Abstract
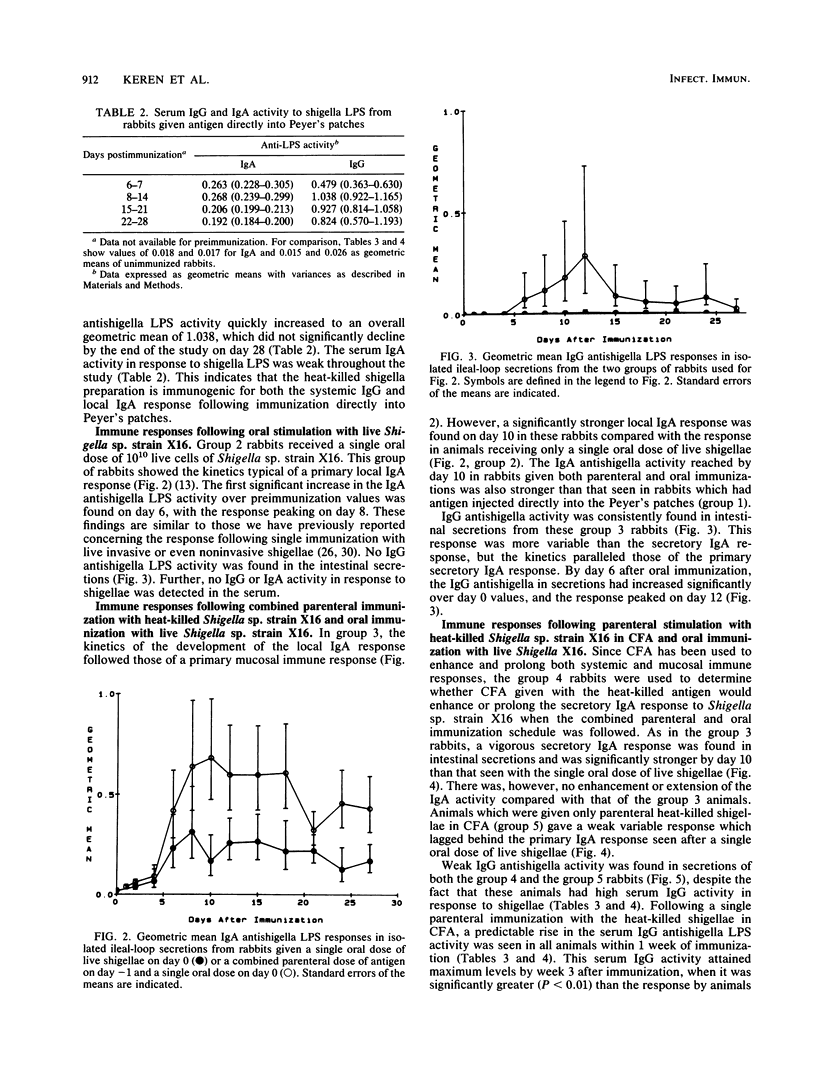
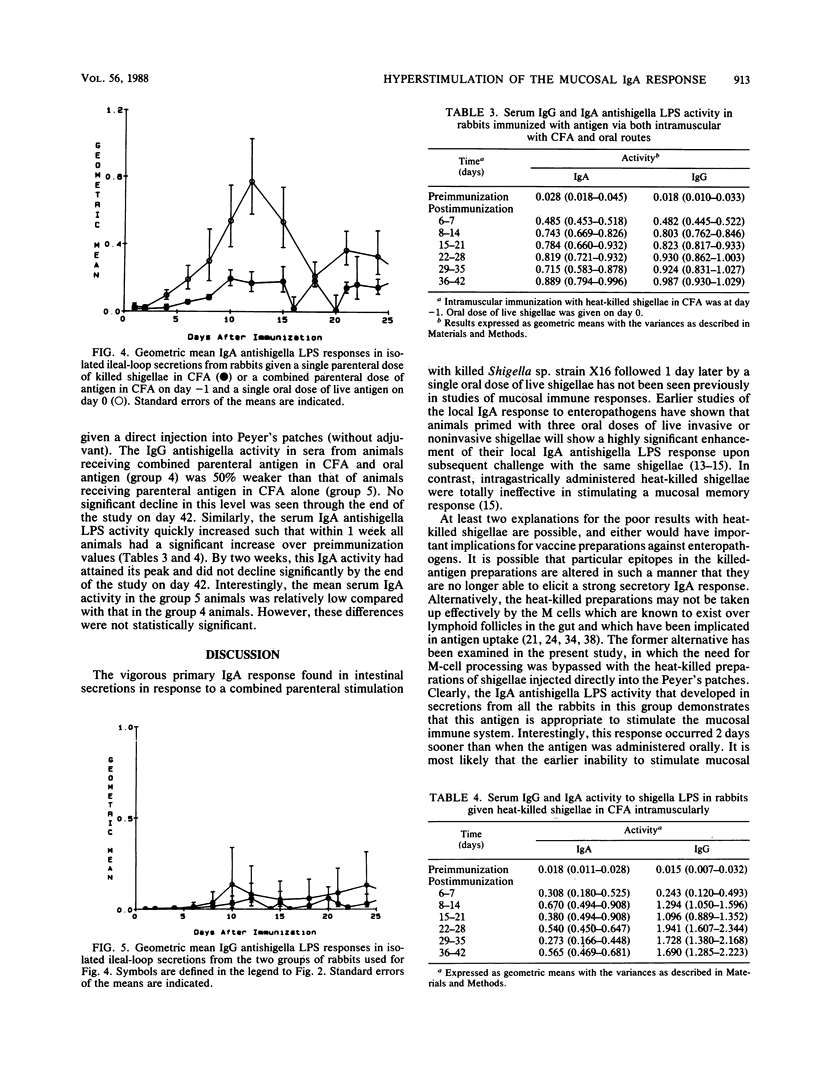
Achieving a vigorous secretory immunoglobulin A (IgA) response in intestinal secretions usually requires multiple doses of antigen given orally, while systemic immunity is more easily attained by parenteral immunization. This study examines the role of combined parenteral and oral immunizations to enhance the early mucosal immune response to an enteropathogen. We have used a chronically isolated intestinal-loop model in rabbits as a probe to monitor kinetically the initial (primary) local immune response to shigella lipopolysaccharide (LPS) following combinations of parenteral immunization intramuscularly (i.m.) and oral stimulation with shigellae. Predictably, effective stimulation of systemic immunity was elicited when heat-killed preparations of Shigella sp. strain X16 were given i.m., as shown by strong serum IgG and weak intestinal IgA activity to shigella LPS. A single oral dose of live Shigella sp. strain X16 given to unprimed rabbits elicited only a typical weak IgA response in intestinal secretions. However, when an i.m. dose of heat-killed shigellae was followed 1 day later by an oral dose of live Shigella sp. strain X16, a hyperstimulation of the early secretory IgA response was elicited, and the response reached levels found previously only after multiple oral administrations of live shigellae. This stimulation did not require the use of an adjuvant. At the same time, the animals receiving this combined oral and i.m. regimen had a lower IgG antishigella LPS activity in serum compared with their response after receiving parenteral antigen in adjuvant alone. These findings indicate that while a dichotomy exists between the systemic and mucosal immune responses, careful orchestration of the stimulatory events can promote a vigorous early local IgA response.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew E., Hall J. G. IgA antibodies in the bile of rats. II. Evidence for immunological memory in secretory immunity. Immunology. 1982 Jan;45(1):177–182. [PMC free article] [PubMed] [Google Scholar]
- Campbell D., Vose B. M. T-cell control of IgA production. I. Distribution, activation conditions and culture of isotype-specific regulatory helper cells. Immunology. 1985 Sep;56(1):81–91. [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., Lyon F. L., Lowe K. L., Farrand A. L., el-Morshidy S. Oral immunization of mice with attenuated Salmonella enteritidis containing a recombinant plasmid which codes for production of the B subunit of heat-labile Escherichia coli enterotoxin. Infect Immun. 1986 Sep;53(3):685–692. doi: 10.1128/iai.53.3.685-692.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORMAL S. B., LABREC E. H., KENT T. H., FALKOW S. ABORTIVE INTESTINAL INFECTION WITH AN ESCHERICHIA COLI-SHIGELLA FLEXNERI HYBRID STRAIN. J Bacteriol. 1965 May;89:1374–1382. doi: 10.1128/jb.89.5.1374-1382.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formal S. B., Hale T. L., Kapfer C., Cogan J. P., Snoy P. J., Chung R., Wingfield M. E., Elisberg B. L., Baron L. S. Oral vaccination of monkeys with an invasive Escherichia coli K-12 hybrid expressing Shigella flexneri 2a somatic antigen. Infect Immun. 