Abstract
Imaging agents targeting β,-amyloid (Aβ) may be useful for diagnosis and treatment of patients with Alzheimer’s disease (AD). Compounds 3e and 4e are fluorinated stilbene derivatives displaying high binding affinities for Aβ plaques in AD brain homogenates (Ki = 15 ± 6 and 5.0 ± 1.2 nM, respectively). In vivo biodistributions of [18F]3e and [18F]4e in normal mice exhibited excellent brain penetrations (5.55 and 9.75 % dose/g at 2 min) and rapid brain washouts were observed, especially for [18F]4e (0.72% dose/g at 60 min). They also showed in vivo plaque labeling in APP/PS1 or Tg2576 transgenic mice, animal models for AD. Autoradiography of postmortem AD brain sections and AD homogenate binding studies confirmed the selective and specific binding properties to Aβ plaques. In conclusion, the preliminary results strongly suggest that these fluorinated stilbene derivatives, [18F]3e and [18F]4e, are suitable candidates as Aβ plaque imaging agents for studying patients with AD.
Introduction
Alzheimer’s disease (AD) is a brain disorder associated with progressive memory loss and decrease of cognitive function. Currently, a definitive diagnosis of AD can only be established by demonstrating the presence of abundant senile plaques and neurofibrillary tangles in the postmortem brain1,2. The senile plaques are extra-cellular deposits of amyloid fibrils ofβ-amyloid (Aβ), and their presence are strongly associated with pathogenesis of the disease. Thus, specific in vivo imaging agents targeting Aβplaques may serve as suitable markers for monitoring the amyloid burden following the disease progression and further provide supporting evidence for therapeutic intervention3. Currently, many efforts focusing on development of therapeutic approach targeting Aβplaques or reversing the effects of the plaque-deposition have been reported 4,5.
Development of imaging agents for direct mapping of Aβ aggregates in the living brain is an active research area in recent years. Several research groups have reported biomarkers for imaging Aβ plaques in the brain6-10. The Aß-plaque-specific imaging agents can be labeled with short-lived isotopes suitable for in vivo imaging studies10. Two isotopes,99mTc (T1/2, 6 h, 140 KeV) and 123I (T1/2, 13 h, 159 KeV), are commonly used for single photon emission computed tomography (SPECT); while 11C (T1/2, 20 min, 511 KeV) and 18F (T1/2, 110 min, 511 KeV) are often used for positron emission tomography (PET). It is generally accepted that SPECT is more convenient and cost-effective, but PET gives a better imaging resolution. Recently, successful preliminary reports using a 11C labeled benzothiazole derivative, [11C]PIB6,11,12, and a 18F labeled probe, [18F]FDDNP9,13,14, for plaque and tangle visualization in living AD patients have demonstrated the potential utility of in vivo imaging (Fig. 1). Similarly, our group has also prepared a 11C labeled PET ligand, [11C]SB-13 (4-N-methylamino-4’-hydroxystilbene), a stilbene derivative7,15 and a 123I labeled SPECT ligand, IMPY (6-iodo-2-(4’-N,N-dimethylamino)phenylimidazo[1,2-a]pyridine)16-18. Both tracersshowed selective and high binding affinities to Aβ plaques19. As expected, [11C]SB-13 displayed a high accumulation in the frontal cortex (presumably an area containing a high density of Aß plaques) in mild to moderate AD patients, but not in age-matched control subjects7; while the SPECT ligand, [123I[IMPY, is currently under active clinical evaluation. Other structurally similar compounds have been recently reported for targeting Aβ plaques in the brain20-22.
Fig.1.
Structures of PET imaging agents proven to target amyloid plaques in AD patients
Initially, we reported an iodinated derivative of stilbene, 3-iodo-4′-diamino-stilbene, targeting amyloid plaques for SPECT imaging23. However, the unfavorable in vivo kinetic properties make it unattractive for further development as a useful in vivo SPECT tracer for amyloid plaques in the brain. We have subsequently identified another stilbene derivative, SB-13, labeled with C-11 as a potential PET tracer for plaque imaging15. It was clinically demonstrated that [11C]SB-13 can detect senile plaques present in AD patients7. However, the short half life (20 min) of C-11 may limit the usefulness of [11C]SB-13 or [11C]PIB for a wide spread clinical application. One major focus of our effort is in development of18F labeled plaque-specific imaging agents, because the longer half-life of F-18 and stability in solution allow the use of the radioligand over a long period of time. While this work was in progress a series of 18F labeled styrylbenzoxazole compounds, such as 2-(4-methylaminostyryl)-6-(2- [18F]fluoroethoxy) benzoxazole, were reported to show promise as potential PET imaging agents targeting amyloid plaques in the brain8.
Reported herein are the synthesis and in vitro and in vivo evaluations of a novel series of two 18F labeled stilbene derivatives as prospective PET radiotracers for imaging amyloid plaques in the brain.
Results and Discussion
Chemistry
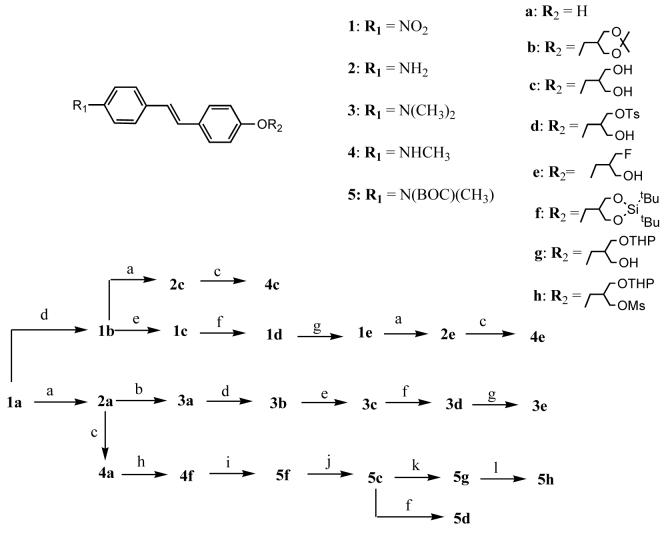
Syntheses of compounds 3e, 4e and radiolabelings of precursors 3d, 5h to prepare [18F]3e and [18F]4e are shown in Scheme 1 and Scheme 4. To prepare compound 3a the nitro group of 4-nitro-4’-hydroxy stilbene, 1a, was reduced with SnCl2/HCl(c) in ethanol to give the corresponding amine 2a. Following a treatment with (CHO)n and NaBH3CN, the dimethylamino compound 3a was obtained. Compound 3b was obtained by reacting the hydroxyl stilbene, 3a, with bromide 8m24, which was separately prepared as shown in Scheme 2, and potassium carbonate in anhydrous DMF. Compound 3c was obtained by treatment of 3b with 1N HCl in acetone to remove the protected group. Reacting diol 3c with 1.5 equivalent of tosyl chloride in pyridine gave a mixture; however, mono tosylate 3d could be isolated from the mixture by silica gel chromatography with 5 % methanol in dichloromethane as the eluent. The tosylate group of 3d was converted to fluoride, 3e, by refluxing with anhydrous TBAF in THF25. The tosyl compound 3d was also used as the starting material to obtain radiolabeled compound, [18F]3e. Nitro compound 1e was similarly synthesized by a coupling reaction of 1a with 8m24 followed by tosylation and fluorination. The synthesis of compound 4e was accomplished by reduction of the nitro group of 1e with SnCl2/EtOH followed by a monomethylation of the amino group with (CHO)n, NaOCH3and NaBH4. The nitro group of the acetal protectecd intermediate, 1b,was reduced to amine, 2c, and then monomethylated to give compound 4c. Similarly, 2a was first monomethylated to give the compound 4a, after which 4a was coupled with 8n to give 5f. Di-tert-butyl siliyl group of 5f was removed with 1N TBAF in THF at room temperature to give diol 5c. The obtained 5c could be either monotosylated to give 5d or mono protected by THP26 to give 5g. From 5g, the desired mesylated precusor 5h for radiolabeling can thus be obtained.
Scheme 1.
a) SnCl2, HCl(c), EtOH; b) (CHO)n, NaBH3CN, AcOH, rt; c) (1) NaOMe, MeOH, (CHO)n; (2) NaBH4; d) 8m, K2CO3, DMF, 100 °C; e) HCl, CH3COCH3, rt; f) TsCl, Py, 0 °C; g) TBAF, THF, reflux; h) 8n, K2CO3, DMF, 100 °C; i) (BOC)2O, THF, reflux; j) TBAF (1 M), THF, rt; k) DHP, PPTS, DCM, rt; l) MsCl, Et3N, DCM, rt.
