriate covariates. These findings, combined with the convergence of data from animal studies with respect to outcomes and gestational timing, underscore the robust relationship between PTE and increased activity and inattention in the offspring.
Acknowledgments
This study was supported by grants from the National Institute on Drug Abuse grant (NIDA 009275) and the National Institute on Alcohol Abuse and Alcoholism (NIAAA 08284; T3207453). Some of these results were presented at the 12th annual meeting of the Society for Research on Nicotine and Tobacco in Orlando, Florida. The authors thank Jennifer Willford for her input on the neurobehavioral effects of prenatal tobacco exposure.
References
- Achenbach TM. Manual for the Child Behavior Checklist/4-18 and 1991 profile. University of Vermont Department of Psychiatry; Burlington, VT: 1991. [Google Scholar]
- Ackerman B, Kogos J, Youngstrom E, Schoff K, Izard C. Family instability and the problem behaviors of children from economically disadvantaged families. Developmental Psychology. 1999;35:258–268. doi: 10.1037//0012-1649.35.1.258. [DOI] [PubMed] [Google Scholar]
- Ajarem JS, Ahmad M. Prenatal nicotine exposure modifies behavior of mice through early development. Pharmacology, Biochemistry, and Behavior. 1998;59:313–318. doi: 10.1016/s0091-3057(97)00408-5. [DOI] [PubMed] [Google Scholar]
- Arnold E, Elliot M, Lindsay R, Molina B, Cornelius M, Vitiello B, Hechtman L, Elliot G, Newcorn J, Epstein J, Wigal T, Swanson J, Wells K. Gestational and postnatal tobacco smoke exposure as predictor of ADHD, comorbid ODD/CD, and treatment response in the MTA. Journal of Clinical Neuroscience Research. 2005;5:295–306. [Google Scholar]
- Atluri P, Fleck M, Shen Q, Mah S, Stadfelt D, Barnes W, Goderie S, Temple S, Schneider A. Functional nicotinic acetylcholine receptors expression in stem and progenitor cells of the early embryonic mouse cerebral cortex. Developmental Biology. 2001;240:143–156. doi: 10.1006/dbio.2001.0453. [DOI] [PubMed] [Google Scholar]
- Baillargeon R, Tremblay R, Willms J. Physical aggression among toddlers. In: Willms JD, editor. Vulnerable children: Findings from Canada’s National Longitudinal Study of Children and Youth. University of Alberta Press; Edmonton: 2002. pp. 121–130. [Google Scholar]
- Batstra L, Hadders-Algra M, Neeleman J. Effect of antenatal exposure to maternal smoking on behavioural problems and academic achievement in childhood: Prospective evidence from a Dutch birth cohort. Early Human Development. 2003;75:21–33. doi: 10.1016/j.earlhumdev.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Berkman L, Syme S. Social networks, host resistance and mortality: A nine-year follow-up study of Alameda County residents. American Journal of Epidemiology. 1979;109:186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- Brennan P, Grekin E, Mednick S. Maternal smoking during pregnancy and adult male criminal outcomes. Archives of General Psychiatry. 1999;56:215–219. doi: 10.1001/archpsyc.56.3.215. [DOI] [PubMed] [Google Scholar]
- Brook J, Brook D, Whiteman M. The influence of maternal smoking during pregnancy on the toddler’s negativity. Archives of Pediatrics Adolescent Medicine. 2000;154:381–385. doi: 10.1001/archpedi.154.4.381. [DOI] [PubMed] [Google Scholar]
- Campbell S, Breaux A. Maternal ratings of activity level and symptomatic behaviors in a nonclinical sample of young children. Journal of Pediatric Psychology. 1983;8:73–82. doi: 10.1093/jpepsy/8.1.73. [DOI] [PubMed] [Google Scholar]