1984 Nov;46(2):465–469. doi: 10.1128/iai.46.2.465-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. A., Cebra J. J. Special features of the priming process for a secretory IgA response. B cell priming with cholera toxin. J Exp Med. 1981 Mar 1;153(3):534–544. doi: 10.1084/jem.153.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husband A. J., Dunkley M. L. Lack of site of origin effects on distribution of IgA antibody-containing cells. Immunology. 1985 Feb;54(2):215–221. [PMC free article] [PubMed] [Google Scholar]
- Husband A. J., Lascelles A. K. The origin of antibody in intestinal secretion of sheep. Aust J Exp Biol Med Sci. 1974 Oct;52(5):791–799. doi: 10.1038/icb.1974.78. [DOI] [PubMed] [Google Scholar]
- Kawanishi H., Saltzman L. E., Strober W. Mechanisms regulating IgA class-specific immunoglobulin production in murine gut-associated lymphoid tissues. I. T cells derived from Peyer's patches that switch sIgM B cells to sIgA B cells in vitro. J Exp Med. 1983 Feb 1;157(2):433–450. doi: 10.1084/jem.157.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren D. F., Elliott H. L., Brown G. D., Yardley J. H. Atrophy of villi with hypertrophy and hyperplasia of Paneth cells in isolated (thiry-Vella) ileal loops in rabbits. Light-microscopic studies. Gastroenterology. 1975 Jan;68(1):83–93. [PubMed] [Google Scholar]
- Keren D. F. Enzyme-linked immunosorbent assay for immunoglobulin G and immunoglobulin A antibodies to Shigella flexneri antigens. Infect Immun. 1979 May;24(2):441–448. doi: 10.1128/iai.24.2.441-448.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren D. F., Holt P. S., Collins H. H., Gemski P., Formal S. B. Variables affecting local immune response in ileal loops: role of immunization schedule, bacterial flora, and postsurgical inflammation. Infect Immun. 1980 Jun;28(3):950–956. doi: 10.1128/iai.28.3.950-956.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren D. F., Kern S. E., Bauer D. H., Scott P. J., Porter P. Direct demonstration in intestinal secretions of an IgA memory response to orally administered Shigella flexneri antigens. J Immunol. 1982 Jan;128(1):475–479. [PubMed] [Google Scholar]
- Keren D. F., McDonald R. A., Formal S. B. Secretory immunoglobulin A response following peroral priming and challenge with Shigella flexneri lacking the 140-megadalton virulence plasmid. Infect Immun. 1986 Dec;54(3):920–923. doi: 10.1128/iai.54.3.920-923.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren D. F., McDonald R. A., Scott P. J., Rosner A. M., Strubel E. Effect of antigen form on local immunoglobulin A memory response of intestinal secretions to Shigella flexneri. Infect Immun. 1985 Jan;47(1):123–128. doi: 10.1128/iai.47.1.123-128.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren D. F., Scott P. J., Bauer D. Variables affecting the local immune response in Thiry-Vella loops. II. Stability of antigen-specific IgG and secretory IgA in acute and chronic Thiry-Vella loops. J Immunol. 1980 Jun;124(6):2620–2624. [PubMed] [Google Scholar]