Scheme 4.
(a) [18F]HF/K2CO3/K222 DMSO, (b) aq HCl
Scheme 2.
a) 7m: (CH3O)2C(CH3)2, TsOH, reflux; 7n: HOBT, Si(t-Bu)2Cl2, Et3N, DCM, reflux; b) CBr4, PPh3, Py, DCM, rt.
A related p-bromobenzyl compound 3j was also synthesized as shown in Scheme 3. The substituted malonate 927 was reduced to diol 10 with DIBALH and then reacted with one equivalent of TBSCl to give 11. The unprotected OH was then converted into bromide 12 with CBr4/PPh3. Compound 12 was reacted with 3a to give 3i which was treated with TBAF to remove TBS group to yield 3j.
Scheme 3.
a) DIBALH, THF, 0 °C; b) TBSCl, Et3N, DCM, rt; c) CBr4, PPh3, DCM, rt; d) K2CO3, 12, DMF, 100 °C; e) TBAF(1 M), THF, rt.
Several starting materials were evaluated in a radiofluoride displacement reaction. To obtain dimethylamino derivative, [18F]3e, the tosylated precursor, 3d, was mixed with [18F]fluoride/potassium carbonate and Kryptofix® 222 in DMSO and heated at 120 °C for 4 min. Crude product was purified by HPLC to attain >99% of the radiochemical purity with 10% radiochemical yield (decay corrected). The procedure took 90 min and specific activity was estimated to be 70 Ci/mmol at the end of synthesis. To obtain the N-monomethyl derivative,[18F]4e, tosylate 5d was first prepared as the precursor for radiolabeling. However, the purification of [18F]4e was tedious and time consuming resulting in a low yield. The situation was greatly improved when using 5h as the precursor for labeling, of which mesylate was used instead of tosylate and the free OH was protected with THP. An advantage of using mesylate (5h) as the radiolabeling precursor is that reaction proceeded more cleanly. As a result, the amount and the number of unknown side-products were significantly reduced. Specific activity estimated by comparing UV peak intensity of labeled compound with reference non-radioactive compound of known concentration improved at least 10 times at the end of synthesis comparing with that obtained from tosylated precursor, 5d. A similar procedure was carried out to obtain [18F]4e from the mesylated precursor, 5h. After the initial reaction in DMSO, the mixture was treated with aqueous HCl to remove BOC and THP groups. Radiochemical purity was >99% after HPLC purification and the radiochemical yield was 20% (decay corrected). The total syntheses took 110 min and specific activity was estimated to be 900-1,000 Ci/mmol at the end of synthesis.
Biological studies
Binding affinities of the new series of stilbene derivatives were examined in a binding assay using AD brain homogenates and [125I]IMPY as the radioligand19. Compound 4a (SB-13), as shown previously, displayed a high binding affinity19. With an additional methyl group attached to the nitrogen atom (dimethylamino vs monomethylamino in 3a vs 4a), a similar high binding affinity was observed (1.1 nM and 1.2 nM for 3a and 4a, respectively)(Table 1). Further modifications of 3a result in epoxi and diol derivatives, 3b and 3c, but they showed lower binding affinities (Ki = 59 and 38 nM, respectively). A relatively high binding affinity (Ki = 15 nM) was obtained with 3e (a fluorinated derivative).
Table 1.
Inhibition constants (Ki, nM) of compounds on I-125-IMPY binding to amyloid plaques in AD brain homogenates
| Compound | Ki ± SEM (nM) |
Compound | Ki ± SEM (nM) |
|---|---|---|---|
| 2a | 95 ± 8.0 | 3e | 15 ± 6 |
| 2e | 15 ± 4.0 | 3j | 80 ± 20 |
| 3a | 1.1 ± 0.2 | 4a (SB-13) | 1.2 ± 0.2* |
| 3b | 59 ± 10 | 4c | 32.5 ± 5.0 |
| 3c | 38 ± 5 | 4e | 5.0 ± 1.2 |
| 3d | 150 ± 30 | PIB | 2.8 ± 0.5 |
Each value was determined three times with duplicate for each measurement.
The value was reported previously 19 .
Since the binding affinity of 3e appeared promising; thus, the corresponding tosylated derivative, 3d, was prepared as a starting material for radiolabeling with 18F. Under the chosen HPLC conditions (Hamilton PRP-1 column, CH3CN/dimethylglutarate buffer (5 mM, pH 7) = 9/1), the tosylated and the diol derivatives can be clearly separated from the desired 18F labeled stilbene, [18F]3e. This HPLC system has been successfully applied to remove the potential pseudo-carrier, the hydroxyl compound, 3c. Similarly, the monomethylamino compounds displayed the same trend with a high affinity for the fluorinated derivative, 4e (Ki = 5 nM), and a lower affinity for the diol derivative, 4c (Ki =32.5 nM). In addition, extra p-bromo-benzyl substituted derivative such as 3j, showed a lower binding affinity (Ki = 80 nM). Interestingly, the free amino derivative, 2e, under a similar binding assay condition, showed a Ki value of 15 ± 4.0 nM which was comparable to the dimethylamino (3e) and monomethylamino (4e) derivatives (competing with [125I]IMPY binding) (Table 1). This result is different from the original stilbene compounds showing a much lower binding affinity for the free amino compound (Ki = 1.1, 1.2 and 95 nM for 3a, 4a(SB-13) and 2a, respectively). Surprisingly, despite the fact that 2e showed reasonably good in vitro binding affinity (a relatively low Ki value), [18F]2e exhibited no specific binding signal for amyloid plaques (using either AD brain section labeling or AD homogenate binding) (data not shown). Therefore, we have abandoned this free amino compound and focused only on [18F]3e and [18F]4e as likely candidates. As expected, both [18F]3e and [18F]4e clearly label plaques by in vitro autoradiography using postmortem AD brain sections showing a high plaque labeling and a low background (see Fig 2). The same results were obtained using brain sections of double transgenic (APP/PS1) mice (data not shown).
Fig.2.
In vitro autoradiography of brain sections from AD patients labeled with[18F]3e(A) and [18F]4e(B). The Aβ plaques were clearly labeled with both 18F tracers with low background labeling.
Previously, using an in vitro binding assay we have reported saturable and high binding affinities in postmortem brain homogenates of AD patients for two potential tracers, [3H]SB-13 (a N-methylamino derivative) and [125I]IMPY (a N,N- dimethylamino derivative)19. Interestingly, [18F]3e contains a N,N-dimethylamino group, but it did not display any specific Aβ plaque binding signals in the brain homogenates binding assay (data not shown). However, using a mono-N-methylated derivative, [18F]4e, in the same binding assay, a distinct binding signal was observed in the gray matter homogenates of AD patients . In the white matter of AD patients, where Aβ plaque deposits were low to non-existent, the binding signal was significantly lower (6-8 times lower) (Fig. 4). Whereas in assays using brain homogenates from control subjects, the binding signals in both gray and white matters were low, suggesting that the binding was highly selective to the presence of Aβ plaque deposits. The specific binding observed for [18F]4e in AD brain homogenates was saturable and the binding capacity (Bmax) was in the range of 10-20 pmole/mg tissue (data not shown). Detailed in vitro binding characterization of [18F]4e will be published elsewhere as a separate paper.
Fig.4.

[18F]4e showed binding to AD and control tissue homogenates prepared from dissected gray and white matter of cortical regions. High specific binding was detected mainly in gray matter with relatively low binding in white matter homogenates. In contrast, homogenates from the control showed significantly lower specific binding of [18F]4e.
After an iv injection, both [18F]3e and [18F]4e displayed good initial brain uptakes in normal mice (5.55% and 9.75% ID/g at 2 min post-injection for [18F]3e and [18F]4e, respectively). The rate of washout of [18F]3e from the brain was slower as compared to that of [18F]4e (1.37 % and 0.31 %ID/g at 120 min post-injection for [18F]3e and [18F]4e, respectively). A relatively higher lipophilicity was observed for [18F]3e (partition coefficient = 1,375 and 889 for [18F]3e and [18F]4e, respectively). This disparity may account for the longer brain retention observed for [18F]3e. Initial blood levels were relatively low for both tracers (1.79% and 2.60% ID/g for [18F]3e and [18F]4e, respectively) and there was a significant reduction at 2 hr post-injection (1.18 and 0.78 %ID/g). In vivo defluorination was likely for both tracers, because the bone uptake showed increasing uptake reaching 7-8%ID/g at 2 hr post-injection.
