Summary
Degradation of maternal mRNA is thought to be essential to undergo the maternal-to-embryonic transition. Messenger RNA is extremely stable during oocyte growth in mouse and MSY2, an abundant germ cell-specific RNA-binding protein, likely serves as a mediator of global mRNA stability. Oocyte maturation, however, triggers an abrupt transition in which most mRNAs are significantly degraded. We report that CDC2A (CDK1)-mediated phosphorylation of MSY2 triggers this transition. Injecting Cdc2a mRNA, which activates CDC2A, overcomes milrinone-mediated inhibition of oocyte maturation, induces MSY2 phosphorylation and the maturation-associated degradation of mRNAs. Inhibiting CDC2A following its activation with roscovitine inhibits MSY2 phosphorylation and prevents mRNA degradation. Expressing non-phosphorylatable dominant-negative forms of MSY2 inhibits the maturation-associated decrease in mRNAs, whereas expressing constitutively-active forms induces mRNA degradation in the absence of maturation and phosphorylation of endogenous MSY2. A positive-feedback loop of CDK1-mediated phosphorylation of MSY2 that leads to degradation of Msy2 mRNA that in turn leads to a decrease in MSY2 protein may ensure that the transition is irreversible.
Introduction
Somatic cells usually divide after doubling their volume, whereas oocytes grow in the absence of cell division; in mouse, oocyte volume increases >200-fold during the growth phase (Schultz and Wassarman, 1977). The accumulated organelles and macromolecules constitute the maternal capital that is inherited by the embryo and partitioned to the blastomeres that form during a series of reductive cleavage divisions prior to implantation. Following implantation there is an increase in mass. During the growth phase that takes ~3 weeks, oocyte mRNA is very stable with a half-life of ~12 days (Bachvarova and DeLeon, 1980; Brower et al., 1981). This stability presumably is a major factor that permits mRNA accumulation, especially because a progressive decrease in transcription initiates around mid-growth, the time of antrum formation (Moore, 1975; Moore and Lintern-Moore, 1978), and results in a fully-grown oocyte that is essentially transcriptionally quiescent.
In females, MSY2 (mouse specific Y-box protein 2), which is a germ cell-specific RNA-binding protein, is implicated in mRNA stability (Brower et al., 1981; Yu et al., 2004; Yu et al., 2001; Yu et al., 2002; Yu et al., 2003). MSY2 belongs to a large protein family that is conserved from bacteria to humans (Gu et al., 1998; Jiang et al., 1997; Salvetti et al., 1998; Swamynathan et al., 1998; Tafuri and Wolffe, 1990; Tekur et al., 1999; Thieringer et al., 1997). Y-box proteins typically consist of a highly divergent Nterminus, a conserved cold-shock domain, and a C-terminal tail domain; the cold-shock domain contains ~67–80 amino acids, which are 43% identical from bacteria to humans (Sommerville and Ladomery, 1996; Wolffe, 1994; Wolffe et al., 1992). This domain contains the RNP1 and RNP2 RNA binding motifs (Schindelin et al., 1994)
In oocytes >70% of MSY2 remains in a Triton X-100 insoluble fraction and its RNA-binding activity is required for this association (Yu et al., 2003). The predominant localization of MSY2 in this insoluble fraction may contribute to mRNA stability during the growth phase because MSY2-associated mRNAs may be protected from degradation. Consistent with this hypothesis is that a 60–70% decrease in the amount of MSY2 using a transgenic RNAi approach results in a 20% decrease in the amount of total mRNA (Yu et al., 2004). Moreover, the developmental competence of these transgenic oocytes is compromised resulting in a marked sub-fertility (Yu et al., 2004) and female mice lacking Msy2 are infertile (Yang et al., 2005b). In male germ cells absence of MSY2 due to gene targeting leads to mRNA instability MSY2 (Yang et al., 2007) but MSY2 is soluble following Triton X-100 treatment (Yu et al., 2003).
There is a growing consensus that degradation of maternal mRNA is essential to undergo successfully the maternal-to-embryonic transition (DeRenzo and Seydoux, 2004; Stitzel and Seydoux, 2007). This transition entails transforming a highly differentiated oocyte—the oocyte is the only cell in the female that can undergo meiosis, expresses oocyte-specific genes (e.g., the zona pellucida genes) and whose cytoplasm is capable of reprogramming a transplanted somatic cell nucleus—to totipotent blastomeres by the 2-cell stage. In contrast to the marked stability of mRNA during oocyte growth, oocyte maturation triggers a substantial decrease in mRNA (Schultz, 1993; Su et al., 2007). The molecular basis underlying this dramatic transition in mRNA stability, however, is not known.