- Campbell S, Pierce E, Moore G, Marakovitz S, Newby K. Boys’ externalizing problems at elementary school: Pathways from early behavior problems, maternal control, and family stress. Developmental Psychopathology. 1996;8:701–720. [Google Scholar]
- Centers for Disease Control and Prevention Annual smoking attributed mortality, years of potential life lost, and economic costs—United States, 1995-1999. Morbidity and Mortality Weekly Report. 2002;51:300–303. [PubMed] [Google Scholar]
- Chassin L, Presson C, Rose J, Sherman S. The natural history of cigarette smoking from adolescence to adulthood: Demographic predictors of continuity and change. Health Psychology. 1996;15:478–484. doi: 10.1037//0278-6133.15.6.478. [DOI] [PubMed] [Google Scholar]
- Chen W, Parnell S, West J. Neonatal alcohol and nicotine exposure limits brain growth and depletes cerebellar Purkinje cells. Alcohol. 1998;15:33–41. doi: 10.1016/s0741-8329(97)00084-0. [DOI] [PubMed] [Google Scholar]
- Coles C, Platzman K, Raskind-Hood C. A comparison of children affected by prenatal alcohol exposure and attention deficit hyperactivity disorder. Alcoholism: Clinical and Experimental Research. 1997;20:150–161. [PubMed] [Google Scholar]
- Cook RD, Weisberg S. Residuals and influence in regression. Chapman & Hall; New York: 1982. [Google Scholar]
- Conger R, Simons R. Life-course contingencies in the development of adolescent antisocial behavior: A matching law approach. In: Thornberry TP, editor. Advances in criminological theory. Vol. 7. Transaction Publishers; New Brunswick, NJ: 1997. pp. 55–99. [Google Scholar]
- Cornelius M, Day N. Effects of prenatal and postnatal tobacco exposures on offspring: A review. Alcohol Health and Research World. 2000;24:242–249. [PMC free article] [PubMed] [Google Scholar]
- Cornelius M, Day N, Richardson G, Taylor P. Epidemiology of substance abuse during pregnancy. In: Ott P, Tarter R, Ammerman R, editors. Sourcebook on substance abuse: Etiology, epidemiology, assessment and treatment. Allyn & Bacon; Needham Heights, MA: 1999. pp. 1–13. [Google Scholar]
- Cornelius M, Geva D, Day N. Patterns and covariates of tobacco use in a recent sample of pregnant teenagers. Journal of Adolescent Health. 1994;15:528–535. doi: 10.1016/1054-139x(94)90135-p. [DOI] [PubMed] [Google Scholar]
- Cornelius M, Goldschmidt L, Day N, Larkby C. Prenatal substance use among pregnant teenagers: A six-year follow-up of effects on offspring growth. Neurotoxicology and Teratology. 2002;24:703–710. doi: 10.1016/s0892-0362(02)00271-4. [DOI] [PubMed] [Google Scholar]
- Cornelius M, Goldschmidt L, Dempsey D. Environmental tobacco smoke exposure in low income six-year-olds: Parent report and urine cotinine measures. Nicotine & Tobacco Research. 2003;5:333–339. doi: 10.1080/1462220031000094141. [DOI] [PubMed] [Google Scholar]
- Cornelius M, Goldschmidt L, Taylor P, Day N. Prenatal alcohol use among teenagers: Effects on neonatal outcomes. Alcoholism: Clinical and Experimental Research. 1999;23:1238–1244. doi: 10.1111/j.1530-0277.1999.tb04284.x. [DOI] [PubMed] [Google Scholar]
- Cornelius M, Leech S, Goldschmidt L. Characteristics of persistent smoking in pregnant teenagers followed to young adulthood. Nicotine & Tobacco Research. 2004;6:159–169. doi: 10.1080/14622200310001656975. [DOI] [PubMed] [Google Scholar]