To examine the in vivo labeling of Aβ plaques in a living brain, we have tested both candidates using mouse models, APP/PS18,28 or Tg2576 transgenic mice29, which are specifically engineered to over produce the Aβ plaques in the brain. When subjected to in vivo labeling of Aβ plaques (after an iv injection) in these models, distinctive Aβ plaque labeling can be observed for both [18F]3e and [18F]4e (Fig. 3A and B). The specific in vivo targeting for Aβ plaques, especially for [18F]4e, demonstrates the feasibility of using it as an in vivo PET imaging agent for detecting senile plaques. Both of these transgenic mouse models have been successfully used in studying relationships between deposit of Aβ plaques and AD30,31. For the purpose of this in vivo Aβ plaque labeling study by a 18Ftracer in a transgenic mouse model we did not make a distinction between using either one of these models. Recently, the presence of soluble oligomer of amyloid-β peptides, prior to their aggregation to fribrillary forms, has attracted attention as the primary underlying feature leading to neuronal toxicity and symptoms of AD32-34. The PET imaging agents reported in this paper will not be able to detect the soluble form of amyloid-β peptides; however, the presence of the oligomers is likely a prelude leading to the formation of Aβ aggregates in the brain. Therefore, from a diagnostic perspective, imaging agents targeting Aβ palques in the brain will still be very useful for the diagnosis of AD. Efforts in developing novel ligands for binding the soluble Aβ oligomers will be an interesting and highly important research topic.
Fig.3.

In vivo plaque labeling visualized by ex vivo autoradiogram (A) [18F]3e in a APP/PS1 mouse brain (B) [18F]4e in a Tg2576 mouse. The animal was injected via tail vein with 270 μCi [18F]3e or 260 μCi [18F]4e and sacrificed 2 hr (for [18F]3e) or 1 hr (for [18F]4e) after tracer injection.
Conclusion
A new series of novel stilbene derivatives displaying high binding affinities to Aβ plaques was successfully prepared as potential PET imaging agents for AD. Both 4-dimethylamino and 4-monomethylamino stilbenes ([18F]3e and [18F]4e) entered the brain of normal mice readily and quickly. [18F]4e showed a fast washout with a low normal brain retention. Specific plaque labeling was demonstrated by in vitro and ex vivo autoradiography of brain sections. Favorable pharmacokinetic properties and specific plaque labeling properties of these 18F labeled stilbene derivatives, especially [18F]4e, warrant further investigation.
Experimental Section
All reagents used in syntheses were commercial products and were used without further purification unless otherwise indicated. 1H NMR spectra were obtained on a Bruker DPX spectrometer (200 MHz) in CDCl3 unless otherwise indicated. Chemical shifts are reported as δ values (parts per million) relative to internal TMS. Coupling constants are reported in Hertz. The multiplicity is defined by s (singlet), d (doublet), t (triplet), br (broad), m (multiplet). Elemental analyses were performed by Atlantic Microlab INC. For each procedure, “standard workup” refers to the following steps: addition of indicated organic solvent, washing the organic layer with water then brine, separation of the organic layer from the aqueous layer, drying off the combined organic layers with anhydrous sodium sulfate, filtering off the sodium sulfate and removing the organic solvent under reduced pressure.
4-Amino-4’-hydroxystilbene (2a)
Stannous chloride (11.8 g, 0.062 mol) was added to a solution of compound 1a (Frinton Lab) (3.0 g, 0.012 mol) in ethanol (100 mL) followed by the addition of concentrated hydrochloric acid (5.0 mL). The solution was brought to reflux for 3 hr and cooled to room temperature stirring overnight. Aqueous sodium hydroxide (1N) was added to adjust the pH to 8-9. After standard workup with dichloromethane, crude product 2a was obtained and was used in the following step without further purifications. 2a (2.6 g,∼100%): 1H NMR (DMSO-d6) δ 9.39 (s, 1H), 7.30 (d, 2H, J = 8.5 Hz), 7.20 (d, 2H, J = 8.5 Hz), 6.80 (m, 2H), 6.72 (d, 2H, J = 8.5 Hz), 6.53 (d, 2H, J = 8.5 Hz), 5.19 (s, 2H).
4-N,N’-Dimethylamino-4’-hydroxystilbene (3a)
To a mixture of 2a (211 mg, 1.0 mmol), paraformaldehyde (300 mg, 10 mmol) and sodium cyanoborohydride (189 mg, 3.0 mmol), acetic acid (10 mL) was added. The whole mixture was stirred at room temperature overnight and then poured into 100 mL of water. Sodium carbonate was added to adjust the pH to 8-9. After standard workup with 5 % methanol in dichloromethane, the residue was purified by silica gel column chromatography (2.5 % methanol in dichloromethane) to afford 3a as a white solid (214 mg, 89.5 %): 1H NMR δ 7.37 (m, 4H), 6.87 (s, 2H), 6.75 (m, 4H), 4.68 (s, 1H), 2.98 (s, 6H).
(4-{2-[4-(2,2-Dimethyl-[1,3]dioxan-5-ylmethoxy)-phenyl]-vinyl}-phenyl)-dimethyl-amine (3b)
Under a nitrogen atmosphere, 3a (100 mg, 0.38 mmol) was dissolved in anhydrous DMF (5.0 mL). Potassium carbonate (140 mg, 1.0 mmol) was added to this solution followed by 5-bromomethyl-2,2-dimethyl-[1,3]dioxane 8m24 (105 mg, 0.5 mmol). The mixture was heated to 100 °C and stirred overnight. After cooling down to room temperature, standard workup with dichloromethane was applied and the residue was purified by silica gel preparative TLC (1% methanol in dichloromethane) to afford compound 3b (100 mg, 72 %): 1H NMR δ 7.38 (m, 4H), 6.88 (m, 4H), 6.70 (d, 2H, J = 8.7 Hz), 4.08 (m, 4H), 3.87 (m, 2H), 2.96 (s, 6H), 2.13 (m, 1H), 1.46 (s, 3H), 1.42 (s, 3H). Anal. (C23H29NO3) C, H, N.
2-{4-[2-(4-Dimethylamino-phenyl)-vinyl]-phenoxymethyl}-propane-1,3-diol (3c)
Compound 3b (180 mg, 0.49 mmol) was suspended in acetone (5.0 mL) and cooled to 0 °C with an ice bath. 1N HCl (5.0 mL, 5.0 mmol) was slowly added over 20 min. The suspension turned into a clear solution during the addition. The solution was stirred at 0 °C for an additional 1.5 hr and then warmed to room temperature in 0.5 hr. Saturated sodium bicarbonate was added to adjust pH to 8-9. After standard workup with dichloromethane, the residue was purified by silica gel preparative TLC (5 % methanol in dichloromethane) to afford compound 3c as a white solid (140 mg, 87 %): 1H NMR δ 7.40 (m, 4H), 6.88 (m, 4H), 6.74 (m, 2H), 4.10 (d, 2H, J = 5.47 Hz), 3.89 (d, 4H, J = 5.28 Hz), 2.98 (s, 6H), 2.22 (m,1H). Anal. (C20H25NO3) C. H. N.
Toluene-4-sulfonic acid-3-{4-[2-(4-dimethylamino-phenyl)-vinyl]-phenoxy}-2-hydroxymethyl-propyl ester (3d)
Compound 3c (158 mg, 0.49 mmol) was dissolved in anhydrous pyridine (15 mL) and cooled to 0 °C with an ice bath. Tosyl chloride (137 mg, 0.72 mmol) was added and the solution was stirred at 0 °C for 2 hr. After standard workup with dichloromethane, the residue was purified by silica gel preparative TLC (5% methanol in dichloromethane) to afford monotosylate compound, 3d, as a white solid (95 mg, 41 %): 1H NMR δ 7.75 (d, 2H, J = 8.26 Hz), 7.37 (m, 4H), 7.26 (m, 2H), 6.88 (m, 2H), 6.72 (m, 4H), 4.26 (d, 2H, J = 5.66 Hz), 3.97 (d, 2H, J = 5.96 Hz), 3.79 (d, 2H, J = 5.24 Hz), 2.95 (s, 6H), 2.38 (m, 4H). Anal. (C27H31NO5S) C, H, N.