MSY2 is phosphorylated during oocyte maturation following germinal vesicle breakdown (GVBD), such that virtually all MSY2 is phosphorylated in the metaphase IIarrested egg (Yu et al., 2001); fertilization triggers the conversion to the dephosphorylated form and by the 2-cell stage MSY2 is essentially absent (Yu et al., 2001). The protein kinase(s) responsible for this phosphorylation—either directly or indirectly—is not known nor is the effect of phosphorylation of MSY2 on its function. We report here that CDC2A mediates, likely directly, MSY2 phosphorylation and that this phosphorylation triggers degradation of endogenous mRNAs.
Materials and Methods
Oocyte collection and culture
Msy2-knock-down (KD) mice have been described (Yu et al., 2004). Unless otherwise stated all experiments were conducted using fully-grown GV-intact oocytes that were harvested from CF1, eCG-primed, 6-week-old females and completely freed of attached cumulus cells via repeated mouth-pipetting. Oocytes were either allowed to mature in vitro in CZB medium (Chatot et al., 1989) or incubated in the presence of 2.5 µM milrinone (Sigma) to inhibit GVBD (Wiersma et al., 1998) for either 6 h or 16–18 h.
Growing meiotically incompetent oocytes were harvested from 13-day-old CF1 female mice (Brower et al., 1981). The small fraction of oocytes that underwent spontaneous GVBD during the first 3 h of incubation was discarded and the remaining oocytes were considered as GVBD-incompetent oocytes. All animal experiments were approved by the Institutional Animal Use and Care Committee and were consistent with National Institutes of Health guidelines.
Protein Kinase Assays
Oocytes were matured in vitro for the different times from 0 to 120 min or overnight, and CDC2A and mitogen-activated protein kinase (MAPK) activities were measured using histone H1 and myelin basic protein (MBP) as substrates, respectively, as previously described (Svoboda et al., 2000).
Triton X-100 treatment, and salt and RNase treatments
The Triton X-100 insoluble fraction was generated as previously described (Yu et al., 2003). Briefly, at least 20 oocytes were treated with 0.1% Triton X-100 containing 100 mM KCl, 5 mM MgCl2, 3 mM EGTA, 20 mM Hepes, pH 6.8, and 1% BSA (intracellular buffer, ICB) for 10 min at room temperature followed by three washes with ICB.
The salt-sensitivity of MSY2 retention in the insoluble preparation was determined by incubating at least 20 oocytes or MII eggs in 0.1% Triton X-100 containing 100 mM to 1000 mM KCl in ICB for 10 min at room temperature. The fraction was then washed three times in ICB. The RNase-sensitivity of MSY2 retention and susceptibility of endogenous mRNA to degradation in the insoluble preparation was determined by subjecting oocytes or MII eggs to RNase treatment at different concentrations (1–100 ng/ml in ICB) for 15 min at room temperature following permeabilization with 0.1% TritonX-100.
To quantify the amount of MSY2, the material was transferred to 2X SDS–PAGE sample buffer prior to immunoblot analysis. To quantify the amount of specific mRNAs, the material was transferred to lysis buffer (Absolutely RNA Microprep Kit, Stratagene, La Jolla, CA) and stored at −80° C prior to Real-Time PCR (qRT-PCR) analysis.
Treatment of Oocytes and MII Eggs with a CDC2A or MAP kinase inhibitor
Oocytes were monitored for GVBD and those that had undergone GVBD within 5 min were rapidly transferred to CZB containing 60 µM roscovitine (Biomol, Plymouth Meeting, PA), a CDC2A inhibitor, or 10 µM U0126 (Biomol), a MAP kinase inhibitor, and cultured without an oil overlay at 37°C in an atmosphere of 5% CO2 in air for 6 h (corresponding to the MI stage) or for 16–18 h when they reached MII stage. MII eggs that matured in vitro in inhibitor-free CZB were incubated with roscovitine or U0126 for 3 h.
DNA Constructs and Site-Directed Mutagenesis
Plasmids pCdc2 (de Vantéry et al., 1997) containing the entire coding region of mouse Cdc2a cDNA and p18.2 (Chapman and Wolgemuth, 1992) containing mouse cyclin B1 (Ccnb1) cDNA were the generous gift of D.J. Wolgemuth (Columbia University). Construct MSY2-pET-28a(+) encoding MSY2 protein bearing a His tag at the amino-terminus has been described (Yu et al., 2003). Construct MSY2IR (hairpin)- pXT7 used in RNAi approach and a construct encoding wild-type Msy2 (MSY2-EGFPpXT7) have been described (Yu et. al, 2004). All Msy2 constructs have an Egfp tag at the carboxyl terminus to monitor protein expression. Threonines at the potential CDK1 phosphorylation sites [(S/T)P] in wild-type Msy2 were mutated to alanine (to produce putative dominant-negative MSY2) or aspartic acid (to produce putative constitutively-active MSY2) by site-directed mutagenesis using the single-stranded Phusion mutagenesis protocol (BioLabs, Ipswich, MA). The following mouse Msy2 mutants were generated: T58A, T67A, T78A, T58/67/78A, T58D, T67D, and T58/67D. All expression constructs were verified by DNA sequencing.