- Cornelius M, Leech S, Goldschmidt L, Day N. Is prenatal tobacco exposure a risk factor for early adolescent smoking? A follow-up study. Neurotoxicology and Teratology. 2005;27:667–676. doi: 10.1016/j.ntt.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Cornelius M, Ryan C, Day N, Goldschmidt L. Prenatal tobacco effects on neuropsychological outcomes in 6-year-old offspring. Journal of Behavioral and Developmental Pediatrics. 2001;22:217–225. doi: 10.1097/00004703-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Cornelius M, Taylor P, Geva D. Prenatal tobacco and marijuana use among adolescents: Effects on offspring gestational age, growth and morphology. Pediatrics. 1995;95:438–443. [PubMed] [Google Scholar]
- Crockenberg S. Predictors and correlates of anger toward the punitive control of toddlers by adolescent mothers. Child Development. 1987;58:964–975. doi: 10.1111/j.1467-8624.1987.tb01432.x. [DOI] [PubMed] [Google Scholar]
- Day N, Cornelius M, Goldschmidt L, Richardson G, Robles N, Taylor P. The effects of prenatal tobacco and marijuana use on offspring growth from birth through age 3 years. Neurotoxicology and Teratology. 1992;14:407–414. doi: 10.1016/0892-0362(92)90051-b. [DOI] [PubMed] [Google Scholar]
- Day N, Richardson G, Goldschmidt L, Cornelius M. Prenatal tobacco exposure and preschooler behavior. Journal of Behavioral and Developmental Pediatrics. 2000;21:180–188. [PubMed] [Google Scholar]
- Day NL, Robles N. Methodological issues in the measurement of substance use. Annals of the New York Academy of Sciences. 1989;562:8–13. doi: 10.1111/j.1749-6632.1989.tb21002.x. [DOI] [PubMed] [Google Scholar]
- DiFranza J, Lew R. Effect of maternal cigarette smoking on pregnancy complications and sudden infant death syndrome. Journal of Family Practice. 1995;40:385–394. [PubMed] [Google Scholar]
- Dohrenwend BS, Dohrenwend BP. Stressful life events: Their nature and effects. Wiley; New York: 1974. [Google Scholar]
- Eskenazi B, Trupin L. Passive and active maternal smoking during pregnancy as measured by serum cotinine and postnatal smoke exposure. 2. Effect on neurodevelopment at age 5 years. American Journal of Epidemiology. 1995;142:S19–S29. doi: 10.1093/aje/142.supplement_9.s19. [DOI] [PubMed] [Google Scholar]
- Fergusson D, Horwood L, Lynskey M. Maternal smoking before and after pregnancy: Effects on behavioral outcomes in middle childhood. Pediatrics. 1993;92:815–822. [PubMed] [Google Scholar]
- Fergusson D, Woodward L, Horwood L. Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Archives of General Psychiatry. 1998;55:721–727. doi: 10.1001/archpsyc.55.8.721. [DOI] [PubMed] [Google Scholar]
- Frankenburg W, Coons C. Home Screening Questionnaire: Its validity in assessing home environment. Journal of Pediatrics. 1986;102:624–626. doi: 10.1016/s0022-3476(86)80853-8. [DOI] [PubMed] [Google Scholar]
- Fraser A, Brockert J, Ward R. Association of young maternal age with adverse reproductive outcomes. The New England Journal of Medicine. 1995;332:1113–1117. doi: 10.1056/NEJM199504273321701. [DOI] [PubMed] [Google Scholar]
- Fried P. Adolescents prenatally exposed to marijuana: Examination of facets of complex behavior and comparison with the influence of in utero cigarettes. Journal of Clinical Pharmacology. 2002;42:97S–102S. doi: 10.1002/j.1552-4604.2002.tb06009.x. [DOI] [PubMed] [Google Scholar]
- Fried P, Watkins B. Differential effects on facets of attention in adolescents prenatally exposed to cigarettes and marihuana. Neurotoxicology and Teratology. 2001;23:421–430. doi: 10.1016/s0892-0362(01)00160-x. [DOI] [PubMed] [Google Scholar]
- Fried P, Watkinson B, Gray R. A follow-up study of attentional behavior in 6-year-old children exposed prenatally to marihuana, cigarettes, and alcohol. Neurotoxicology and Teratology. 1992;14:299–311. doi: 10.1016/0892-0362(92)90036-a. [DOI] [PubMed] [Google Scholar]