3-{4-[2-(4-Dimethylamino-phenyl)-vinyl]-phenoxy}-2-fluoromethyl-propan-1-ol (3e)
Compound 3d (40 mg, 0.083 mmol) was dissolved in anhydrous THF (5.0 mL). Under the nitrogen atmosphere, anhydrous TBAF 25 (150 mg, 0.5 mmol) in anhydrous THF (1.0 mL) was slowly added. The solution was then heated to reflux for 3 hr. After cooled down to room temperature, standard workup with dichloromethane was applied and the residue was applied for silica gel preparative TLC (5 % methanol in dichloromethane) to afford product 3e (17 mg, 62 %): 1H NMR δ 7.40 (m, 4H), 6.89 (m, 4H), 6.70 (d, 2H, J = 8.82 Hz), 4.67 (d d, 2H, J1= 47.1 Hz, J2= 5.46 Hz), 4.10 (d, 2H, J = 5.86Hz), 3.88 (d, 2H, J = 5.24 Hz), 2.97 (s, 6H), 2.40 (m, 1H), 1.76 (s, 1H). Anal. (C20H24FNO2) C, H, N.
2,2-Dimethyl-5-{4-[2-(4-nitro-phenyl)-vinyl]-phenoxymethyl}-[1,3]dioxane(1b)
Compound 1b was prepared from 1a (241 mg, 1.0 mmol) with the same procedure described for compound 3b. 1b (260 mg, 70 %): 1H NMR β 8.19 (d,2H, J = 8.80 Hz), 7.49 (m, 4H), 7.07 (m, 2H), 6.90 (d, 2H, J = 8.80 Hz), 4.12 (m,4H), 3.89 (d, 2H), 2.10 (m, 1H), 1.48 (s, 3H), 1.43 (s, 3H). Anal. calcd. (C21H23NO5) C, H, N.
2-{4-[2-(4-Nitro-phenyl)-vinyl]-phenoxymethyl}-propane-1,3-diol (1c)
Compound 1c was prepared from 1b (260 mg, 0.7 mmol) with the same procedure described for compound 3c. 1c (190 mg, 82 %): 1H NMR (CD3OD) δ 8.19 (d, 2H, J = 8.80 Hz), 7.72 (d, 2H, J = 8.80 Hz), 7.55 (d, 2H, J = 8.70 Hz), 7.24 (q, 2H), 6.96 (d, 2H, J = 8.70 Hz), 4.09 (d, 2H, J = 5.78 Hz), 3.74 (d, 4H, J = 5.94 Hz), 2.14 (m, 1H). Anal. (C18H19NO5) C, H, N.
Toluene-4-sulfonic acid 2-hydroxymethyl-3-{4-[2-(4-nitro-phenyl)-vinyl]-phenoxy}-propyl ester (1d)
Compound 1d was prepared from 1c (80 mg, 0.24 mmol) with the same procedure described for compound 3d. 1d (66mg, 56 %): 1HNMR δ 8.18 (d, 2H, J = 8.82 Hz), 7.77 (d, 2H, J = 8.32 Hz), 7.58 (d, 2H, J = 8.82 Hz), 7.45 (d, 2H, J = 8.73 Hz), 7.28 (d, 2H, J = 8.18 Hz), 7.09 (q, 2H), 6.81 (d, 2H, J = 8.73 Hz), 4.27 (d, 2H, J = 5.70 Hz), 4.01 (m, 2H), 3.80 (d, 2H, J = 5.61 Hz), 2.40 (m, 4H), 2.02 (s, 1H). Anal. (C25H25NO7S) C, H, N.
2-Fluoromethyl-3-{4-[2-(4-nitro-phenyl)-vinyl]-phenoxy}-propan-1-ol (1e).
Compound 1e was prepared from 1d (33 mg, 0.069 mmol) with the same procedure described for compound 3e. 1e (20 mg, 88 %): 1H NMR δ 8.19 (d, 2H,J = 8.83 Hz), 7.58 (d, 2H, J = 8.84 Hz), 7.48 (d, 2H, J = 8.74 Hz), 7.10 (q, 2H), 6.94 (d, 2H, J = 8.68 Hz), 4.69 (d d, 2H, J1= 47.1 Hz, J2 = 5.36 Hz), 4.15 (d, 2H, J= 5.89Hz), 3.90 (d, 2H, J = 5.43 Hz), 2.43 (m, 1H), 1.74 (s, 1H). Anal. (C18H18FNO4) C, H, N.
3-{4-[2-(4-Amino-phenyl)-vinyl]-phenoxy}-2-fluoromethyl-propan-1-ol (2e)
Compound 2e was prepared from 1e (37 mg, 0.11 mmol) with the same procedure described for compound 2a. 2e (24 mg, 71 %): 1H NMR β 7.35 (m, 4H), 6.90 (m, 4H), 6.66 (d, 2H, J = 8.54 Hz), 4.69 (d d, 2H, J1 = 47.1 Hz, J2 = 5.46 Hz), 4.12 (d, 2H, J = 5.84 Hz), 3.90 (d, 2H, J = 5.56 Hz), 3.70 (s, 2H), 2.39 (m, 1H), 1.71 (s, 1H). Anal. (C18H20FNO2) C, H, N.
2-Fluoromethyl-3-{4-[2-(4-methylamino-phenyl)-vinyl]-phenoxy}-propan-1-ol (4e)
Under the nitrogen atmosphere, sodium methoxide (22 mg, 0.4 mmol) was added to a suspension of compound 2e (24 mg, 0.08 mmol) in methanol (6mL) followed by paraformaldehyde (12 mg, 0.4 mmol). The solution was heated to reflux for 2 hr and cooled to 0 °C with an ice bath. Sodium borohydride (15mg, 0.4 mmol) was added in portions. Reaction mixture was brought to reflux again for 1 hr and poured onto crushed ice. After standard workup with dichloromethane, the residue was applied for silica gel preparative TLC (4.5 % methanol in dichloromethane) to afford product 4e (23 mg, 92 %): 1H NMR δ 7.37 (m, 4H), 6.87 (m, 4H), 6.59 (d, 2H, J = 8.56 Hz), 4.69 (d, d, 2H, J1 = 47.1 Hz, J2 = 5.44 Hz), 4.12 (d, 2H, J = 5.86 Hz), 4.00 (s, 1H), 3.89. (d, 2H, J = 5.52 Hz), 2.86 (s, 3H), 2.41 (m, 1H), 1.75 (s, 1H). Anal. (C19H22FNO2) C, H, N.
4-N-Methylamino-4’-hydroxystilbene (4a)
Compound 4a was prepared from 2a (105 mg, 0.5 mmol) with the same procedure as described for compound 4e.4a (100 mg, 89 %): 1H NMR δ 7.34 (m, 4H), 6.86 (s, 2H), 6.79 (d, 2H, J = 8.58 Hz), 6.60 (d, 2H, J = 8.58 Hz), 2.85 (s, 3H).
(2,2-Di-tert-butyl-[1,3,2]dioxasilinan-5-yl)-methanol (7n)
To the solution of 2-hydroxypropyl-1,3-diol 6 (500 mg, 4.7 mmol) in anhydrous dichloromethane (15mL), HOBT(135 mg, 1.0 mmol) was added. Under the nitrogen atmosphere, triethylamine (6.5 mL, 4.9 g, 48 mmol) was added via a syringe followed by di-tert-butyl-dichlorosilane (1.05 g, 5.0 mmol). The solution was gently refluxed for 1 hr and cooled to room temperature. After standard workup with dichloromethane, the residue was applied for silica gel column chromatography (1% methanol in dichloromethane) to afford product 7n, which was used for the following step without further purification. 7n (1.03 g, 89 %): 1H NMR δ 4.17 (m, 2H), 3.92 (t, 2H), 3.50 (d, 2H, J = 5.78 Hz), 2.30 (m, 1H), 1.39 (s, 1H), 1.04 (s, 9H), 1.02 (s, 9H).