- Keren D. F., Scott P. J., McDonald R. A., Wiatrak M. Effect of parenteral immunization on the local immunoglobulin A response of the intestine to Shigella flexneri antigens. Infect Immun. 1983 Oct;42(1):202–207. doi: 10.1128/iai.42.1.202-207.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyono H., McGhee J. R., Wannemuehler M. J., Frangakis M. V., Spalding D. M., Michalek S. M., Koopman W. J. In vivo immune response to a T-cell-dependent antigen by cultures of disassociated murine Peyer's patch. Proc Natl Acad Sci U S A. 1982 Jan;79(2):596–600. doi: 10.1073/pnas.79.2.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F., Clements J. D. Arousal of mucosal secretory immunoglobulin A antitoxin in rats immunized with Escherichia coli heat-labile enterotoxin. Infect Immun. 1982 Sep;37(3):1086–1092. doi: 10.1128/iai.37.3.1086-1092.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F. Respective contributions to protection of primary and booster immunization with Escherichia coli heat-labile enterotoxin in rats. Infect Immun. 1981 Jan;31(1):252–260. doi: 10.1128/iai.31.1.252-260.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeFevre M. E., Vanderhoff J. W., Laissue J. A., Joel D. D. Accumulation of 2-micron latex particles in mouse Peyer's patches during chronic latex feeding. Experientia. 1978 Jan 15;34(1):120–122. doi: 10.1007/BF01921939. [DOI] [PubMed] [Google Scholar]
- Mattingly J. A., Kaplan J. M., Janeway C. A., Jr Two distinct antigen-specific suppressor factors induced by the oral administration of antigen. J Exp Med. 1980 Sep 1;152(3):545–554. doi: 10.1084/jem.152.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly J. A., Waksman B. H. Immunologic suppression after oral administration of antigen. I. Specific suppressor cells formed in rat Peyer's patches after oral administration of sheep erythrocytes and their systemic migration. J Immunol. 1978 Nov;121(5):1878–1883. [PubMed] [Google Scholar]
- Owen R. L., Pierce N. F., Apple R. T., Cray W. C., Jr M cell transport of Vibrio cholerae from the intestinal lumen into Peyer's patches: a mechanism for antigen sampling and for microbial transepithelial migration. J Infect Dis. 1986 Jun;153(6):1108–1118. doi: 10.1093/infdis/153.6.1108. [DOI] [PubMed] [Google Scholar]
- Owen R. L. Sequential uptake of horseradish peroxidase by lymphoid follicle epithelium of Peyer's patches in the normal unobstructed mouse intestine: an ultrastructural study. Gastroenterology. 1977 Mar;72(3):440–451. [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr, Sacci J. B., Jr, Craig J. P., Germanier R., Fürer E. Procholeragenoid: a safe and effective antigen for oral immunization against experimental cholera. Infect Immun. 1983 Jun;40(3):1112–1118. doi: 10.1128/iai.40.3.1112-1118.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Cray W. C., Jr, Sircar B. K. Induction of a mucosal antitoxin response and its role in immunity to experimental canine cholera. Infect Immun. 1978 Jul;21(1):185–193. doi: 10.1128/iai.21.1.185-193.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F. Induction of optimal mucosal antibody responses: effects of age, immunization route(s), and dosing schedule in rats. Infect Immun. 1984 Jan;43(1):341–346. doi: 10.1128/iai.43.1.341-346.