- Fung Y, Lau Y. Receptor mechanisms of nicotine-induced locomotor hyperactivity in chronic nicotine-treated rats. European Journal of Pharmacology. 1988;152:263–271. doi: 10.1016/0014-2999(88)90721-2. [DOI] [PubMed] [Google Scholar]
- Furstenberg F, Levine J, Brooks-Gunn J. The children of teenage mothers: Patterns of early childbearing in two generations. Family Planning Perspectives. 1990;22:54–61. [PubMed] [Google Scholar]
- Gilchrist L, Hussey J, Gillmore M, Lohr M, Morrison D. Drug use among adolescent mothers: Pregnancy to 18 months postpartum. Journal of Adolescent Health. 1996;19:337–344. doi: 10.1016/S1054-139X(96)00052-3. [DOI] [PubMed] [Google Scholar]
- Gold M. Marijuana. Plenum; New York: 1989. [Google Scholar]
- Goldschmidt L, Day N, Richardson G. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicology and Teratology. 2000;22:325–336. doi: 10.1016/s0892-0362(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Greenberg M, Lengua L, Coie J, Pinderhughes E. Predicting developmental outcomes at school entry using a multiple risk model: Four American communities. Developmental Psychology. 1999;35:403–417. doi: 10.1037//0012-1649.35.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hechtman L. Teenage mothers and their children: Risks and problems: A review. Canadian Journal of Psychiatry. 1989;34:569–575. doi: 10.1177/070674378903400615. [DOI] [PubMed] [Google Scholar]
- Huizink A, Mulder E. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neuroscience and Biobehavioral Reviews. 2006;30:24–41. doi: 10.1016/j.neubiorev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Jacobson J, Jacobson S. Effects of prenatal alcohol exposure on child development. Alcohol Research & Health. 2002;26:282–286. [PMC free article] [PubMed] [Google Scholar]
- Johns J, Louis T, Becker R, Means L. Behavioral effects of prenatal exposure to nicotine in guinea pigs. Neurotoxicology and Teratology. 1982;4:365–369. [PubMed] [Google Scholar]
- Kandel D, Wu P, Davies M. Maternal smoking during pregnancy and smoking by adolescent daughters. American Journal of Public Health. 1994;84:1407–1413. doi: 10.2105/ajph.84.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan K, Shaw D. The development of coercive family processes: The interaction between aversive toddler behavior and parenting factors. In: McCord J, editor. Coercion and punishment in long-term perspectives. Cambridge University Press; Cambridge, MA: 1995. [Google Scholar]
- Knopik V, Heath A, Jacob T, Slutske W, Bucholz K, Madden P, Waldron M, Martin N. Maternal alcohol use disorder and offspring ADHD: Disentangling genetic and environmental effects using a children-of-twins design. Psychological Medicine. 2006;36:1461–1471. doi: 10.1017/S0033291706007884. [DOI] [PubMed] [Google Scholar]
- Kotimaa A, Moilanen I, Taanila A, Ebeling H, Smalley S, McGough J, Hartikainen A, Järvelin M. Maternal smoking and hyperactivity in 8-year old children. American Academy of Child and Adolescent Psychiatry. 2003;42:826–833. doi: 10.1097/01.CHI.0000046866.56865.A2. [DOI] [PubMed] [Google Scholar]
- Kristjansson E, Fried P, Watkinson B. Maternal smoking during pregnancy affects children’s vigilance performance. Drug and Alcohol Dependence. 1989;24:11–19. doi: 10.1016/0376-8716(89)90003-3. [DOI] [PubMed] [Google Scholar]
- Langley K, Rice F, van den Bree M, Thapar A. Maternal smoking during pregnancy as an environmental risk factor for attention deficit hyperactivity disorder behaviour. A review. Minerva Pediatrica. 2005;57:359–371. [PubMed] [Google Scholar]