5-Bromomethyl-2,2-di-tert-butyl-[1,3,2]dioxasilinan (8n)
Compound 7n(123 mg, 0.5 mmol) was dissolved in dichloromethane (10 mL) and cooled to -10 °C with an ethanol-ice bath. Pyridine (1 mL) was added followed by carbon tetrabromide (220 mg, 0.66 mmol). Triphenylphosphine (174 mg, 0.66 mmol) was added in portions and the solution was stirred at -10 °C for 2 hr then raised to room temperature overnight. Solvent was removed under reduced pressure and residue was applied for silica gel column chromatography (10 % ethyl acetate in hexane) to afford compound 8n, which was used in the following step without further purification. 8n (130 mg, 84 %): 1H NMR δ 4.20 (m, 2H), 3.93 (t, 2H), 3.20 (d, 2H, J = 6.19 Hz), 2.39 (m, 1H), 1.04 (s, 9H), 1.01 (s, 9H).
(4-{2-[4-(2,2-Di-tert-butyl-[1,3,2]dioxasilinan-5-ylmethoxy)-phenyl]-vinyl}-phenyl)-methyl-amine (4f)
Under the nitrogen atmosphere, compound 4a (90 mg, 0.4 mmol) was dissolved in anhydrous DMF (15.0 mL). Potassium carbonate (560 mg, 4.0 mmol) was added followed by 5-bromomethyl-2,2-di-tert-butyl-[1,3,2]dioxasilinan, 8n (127 mg, 0.4 mmol). The suspension was heated to 100 °C and stirred overnight. After cooling to room temperature, standard workup with dichloromethane was applied and the residue was purified by silica gel preparative TLC (dichloromethane) to afford compound 4f (115 mg, 63 %): 1HNMR δ 7.38 (m, 4H), 6.88 (s, 2H), 6.82 (d, 2H, J = 8.64 Hz), 6.73 (d, 2H, J =8.42), 5.80 (s, 1H), 4.26 (m, 2H), 4.04 (t, 2H), 3.81 (d, 2H, J = 5.82 Hz), 2.89 (s, 3H), 2.58 (m, 1H), 1.06 (s, 9H), 1.04 (s, 9H). Anal. (C27H39NO3Si) C, H, N.
(4-{2-[4-(2,2-Di-tert-butyl-[1,3,2]dioxasilinan-5-ylmethoxy)-phenyl]-vinyl}-phenyl)-methyl-carbamic acid tert-butyl ester (5f)
BOC anhydride (320 mg, 1.46 mmol) was added to a solution of compound 4f (110 mg, 0.24 mmol) in anhydrous THF (10 mL). Under the protection of nitrogen, triethylamine (1.0 mL) was added via a syringe. The solution was then refluxed for 34 hr. After cooling down to room temperature, standard workup with dichloromethane was applied. Organic solvent was removed under reduced pressure and the residue was purified through silica gel column chromatography to afford compound 5f, which was used in the following step without further purification. 5f (122 mg, 91 %): 1HNMR δ 7.42 (d, 4H, J = 7.52 Hz), 7.20 (d, 2H, J = 8.52 Hz), 6.98 (m, 2H), 6.84 (d, 2H, J = 8.72 Hz), 4.26 (m, 2H), 4.05 (t, 2H), 3.82 (d, 2H, J = 5.84 Hz), 3.27 (s, 3H), 2.58 (m, 1H), 1.46 (s, 9H), 1.06 (s, 9H), 1.04 (s, 9H).
(4-{2-[4-(3-Hydroxy-2-hydroxymethyl-propoxy)-phenyl]-vinyl}-phenyl)-methyl-carbamic acid tert-butyl ester (5c)
Compound 5f (120 mg, 0.22 mmol) was dissolved in anhydrous THF (10 mL) and the solution was cooled to 0 °C with an ice bath. Under the nitrogen atmosphere, TBAF (0.44 mL, 1M in THF, 0.44 mmol) was added via a syringe. The solution was stirred at 0 °C for 0.5 hr and then brought to room temperature for another 2 hr. After standard workup with dichloromethane, the residue was applied for silica gel preparative TLC (5 % methanol in dichloromethane) to afford compound 5c (89 mg, 99 %): 1H NMR δ 7.43 (d, 4H, J = 8.68 Hz), 7.20 (d, 2H, J = 8.56 Hz), 6.98 (m, 2H), 6.90 (d, 2H, J= 8.74 Hz), 4.14 (d, 2H, J = 5.96 Hz), 3.95 (d, 4H, J = 5.24 Hz), 3.27 (s, 3H), 2.27(m, 1H), 1.70 (s, 2H), 1.46 (s, 9H). Anal. (C24H31NO5) C, H, N.
[4-(2-{4-[2-Hydroxymethyl-3-(tetrahydro-pyran-2-yloxy)-propoxy]-phenyl}-vinyl)-phenyl]-methyl-carbamic acid tert-butyl ester (5g)
A solution of 5c (53mg, 0.13 mmol) and 3, 4-dihydropyran (12.9 mg, 0.15 mmol) in drydichloromethane (12 ml) containing pyridinium p-toluene sulfonate 26. (3.3 mg, 0.013 mol) was stirred at room temperature for 4 hr. After standard workup with dichloromethane, the residue was applied for silica gel preparative TLC (5 % methanol in dichloromethane) to afford compound 5g, which was used in the following step without further purifications. 5g (43 mg, 67 %): 1H NMR δ 7.43 (d, 4H, J = 8.46 Hz), 7.20 (d, 2H, J = 8.46 Hz), 6.97 (m, 2H), 6.90 (d, 2H, J = 8.62 Hz), 4.60(b, 1H), 3.95 (m, 6H), 3.70 (m, 1H), 3.54 (m, 1H), 3.26 (s, 3H), 2.34 (m, 1H), 1.70 (m, 6H), 1.46 (s, 9h).
Methanesulfonic acid 3-(4-{2-[4-(tert-butoxycarbonyl-methyl-amino)-phenyl]-vinyl}-phenoxy)-2-(tetrahydro-pyran-2-yloxymethyl)-propyl ester(5h)
Triethylamine (0.2 ml) was added to a solution of compound 5g (43 mg, 0.087 mmol) and methanesulfonyl chloride (29.7 mg, 0.26 mmol)in drydichloromethane (10 ml). The solution was stirred at room temperature for 3.5 hr. After standard work up with dichloromethane, the residue was applied for silicagel preparative TLC (2 % methanol in dichloromethane) to afford compound 5h(43 mg, 86 %): 1H NMR δ 7.43 (d, 4H, J = 8.58 Hz), 7.20 (d, 2H, J = 8.46 Hz), 6.97 (m, 2H), 6.90 (d, 2H, J = 8.62 Hz), 4.59(b, 1H), 4.46 (d, 2H, J = 5.66 Hz), 4.11 (m, 2H), 3.85 (m, 2H), 3.55 (m, 2H), 3.27 (s, 3H), 3.00 (s, 3H), 2.59 (m, 1H), 1.70 (m, 6H), 1.46 (s, 9h). HRMS m/Z calcd. For C30H41NO8S (M+Na+): 598.2451. Found: 598.2444.
2-{4-[2-(4-Amino-phenyl)-vinyl]-phenoxymethyl}-propane-1,3-diol (2c)
Compound 2c was prepared from 1b (200 mg, 0.54 mmol) with the same procedure described for 2a and was used in the following step without further purifications. 2c (144 mg, 89 %): 1H NMR (DMSO-d6) δ 7.40 (d, 2H, J = 8.58 Hz), 7.22 (d, 2H, J = 8.30 Hz), 6.91 (m, 4H), 6.54 (d, 2H, J = 8.30 Hz), 5.22 (s, 2H), 4.51 (t, 2H, J = 5.11 Hz), 3.97 (d, 2H, J = 5.85 Hz), 3.51 (t, 4H), 1.96 (m, 1H).
2-{4-[2-(4-Methylamino-phenyl)-vinyl]-phenoxymethyl}-propane-1,3-diol(4c)
Compound 4c was prepared from 2c (100 mg, 0.33 mmol) with the same procedure described for 4a. 4c (104 mg, 99%): 1H NMR (DMSO-d6) δ 7.42 (d, 2H, J = 8.58 Hz), 7.30 (d, 2H, J = 8.46 Hz), 6.88 (m, 4H), 6.52 (d, 2H, J = 8.42Hz), 5.80 (m, 1H), 4.51 (t, 2H), 3.97 (d, 2H, J = 5.85 Hz), 3.51 (t, 4H, J = 5.95 Hz), 2.68 (d, 3H, J = 4.7 Hz), 1.95 (m, 1H). Anal. (C19H23NO3) C, H, N.