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Kaper J. B., Mekalanos J. J., Cray W. C., Jr, Richardson K. Determinants of the immunogenicity of live virulent and mutant Vibrio cholerae O1 in rabbit intestine. Infect Immun. 1987 Feb;55(2):477–481. doi: 10.1128/iai.55.2.477-481.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Sacci J. B., Jr Enhanced mucosal priming by cholera toxin and procholeragenoid with a lipoidal amine adjuvant (avridine) delivered in liposomes. Infect Immun. 1984 May;44(2):469–473. doi: 10.1128/iai.44.2.469-473.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F. The role of antigen form and function in the primary and secondary intestinal immune responses to cholera toxin and toxoid in rats. J Exp Med. 1978 Jul 1;148(1):195–206. doi: 10.1084/jem.148.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter P., Kenworthy R., Noakes D. E., Allen W. D. Intestinal antibody secretion in the young pig in response to oral immunization with Escherichia coli. Immunology. 1974 Nov;27(5):841–853. [PMC free article] [PubMed] [Google Scholar]
- Richman L. K., Graeff A. S., Yarchoan R., Strober W. Simultaneous induction of antigen-specific IgA helper T cells and IgG suppressor T cells in the murine Peyer's patch after protein feeding. J Immunol. 1981 Jun;126(6):2079–2083. [PubMed] [Google Scholar]
- Rosner A. J., Keren D. F. Demonstration of M cells in the specialized follicle-associated epithelium overlying isolated lymphoid follicles in the gut. J Leukoc Biol. 1984 Apr;35(4):397–404. doi: 10.1002/jlb.35.4.397. [DOI] [PubMed] [Google Scholar]
- Rubin D., Weiner H. L., Fields B. N., Greene M. I. Immunologic tolerance after oral administration of reovirus: requirement for two viral gene products for tolerance induction. J Immunol. 1981 Oct;127(4):1697–1701. [PubMed] [Google Scholar]
- Suzuki I., Kitamura K., Kiyono H., Kurita T., Green D. R., McGhee J. R. Isotype-specific immunoregulation. Evidence for a distinct subset of T contrasuppressor cells for IgA responses in murine Peyer's patches. J Exp Med. 1986 Aug 1;164(2):501–516. doi: 10.1084/jem.164.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsmann D., Klein J. P., Schöller M., Frank R. M. Local and systemic immune response to orally administered liposome-associated soluble S. mutans cell wall antigens. Immunology. 1985 Jan;54(1):189–193. [PMC free article] [PubMed] [Google Scholar]
- Wolf J. L., Dambrauskas R., Sharpe A. H., Trier J. S. Adherence to and penetration of the intestinal epithelium by reovirus type 1 in neonatal mice. Gastroenterology. 1987 Jan;92(1):82–91. doi: 10.1016/0016-5085(87)90842-0. [DOI] [PubMed] [Google Scholar]
- Wolf J. L., Kauffman R. S., Finberg R., Dambrauskas R., Fields B. N., Trier J. S. Determinants of reovirus interaction with the intestinal M cells and absorptive cells of murine intestine. Gastroenterology. 1983 Aug;85(2):291–300. [PubMed] [Google Scholar]
- Yardley J. H., Keren D. F., Hamilton S. R., Brown G. D. Local (immunoglobulin A) immune response by the intestine to cholera toxin and its partial suppression with combined systemic and intra-intestinal immunization. Infect Immun. 1978 Feb;19(2):589–597. doi: 10.1128/iai.19.2.589-597.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