- Leech S, Richardson G, Goldschmidt L, Day N. Prenatal substance exposure: Effects on attention and impulsivity of 6-year-olds. Neurotoxicology and Teratology. 1999;21:109–118. doi: 10.1016/s0892-0362(98)00042-7. [DOI] [PubMed] [Google Scholar]
- Levin E, Slotkin T. Developmental neurotoxicity of nicotine. In: Slikker W, Chang LW, editors. Handbook of developmental neurotoxicology. Academic Press; San Diego: 1998. pp. 587–615. [Google Scholar]
- Linnet K, Wisborg K, Obel C, Secher N, Thomsen P, Agerbo E, Henriksen B. Smoking during pregnancy and the risk for hyperkinetic disorder in offspring. Pediatrics. 2005;116:462–467. doi: 10.1542/peds.2004-2054. [DOI] [PubMed] [Google Scholar]
- Martin S, Burchinal M. Young women’s antisocial behavior and the later emotional and behavioral health of their children. American Journal of Public Health. 1992;82:1007–1010. doi: 10.2105/ajph.82.7.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews T. Smoking during pregnancy in the 1990’s. National Center for Health Statistics; Hyattsville, MD: 2001. [Google Scholar]
- Maughan B, Taylor A, Caspi A, Moffitt T. Prenatal smoking and early childhood conduct problems: Testing genetic and environmental explanations of the association. Archives of General Psychiatry. 2004;61:836–843. doi: 10.1001/archpsyc.61.8.836. [DOI] [PubMed] [Google Scholar]
- Meade C, Ickovics J. Systematic review of sexual risk among pregnant and mothering teens in the USA: Pregnancy as an opportunity for integrated prevention of STD and repeat pregnancy. Social Science and Medicine. 2005;60:661–678. doi: 10.1016/j.socscimed.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Miao H, Liu C, Bishop K, Gong Z, Nordberg A, Zhang X. Nicotine exposure during a critical period of development leads to persistent changes in nicotinic acetylcholine receptors of adult rat brain. Journal of Neurochemistry. 1998;70:752–762. doi: 10.1046/j.1471-4159.1998.70020752.x. [DOI] [PubMed] [Google Scholar]
- Mick E, Biederman J, Faraone S, Sayer J, Kleinman S. Case-control study of attention-deficit hyperactivity disorder and maternal smoking, alcohol use, and drug use during pregnancy. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:378–385. doi: 10.1097/00004583-200204000-00009. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone S, Chen L, Jones J. Is maternal smoking during pregnancy a risk factor for attention deficit hyperactivity disorder in children? American Journal of Psychiatry. 1996;153:1138–1142. doi: 10.1176/ajp.153.9.1138. [DOI] [PubMed] [Google Scholar]
- Newberger C. Cognitive structure of parenthood: Designing descriptive measures. New Directions in Child Development. 1983;7:45–67. [Google Scholar]
- Olds D. Tobacco exposure and impaired development: A review of the evidence. Mental Retardation and Developmental Disabilities Research Review. 1997;3:257–269. [Google Scholar]
- Orlebeke J, Knol D, Verhulst F. Increase in child behavior problems resulting from maternal smoking during pregnancy. Archives of Environmental Health. 1997;52:317–321. doi: 10.1080/00039899709602205. [DOI] [PubMed] [Google Scholar]
- Osofsky J, Culp A, Ware L. Intervention challenges with adolescent mothers and their infants. Psychiatry. 1988;51:236–241. doi: 10.1080/00332747.1988.11024397. [DOI] [PubMed] [Google Scholar]
- Pelham W, Bender M. Peer relationships in hyperactive children. Description and treatment. Advances in Learning Behavior Diseases. 1982;1:365–436. [Google Scholar]
- Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measures. 1977;1:385–401. [Google Scholar]