2-(4-Bromo-benzyl)-propane-1,3-diol (10)
2-(4-Bromo-benzyl)-malonic acid diethyl ester 9 27 (1.5 g, 3.8 mmol) was dissolved in 5 mL THF and the solution was added slowly to DIBALH (1M in toluene, 25 mL) via a syringe at 0 °C and stirred at the same temperature for 3 hr. HCl (2N, 50 mL) was then added to break the complex. After standard work up with ethyl acetate, crude product 10 (0.9 g, 99 %) was obtained, which was used directly for the next step without further purification: 1H NMR δ 7.39 (d, 2H, J = 8.2 Hz), 7.04 (d, 2H, J = 8.2 Hz), 3.60 (m, 4H), 2.55 (d, 2H, J = 7.4 Hz), 1.96 (m, 1H).
3-(4-Bromo-phenyl)-2-(tert-butyl-dimethyl-silanyloxymethyl)-propan-1-ol>(11)
Under the nitrogen atmosphere, tert-butyl-chloro-dimethyl silane (246 mg, 1.63 mmol) was added to a solution of compound 10 (400 mg, 1.63 mmol) in dichloromethane (10 mL) at 0 °C, followed by triethylamine (412 mg, 4.07 mmol). The solution was gradually warmed to room temperature and stirred overnight. After standard work up with dichloromethane, the residue was purified by silica gel preparative TLC (40 % ethyl acetate in hexane) to afford compound 11 (370 mg, 63.2 %): 1H NMR δ 7.41 (d, 2H, J = 6.6 Hz), 7.08 (d, 2H, J = 6.6 Hz), 3.66 (m, 4 H), 2.60 (m, 2H), 1.94 (m, 1H), 0.91 (s, 9H), 0.06 (s, 6H).
[2-Bromomethyl-3-(4-bromo-phenyl)-propoxy]-tert-butyl-dimethyl-silane(12)
Compound 12 was prepared from 11 (80 mg, 0.22 mmol) with the same procedure described for 8m 24 and was used in the following step without further purifications. 12 (70 mg, 75 %): 1H NMR δ 7.40 (d, 2H, J = 6.6 Hz), 7.07 (d, 2H, J = 6.6 Hz), 3.46 (m, 4H), 2.73 (d, 2H, J = 7.2 Hz), 2.03 (m, 1H), 0.94 (s, 9H), 0.06 (s, 6H).
[4-(2-{4-[2-(4-Bromo-benzyl)-3-(tert-butyl-dimethyl-silanyloxy)-propoxy]-phenyl}-vinyl)-phenyl]-dimethyl-amine (3i)
Compound 3i was prepared from12 (50 mg, 0.12 mmol) and 3a (28 mg, 0.12 mmol) with the same procedure for 3b. This product was used in the following step without further purifications. 3i (40 mg, 59 %). 1H NMR δ 7.40 (m, 6H), 7.09 (d, 2H, J = 8.2 Hz), 6.84 (m, 4H), 6.72 (d, 2H, J = 8.8 Hz), 3.91 (d, 2H, J = 5.4 Hz), 3.67 (m, 2H), 2.99 (s, 6H), 2.75 (d, 2H, J = 7.4 Hz), 2.20 (m, 1H), 0.91 (s, 9H), 0.04 (s, 6H).
2-(4-Bromo-benzyl)-3-{4-[2-(4-dimethylamino-phenyl)-vinyl]-phenoxy}-propan-1-ol (3j)
Compound 3j was prepared from 3i (40 mg, 0.069 mmol) with the same procedure described for 5c. 3j (19 mg, 59 %): 1H NMR δ: 7.40 (m, 4H), 7.07 (d, 2H, J = 8.2 Hz), 6.87 (m, 4H), 6.70 (d, 2H, J = 8.2 Hz), 3.94(m, 2H), 3.75 (b, 2H), 2.97 (s, 6H), 2.76 (d, 2H, J = 7.4 Hz), 2.23 (m, 1H), 1.78 (s, 1H). Anal. (C26H28BrNO2) C, H, N.
[18F]3-{4-[2-(4-Dimethylamino-phenyl)-vinyl]-phenoxy}-2-fluoromethyl-propan-1-ol ([18F]3e)
[18F]Fluoride, produced by a cyclotron using 18O(p,n)18F reaction, was passed through a Sep-Pak Light QMA cartridge as an aqueous solution in [18O]-enriched water. The cartridge was dried by airflow, and the 18F activity was eluted with 2 mL of Kryptofix 222 (K222)/K2CO3 solution (22 mg of K222 and 4.6 mg of K2CO3 in CH3CN/H2O 1.77/0.23). The solvent was removed at 120 °C under an argon stream. The residue was azeotropically dried with 1 mL of anhydrous CH3CN twice at 120°C under an argon stream. A solution of tosylate precursor 3d (4 mg) in DMSO (0.2 mL) was added to the reaction vessel containing the dried 18F activities. The solution was heated at 120°C for 4 min. Water (2 mL) was added, and the mixture was extracted with ethyl acetate (1 mL × 2). The combined organic layer was dried (Na2SO4), and the solvent was removed using an argon stream with gentle heating (55-60°C). The residue was dissolved in CH3CN and injected to HPLC for purification [Hamilton PRP-1 column (7.0 x 305 mm, 10 μm); CH3CN/dimethylglutarate buffer (5 mM, pH 7)=9/1; flow rate = 2mL/min). Retention time of 3e was 11 min and well separated from precursor 3d (rt = 13 min). Same HPLC condition was used for quality control (RCP >99%). Specific activity was estimated by comparing UV peak intensity of purified [18F]3e reference non-radioactive compound of known concentration. Specific activity was estimated to be 70 Ci/mmol after the preparation.
[18F]2-Fluoromethyl-3-{4-[2-(4-methylamino-phenyl)-vinyl]-phenoxy}-propan-1-ol ([18F]4e)
The labeling reaction was carried out as described above for dimethyl amino compound. The mesylate 5h (4 mg) was used as the precursor for the labeling. After the initial reaction at 120°C in DMSO, 1 mL of H2O was added and the solution was cooled down for 1 min. 1 mL of 10% HCl was then added and the mixture was heated at 120°C again for 10 min. Aqueous solution of NaOH was added to adjust the pH to basic. The mixture was extracted with ethyl acetate (1 mL × 2) and the combined organic layer was dried (Na2SO4), and the solvent removed under argon stream with gentle heating (55-60°C). The residue was dissolved in CH3CN and injected to HPLC for purification [Hamilton PRP-1 column (7.0 x 305 mm, 10 μm); CH3CN/dimethylglutarate buffer (5 mM, pH 7)=9/1; flow rate = 2mL/min). Retention time of 4e was 10 min and well separated from precursor 5h (rt = 13 min) as well as the hydrolysis by-product of precursor (rt = 8 min). Same HPLC condition was used for quality control (RCP >99%). Specific activity was estimated by comparing UV peak intensity of purified [18F]4e with reference non-radioactive compound of known concentration. Specific activity was estimated to be 900-1000 Ci/mmol after the preparation.
Preparation of brain tissue homogenates
Postmortem brain tissues were obtained from control and AD patients at autopsy, and neuropathological diagnosis was confirmed by current criteria (NIA-Reagan Institute Consensus Group, 1997). Homogenates were then prepared from dissected gray and white matters from pooled control and AD patients in phosphate buffered saline (PBS, pH 7.4) at the concentration of approximately 100 mg wet tissue/ml (motor-driven glass homogenizer with setting of 6 for 30sec). The homogenates were aliquoted into 1 ml-portions and stored at —70°C for 3-6 month without loss of binding signal.
Binding studies
[125I]IMPY with 2200 Ci/mmole specific activity and greater than 95% radiochemical purity was prepared using the standard iododestannylation reaction,and purified by a simplified C-4 mini column as described previously 18. Binding assays were carried out in 12 × 75 mm borosilicate glass tubes. The reaction mixture contained 50 μl of brain homogenates (20-50 μg), 50 μl of [125I]IMPY (0.04-0.06 nM diluted in PBS) and 50 μl of inhibitors (10-5-10-10 M diluted serially in PBS containing 0.1 % bovine serum albumin, BSA) in a final volume of 1 ml. Nonspecific binding was defined in the presence of IMPY (600 nM) in the same assay tubes. A similar assay condition for [18F]4e was used for binding in a range of concentration between 0.3-0.5 nM. The non-specific binding was defined in the presence of nonradioactive 4e (1,000 nM). The mixture was incubated at 37°C for 2 hr and the bound and the free radioactivity were separated by vacuum filtration through Whatman GF/B filters using a Brandel M-24R cell harvester followed by 2 × 3 ml washes of PBS at room temperature. Filters containing the bound 125I or 18F ligand were assayed for radioactivity content in a gamma counter (Packard 5000) with 70% counting efficiency. Under the assay conditions, the specifically bound fraction was less than 15% of the total radioactivity. The results of inhibition experiments were subjected to nonlinear regression analysis using EBDA by which Ki values were calculated.
