Figure 8.
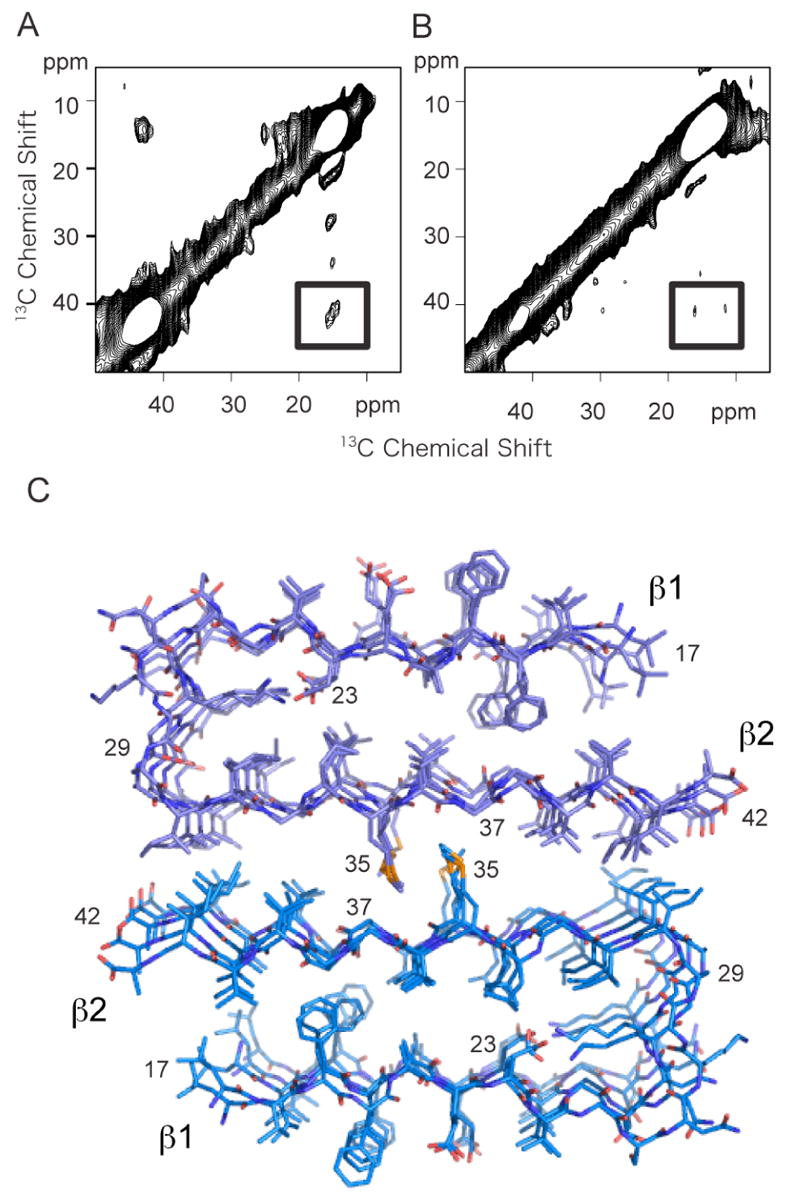
Solid-state 13C NMR of Aβ42. A. 2D 13C DARR NMR spectrum of Aβ42 fibrils. The spectrum was obtained using fibrils formed from an equimolar mixture of two 13C-labeled Aβ42 peptides having the same labeling scheme as in (A–C). A crosspeak (boxed) is observed only between 2-13C-Gly37 and 5-13C-Met35, and not between 1-13C-Gly33 and 5-13C-Met35. B. 2D 13C DARR NMR spectrum of Aβ42 fibrils formed using only 5-13C-Met35 Aβ42. The samples in (A) and (B) were prepared in parallel. The absence of a crosspeak in (B) demonstrates that the crosspeak observed in (A) is not due to natural abundance 13C. C. Structural model of the Aβ42 fibril. The model was based on the protofilament subunit structure of Aβ42 proposed by Riek and co-workers (56) (PDB accession code 2BEG), and the solid-state NMR contacts between Met35 and Gly37. The protofilament subunits were docked with opposite orientations to satisfy the solid-state NMR constraint that only a contact between Met35 and Gly37 was observed. The docked structure was energy minimized using standard methods. The difference in the Met- Gly contacts between Aβ40 and Aβ42 results in a shift of the two protofilament subunits relative to one another in the two structures.