- Rasanen P, Hakko H, Isohanni M, Hodgins S, Jarvelin M, Tihonen J. Maternal smoking during pregnancy and risk of criminal behavior among adult male offspring in Northern Finland 1966 birth cohort. American Journal of Psychiatry. 1999;156:857–862. doi: 10.1176/ajp.156.6.857. [DOI] [PubMed] [Google Scholar]
- Richardson S, Tizabi Y. Hyperactivity in the offspring of nicotine-treated rats: Role of the mesolimbic and nigrostriatal dopaminergic pathways. Pharmacology, Biochemistry, and Behavior. 1994;47:331–337. doi: 10.1016/0091-3057(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Royal College of Physicians . Smoking and the young. Author; London: 1992. [Google Scholar]
- Routh D, Schroeder C, O’Tuama L. Development of activity level in children. Developmental Psychology. 1974;10:163–168. [Google Scholar]
- Scaramella L, Leve L. Clarifying parent-child reciprocities during early childhood: The early childhood coercion model. Clinical Child and Family Psychological Review. 2004;7:89–107. doi: 10.1023/b:ccfp.0000030287.13160.a3. [DOI] [PubMed] [Google Scholar]
- Schneider A, Atluri P, Shen Q, Barnes W, Mah S, Stadfelt D, Goderie S, Temple S, Fleck M. Functional nicotinic acetylcholine receptor expression on stem and progenitor cells of the early embryonic nervous system. Annals of New York Academy of Sciences. 2002;971:135–138. doi: 10.1111/j.1749-6632.2002.tb04447.x. [DOI] [PubMed] [Google Scholar]
- Serbin L, Cooperman J, Peters P, Lehoux P, Stack D, Schwartzman A. Intergenerational transfer of psychosocial risk in women with childhood histories of aggression, withdrawal, or aggression and withdrawal. Developmental Psychology. 1998;34:1246–1262. doi: 10.1037//0012-1649.34.6.1246. [DOI] [PubMed] [Google Scholar]
- Serbin L, Stack D, DeGenna N, Grunzeweig N, Temcheff C, Schwartzman A, Ledingham J. When aggressive girls become mothers: Problems in parenting, health, and development across two generations. In: Bierman MPAK, editor. Aggression, antisocial behavior, and violence among girls: A developmental perspective. Guilford Press; New York: 2004. [Google Scholar]
- Shaw D, Gilliom M, Ingoldsby E, Nagin D. Trajectories leading to school-age conduct problems. Developmental Psychology. 2003;39:189–200. doi: 10.1037//0012-1649.39.2.189. [DOI] [PubMed] [Google Scholar]
- Silberg J, Parr T, Neale M, Rutter M, Angold A, Eaves L. Maternal smoking during pregnancy and risk to boys’ conduct disturbance: An examination of the causal hypothesis. Biological Psychiatry. 2003;53:130–135. doi: 10.1016/s0006-3223(02)01477-4. [DOI] [PubMed] [Google Scholar]
- Slaughter D. Early intervention and its effects on maternal and child development. Monographs of the Society for Research in Child Development. 1983;48(4) [Google Scholar]
- Slotkin T. Prenatal exposure to nicotine: What can we learn from animal models? In: Zagon IS, Slotkin TA, editors. Maternal substance abuse and the developing nervous system. Academic Press; San Diego: 1992. pp. 97–124. [Google Scholar]
- Slotkin T. Fetal nicotine or cocaine exposure: Which one is worse? Journal of Pharmacology and Experimental Therapeutics. 1998;285:931–945. [PubMed] [Google Scholar]
- Slotkin T. Developmental cholinotoxicants: Nicotine and chlorpyrifos. Environmental Health Perspectives. 1999;107:71–80. doi: 10.1289/ehp.99107s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T, Lappi S, Tayyeb M, Seidler F. Chronic prenatal nicotine exposure sensitizes rat brain to acute post-natal nicotine challenge as assessed with ornithine decarboxylase. Life Sciences. 1991;49:655–670. doi: 10.1016/0024-3205(91)90113-p. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R. Manual for the State Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1970. [Google Scholar]