Film autoradiography
Brain sections from AD subjects were obtained by freezing the brain in powdered dry ice and cut into 20 micrometer-thick sections. The sections were incubated with [18F]tracers (200,000-250,000 cpm/200 μ1) for 1 hr at room temperature. The sections were then dipped in saturated Li2CO3 in 40% EtOH (two two-minute washes) and washed with 40% EtOH (one two-minute wash) followed by rinsing with water for 30 sec. After drying, the 18F-labeled sections were exposed to Kodak MR film overnight.
In vivo plaque labeling with [18F]3e and [18F]4e
The in vivo evaluation was performed using either double transgenic APP/PS1 or single transgenic APP2576 mice which were kindly provided by AstraZeneca. After anesthetizing with 1% isoflurane, 250-300 μCi of [18F]3e or [18F]4e in 200 μl of 0.1% BSA solution was injected through the tail vein. The animals were allowed to recover for 60-120 min and then killed by decapitation. The brains were immediately removed and frozen in powdered dry ice. Sections of 20 micrometers were cut and exposed to Kodak MR film for overnight.Exvivofilm autoradiograms were thus obtained.
Organ distribution in normal mice
While under isoflurane anesthesia, 0.15 mL of a 0.1% bovine serum albumin solution containing [18F]tracers (5-10 μCi) were injected directly into the tail vein of ICR mice (22-25 g, male) The mice (n = 3 for each time point) were sacrificed by cervical dislocation at 120 min post injection. The organs of interest were removed and weighed, and the radioactivity was assayed for radioactivity content with an automatic gamma counter. The percentage dose per organ was calculated by a comparison of the tissue counts to suitably diluted aliquots of the injected material. Total activities of blood were calculated under the assumption that they were 7% of the total body weight. The % dose/g of samples was calculated by comparing the sample counts with the count of the diluted initial dose.
Partition coefficient
Partition coefficients were measured by mixing the [18F]tracer with 3 g each of 1-octanol and buffer (0.1 M phosphate, pH 7.4) in a test tube. The test tube was vortexed for 3 min at room temperature, followed by centrifugation for 5 min. Two weighed samples (0.5 g each) from the 1-octanol and buffer layers were counted in a well counter. The partition coefficient was determined by calculating the ratio of cpm/g of 1-octanol to that of buffer. Samples from the 1-octanol layer were re-partitioned until consistent partitions of coefficient values were obtained (usually the 3rd or 4th partition). The measurement was done in triplicate and repeated three times.
Supplementary Material
Table 2.
Biodistribution in ICR mice after an iv injection of [18F]tracers in 0.1% BSA (%dose/g, avg of 3 mice ± SD)
| [18F]3e (Partition Coefficient = 1375) | ||||
|---|---|---|---|---|
| Organ | 2 min | 30 min | 1 hr | 2 hr |
| Blood | 1.79 ±0.25 | 1.65 ± 0.15 | 1.51 ±0.20 | 1.18 ±0.08 |
| Heart | 10.43 ±0.96 | 2.76 ±0.26 | 1.45 ±0.17 | 0.91 ±0.04 |
| Muscle | 0.84 ±0.30 | 1.53 ±0.30 | 1.22 ±0.20 | 0.66 ±0.13 |
| Lung | 93.40 ±16.91 | 12.93 ±4.75 | 5.00 ±0.84 | 3.74 ±0.35 |
| Kidney | 13.32 ±1.41 | 4.64 ±0.26 | 2.97 ±0.29 | 2.19 ±0.26 |
| Spleen | 4.43 ±0.26 | 2.45 ±0.22 | 1.24 ±0.11 | 0.99 ±0.14 |
| Liver | 14.13 ±0.73 | 14.65 ±1.73 | 13.46 ±2.62 | 9.51 ±1.04 |
| Skin | 0.67 ±0.02 | 1.69 ±0.38 | 1.65 ±0.15 | 1.00 ±0.07 |
| Brain | 5.55 ±0.64 | 5.21 ±0.66 | 2.97 ±0.43 | 1.37 ±0.14 |
| Bone | 1.57 ±0.12 | 2.06 ±0.35 | 6.50 ±2.92 | 8.63 ±1.78 |
| [18F]4e(Partition Coefficient = 889) | ||||
| Organ | 2 min | 30 min | 1 hr | 2 hr |
| Blood | 2.60 ±0.23 | 2.47 ±0.25 | 1.82 ±0.30 | 0.78 ±0.02 |
| Heart | 7.49 ±0.95 | 2.06 ±0.24 | 1.54 ±0.12 | 0.65 ±0.02 |
| Muscle | 0.74 ±0.11 | 1.31 ±0.45 | 0.85 ±0.41 | 0.41 ±0.04 |
| Lung | 26.13 ±1.91 | 8.90 ±1.39 | 7.51 ±1.01 | 4.15 ±0.61 |
| Kidney | 11.70 ±1.49 | 4.13 ±0.35 | 3.06 ±0.17 | 1.50 ±0.08 |
| Spleen | 5.01 ±1.64 | 2.41 ±0.57 | 1.37 ±1.08 | 1.22 ±0.34 |
| Liver | 20.61 ±2.00 | 19.60 ±1.40 | 14.58 ±1.35 | 8.14 ±2.33 |
| Skin | 0.92 ±0.23 | 1.49 ±0.25 | 1.22 ±0.21 | 0.57 ±0.08 |
| Brain | 9.75 ±1.38 | 1.70 ±0.41 | 0.72 ±0.13 | 0.31 ±0.04 |
| Bone | 1.53 ±0.32 | 2.13 ±0.19 | 5.30 ±1.94 | 7.76 ±0.29 |
Acknowledgements
This work was supported by the grant from the National Institute of Health (AG022559 to H.F.K). APP/PS1 and Tg2576 transgenic mice were kindly provided by AstraZeneca. PIB was kindly provided by Dr. A Verbruggen in Katholieke Universiteit, Leuven.
Footnotes
Supporting Information Available: Elemental analysis data are available for the preparation of all compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’ disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- (2).Ginsberg SD, Schmidt ML, Crino PB, Eberwine JH, Lee VM-Y, Trojanowski JQ. Cerebralcortex: neurodegenerative and age-related changes in structure and function of cerebral cortex. Kluwer Academic/Plenum; New York: 1999. Molecular pathology of Alzheimer‘s disease and related disorders; pp. 603–654. [Google Scholar]
- (3).Selkoe DJ. Imaging Alzheimer’ amyloid. Nature Biotech. 2000;18:823–824. doi: 10.1038/78422. [DOI] [PubMed] [Google Scholar]
- (4).Wolfe MS. Therapeutic strategies for Alzheimer’ disease. Nat Rev Drug Discov. 2002;1:859–866. doi: 10.1038/nrd938. [DOI] [PubMed] [Google Scholar]
- (5).Bachurin SO. Medicinal chemistry approaches for the treatment and prevention of Alzheimer’ disease. Med Res Rev. 2003;23:48–88. doi: 10.1002/med.10026. [DOI] [PubMed] [Google Scholar]
- (6).Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang G.-f., Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Imaging Brain Amyloid in Alzheimer’ Disease with Pittsburgh Compound-B. Ann. Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- (7).Verhoeff NP, Wilson AA, Takeshita S, Trop L, Hussey D, Singh K, Kung HF, Kung M-P, Houle S. In vivo imaging of Alzheimer disease beta-amyloid with [11C]SB-13 PET. Am. J. Geriatr. Psychiatry. 2004;12:584–595. doi: 10.1176/appi.ajgp.12.6.584. [DOI] [PubMed] [Google Scholar]
- (8).Okamura N, Suemoto T, Shimadzu H, Suzuki M, Shiomitsu T, Akatsu H, Yamamoto T, Staufenbiel M, Yanai K, Arai H, Sasaki H, Kudo Y, Sawada T. Styrylbenzoxazole derivatives for in vivo imaging of amyloid plaques in the brain. J. Neurosci. 2004;24:2535–2541. doi: 10.1523/JNEUROSCI.4456-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Shoghi-Jadid K, Small GW, Agdeppa ED, Kepe V, Ercoli LM, Siddarth P, Read S, Satyamurthy N, Petric A, Huang SC, Barrio JR, Liu J, Flores-Torres S, Cole GM. Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with Alzheimer disease: Binding characteristics of radiofluorinated 6-dialkylamino-2-naphthylethylidene derivatives as positron emission tomography imaging probes for beta-amyloid plaques in Alzheimer disease. Am. J. Geriatr. Psychiatry. 2002;10:24–35. [PubMed] [Google Scholar]
- (10).Mathis CA, Wang Y, Klunk WE. Imaging b-amyloid plaques and neurofibrillary tangles in the aging human brain. Curr. Pharm. Des. 2004;10:1469–1492. doi: 10.2174/1381612043384772. [DOI] [PubMed] [Google Scholar]
- (11).Mathis CA, Wang Y, Holt DP, Huang G.-f., Debnath ML, Klunk WE. Synthesis and Evaluation of 11C-Labeled 6-Substituted 2- Arylbenzothiazoles as Amyloid Imaging Agents. J. Med. Chem. 2003;46:2740–2754. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- (12).Wang Y, Klunk WE, Debnath ML, Huang GF, Holt DP, Shao L, Mathis CA. Development of a PET/SPECT agent for amyloid imaging in Alzheimer’ disease. J Mol Neurosci. 2004;24:55–62. doi: 10.1385/JMN:24:1:055. [DOI] [PubMed] [Google Scholar]
- (13).Agdeppa ED, Kepe V, Liu J, Flores-Torres S, Satyamurthy N, Petric A, Cole GM, Small GW, Huang SC, Barrio JR. Binding characteristics of radiofluorinated 6-dialkylamino-2-naphthylethylidene derivatives as positron emission tomography imaging probes for β-amyloid plaques in Alzheimer’ disease. J. Neurosci. 2001;21:RC189. doi: 10.1523/JNEUROSCI.21-24-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Nordberg A. PET imaging of amyloid in Alzheimer’ disease. Lancet Neurol. 2004;3:519–527. doi: 10.1016/S1474-4422(04)00853-1. [DOI] [PubMed] [Google Scholar]
- (15).Ono M, Wilson A, Nobrega J, Westaway D, Verhoeff P, Zhuang Z-P, Kung M-P, Kung HF. C-Labeled Stilbene Derivatives as Aβ-aggregate-specific PET Imaging Agents for Alzheimer’ Disease. Nucl. Med. Biol. 2003;30:565–571. doi: 10.1016/s0969-8051(03)00049-0. [DOI] [PubMed] [Google Scholar]
- (16).Zhuang ZP, Kung MP, Wilson A, Lee CW, Plossl K, Hou C, Holtzman DM, Kung HF. Structure-activity relationship of imidazo[1,2-a]pyridines as ligands for detecting beta-amyloid plaques in the brain. J. Med. Chem. 2003;46:237–243. doi: 10.1021/jm020351j. [DOI] [PubMed] [Google Scholar]
- (17).Kung MP, Hou C, Zhuang Z-P, Zhang B, Skovronsky DM, Gur T, Lee VM-Y, Trojanowski JQ, Kung HF. IMPY: An improved thioflavin-T derivative for in vivo Labeling of β-amyloid plaques. Brain Res. 2002;956:202–210. doi: 10.1016/s0006-8993(02)03436-4. [DOI] [PubMed] [Google Scholar]
- (18).Kung M-P, Hou C, Zhuang Z-P, Cross AJ, Maier DL, Kung HF. Characterization of IMPY as a potential imaging agent for β-amyloid plaques in double transgenic PSAPP mice. Euro. J. Nucl. Med. Mol. Imag. 2004;31:1136–1145. doi: 10.1007/s00259-004-1487-z. [DOI] [PubMed] [Google Scholar]
- (19).Kung M-P, Hou C, Zhuang Z-P, Skovronsky D, Kung HF. Binding of two potential imaging agents targeting amyloid plaques in postmortem brain tissues of patients with Alzheimer’ disease. Brain Res. 2004;1025:89–105. doi: 10.1016/j.brainres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- (20).Cai L, Chin FT, Pike VW, Toyama H, Liow J-S, Zoghbi SS, Modell K, Briard E, Shetty UH, Sinclair K, Donohue S, Tipre D, Kung M-P, Dagostin C, Widdowson DA, Green M, Gao W, Herman MM, Ichise M, Innis RB. Synthesis and Evaluation of Two 18F-Labeled 6-Iodo-2-(4'-N,N-dimethylamino)phenylimidazo[1,2-a]pyridine Derivatives as Prospective Radioligands for β-Amyloid in Alzheimer’ Disease. J. Med. Chem. 2004;47:2208–2218. doi: 10.1021/jm030477w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Lee C-W, Kung M-P, Hou C, Kung HF. Dimethylamino-fluorenes: Ligands for Detecting β-Amyloid Plaques in the Brain. Nucl. Med. Biol. 2003;30:573–580. doi: 10.1016/s0969-8051(03)00050-7. [DOI] [PubMed] [Google Scholar]
- (22).Suemoto T, Okamura N, Shiomitsu T, Suzuki M, Shimadzu H, Akatsu H, Yamamoto T, Kudo Y, Sawada T. In vivo labeling of amyloid with BF-108. Neurosci. Res. (Amsterdam) 2004;48:65–74. doi: 10.1016/j.neures.2003.09.005. [DOI] [PubMed] [Google Scholar]
- (23).Kung HF, Lee C-W, Zhuang ZP, Kung MP, Hou C, Plossl K. Novel stilbenes as probes for amyloid plaques. J. Am. Chem. Soc. 2001;123:12740–12741. doi: 10.1021/ja0167147. [DOI] [PubMed] [Google Scholar]
- (24).Yuan W, Berman RJ, Gelb MH. Synthesis and evaluation of phospholipid analogs as inhibitors of cobra venom phospholipase A2. J. Am. Chem. Soc. 1987;109:8071–8081. [Google Scholar]
- (25).Cox DP, Terpinski J, Lawrynowicz W. “Anhydrous” tetrabutylammonium fluoride: a mild but highly efficient source of nucleophilic fluoride ion. J. Org. Chem. 1984;49:3216–3219. [Google Scholar]
- (26).Miyashita N, Yoshikoshi A, Grieco AP. Pyridinium p-toluenesulfonate. A mild and efficient catalyst for the tetrahydropyranylation of alcohols. J. Org. Chem. 1977;42:3772–3774. [Google Scholar]
- (27).Musso DL, Cochran FR, Kelley JL, McLean EW, Selph JL. Indanylidenes.1. Design and Synthesis of (E)-2-(4,6-Difluoro-1-indanylidene)acetamide, a Potent, Centrally Acting Muscle Relaxant with Antiinflammatory and Analgesic Activity. J. Med. Chem. 2003;46:399–408. doi: 10.1021/jm020067s. [DOI] [PubMed] [Google Scholar]
- (28).McGowan E, Sanders S, Iwatsubo T, Takeuchi A, Saido T, Zehr C, Yu X, Uljon S, Wang R, Mann D, Dickson D, Duff K. Amyloid phenotype characterization of transgenic mice overexpressing both mutant amyloid precursor protein and mutant presenilin 1 transgenes. Neurobiol. Dis. 1999;6:231–244. doi: 10.1006/nbdi.1999.0243. [DOI] [PubMed] [Google Scholar]
- (29).Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer’ disease. J. Neurosci. 2002;22:1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).van Dooren T, Dewachter I, Borghgraef P, van Leuven F. Transgenic mouse models for APP processing and Alzheimer’s disease: early and late defects. Subcell Biochem. 2005;38:45–63. doi: 10.1007/0-387-23226-5_2. [DOI] [PubMed] [Google Scholar]
- (31).Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- (32).Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- (33).Bitan G, Kirkitadze MD, Lomakin A, Vollers SS, Benedek GB, Teplow DB. Amyloid beta -protein (Abeta) assembly: Abeta 40 and Abeta 42 oligomerize through distinct pathways. Proc. Natl. Acad. Sci. U.S.A. 2003;100:330–335. doi: 10.1073/pnas.222681699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Serpell LC, Sunde M, Benson MD, Tennent GA, Pepys MB, Fraser PE. The protofilament substructure of amyloid fibrils. J. Mol. Biol. 2000;300:1033–1039. doi: 10.1006/jmbi.2000.3908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.