- Thapar A, Fowler T, Rice F, Scourfield J, Van den Bree M, Thomas H, Harold G, Hay D. Maternal smoking during pregnancy and attention deficit hyperactivity disorder symptoms in offspring. American Journal of Psychiatry. 2003;160:1985–1989. doi: 10.1176/appi.ajp.160.11.1985. [DOI] [PubMed] [Google Scholar]
- Thomas J, Garrison M, Slawecki C, Ehlers C, Riley E. Nicotine exposure during the neonatal brain growth spurt produces hyperactivity in preweanling rats. Neurotoxicology and Teratology. 2000;22:695–701. doi: 10.1016/s0892-0362(00)00096-9. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Popke E, Rahman M, Nespor S, Grunberg N. Hyperactivity induced by prenatal nicotine exposure is associated with an increase in cortical nicotinic receptors. Pharmacology, Biochemistry, and Behavior. 1997;58:141–146. doi: 10.1016/s0091-3057(96)00461-3. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Public Health Service, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health . Women and smoking: A report of the surgeon general. Author; Atlanta, GA: 2001. [Google Scholar]
- U.S. Department of Health and Human Services. Substance Abuse and Mental Health Services Administration . National Survey on Drug Use and Health: Substance use during pregnancy: 2002 and 2003 update. Author; Rockville, MD: 2005. [Google Scholar]
- Vaglenova J, Birru S, Pandiella N, Breese C. An assessment of the long-term developmental and behavioral teratogenicity of prenatal nicotine exposure. Behavioral Brain Research. 2004;150:159–170. doi: 10.1016/j.bbr.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Ventura S, Martin J, Curtin S, Menacker F, Hamilton B. Births: Final data for 1999. National Vital Statistics Reports. 2001;49 [PubMed] [Google Scholar]
- Wakschlag L, Hans S. Maternal smoking during pregnancy and conduct problems in high-risk youth: A developmental framework. Developmental Psychopathology. 2002;14:351–369. doi: 10.1017/s0954579402002092. [DOI] [PubMed] [Google Scholar]
- Wakschlag L, Lahey B, Loeber R, Green S, Gordon R, Leventhal B. Maternal smoking during pregnancy and the risk of conduct disorder in boys. Archives of General Psychiatry. 1997;54:670–676. doi: 10.1001/archpsyc.1997.01830190098010. [DOI] [PubMed] [Google Scholar]
- Weissman M, Warner V, Wickramaratne P, Kandel D. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:7. doi: 10.1097/00004583-199907000-00020. [DOI] [PubMed] [Google Scholar]
- Weitzman M, Gortmaker S, Sobol A. Maternal smoking and behavior problems of children. Pediatrics. 1992;90:342–349. [PubMed] [Google Scholar]
- Willford J, Day N, Cornelius M. Maternal tobacco use during pregnancy: Epidemiology and effects on offspring. In: Miller M, editor. Development of the mammalian central nervous system: Lessons learned from studies on alcohol and nicotine exposure. Oxford University Press; New York: 2006. pp. 315–328. [Google Scholar]
- Williams G, O’Callaghan M, Najman J, Bor W, Andersen M, Richards D, Chunley U. Maternal cigarette smoking and child psychiatric morbidity: A longitudinal study. Pediatrics. 1998;102:e11. doi: 10.1542/peds.102.1.e11. [DOI] [PubMed] [Google Scholar]
- Zhang X, Liu C, Miao H, Gong Z, Nordberg A. Postnatal changes of nicotinic acetylcholine receptor α2, α3, α4, α7 and β2 subunits genes expression in rat brain. International Journal of Developmental Neuroscience. 1998;16:507–518. doi: 10.1016/s0736-5748(98)00044-6. [DOI] [PubMed] [Google Scholar]