mRNA Preparation and Microinjection
To prepare sense transcripts, pCdc2 was linearized with XbaI and p18.2 was linearized with XhoI as described (de Vantéry et al., 1997). Polyadenylated, capped Cdc2a mRNA was obtained using mMESSAGE mMACHINE Ultra T7 Kit (Ambion, Austin, TX). In vitro transcription of Ccnb1 mRNA was performed using the mMESSAGE mMACHINE T3 Kit and Poly(A) Tailing Kit (Ambion). Constructs MSY2IR (hairpin)-pXT7, MSY2-EGFP-pXT7, and all phosphorylation site-mutated Msy2 constructs were linearized with SacI and in vitro transcription was carried out using the mMESSAGE mMACHINE T7 kit (Ambion). mRNAs from pCdc2 and p18.2 were resuspended at a concentration of 1 µg/µl, and all other mRNAs were resuspended at a concentration of 2 µg/µl in TE buffer, and stored at -80°C prior to use for microinjection.
In the Cdc2a induction experiment, fully-grown oocytes were microinjected with 5–10 pl of Cdc2a mRNA and then incubated in CZB supplemented with 0.7 µM milrinone, which we determined was a minimal concentration required to inhibit GVBD. We selected this minimal concentration in order to maximize the ability of the injected oocytes to overcome the milrinone block of GVBD. Under these conditions we observed that 60–70% of Cdc2a mRNA-injected oocytes underwent GVBD. Oocytes that underwent GVBD within 1 h following injection were then incubated for an additional 6 h or for 16–18 h before they were harvested and stored at −80°C until used for the immunoblot or qRT-PCR analysis. Meiotically-incompetent oocytes were injected with both Cdc2a and Ccnb1 mRNAs (0.5 µg/µl of each) to induce GVBD. The oocytes were incubated in CZB for 18h, and typically all the microinjected meiotically incompetent oocytes underwent GVBD.
For the RNAi experiment, 5–10 pl of Msy2 dsRNA was microinjected to cytoplasm of fully-grown oocytes followed by 18 h incubation in the presence of 2.5 µM milrinone. Oocytes were then harvested and stored at −80°C until used for the immunoblot or qRT-PCR analysis.
When dominant-negative forms of Msy2 were used, oocytes were injected with the appropriate mRNA as described above and then incubated in CZB supplemented with 200 µM IBMX to inhibit maturation for 24 h. The oocytes were divided in two groups in which one group remained in IBMX-containing CZB for another 18 h, whereas oocytes in the other group were washed, transferred to IBMX-free CZB and then cultured for 18 h before they were harvested and stored at −80°C until used for immunoblot or qRT-PCR analysis. When constitutively active forms of Msy2 were used, oocytes were injected with the appropriate mRNA and incubated in CZB supplemented with 200 µM IBMX for 40 h before they were harvested and stored at −80°C until used for the immunoblot or qRT-PCR analysis.
Immunoblotting
Samples were separated in a 10% SDS-PAGE gel and transferred to an Immobilon P membrane (Millipore, Bedford, MA) using semidry transfer. Membranes were processed and developed, and the signal quantified as previously described (Yu et al., 2003). The α-MSY2 anti-body was used in 1:2000 dilution. Detection and quantification of signal was done as previously described (Yu et al., 2003).
qRT-PCR analysis
The mRNA levels of the panel of 20 randomly selected oocyte-expressed genes were quantified using qRT-PCR. Briefly, total RNA from 80 to 100 oocytes or MII eggs was extracted using the Absolutely RNA Microprep Kit (Stratagene, La Jolla, CA) including a DNase step to reduce genomic DNA to undetectable levels. In addition, 230 µM sodium acetate (pH 5.2) and 40 µg glycogen (Roche Applied Science, Indianapolis, IN) as a carrier, were added prior to precipitation with ice-cold ethanol. Equal amounts of human GAPDH mRNA (PE Applied Biosystems, Foster City, CA) were added to each sample before RNA extraction to normalize for RNA extraction and reverse transcription efficiency. First-strand complimentary DNA was synthesized by priming with random hexamers using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA) following the manufacturer's instructions.
One oocyte equivalent of the resulting cDNA was used for each real-time qPCR reaction with a minimum of three replicates (from independently collected oocytes per replicate). qPCR reactions were performed with TaqMan PCR Master reagents and 7700 sequence detector (PE Applied Biosystems, Foster City, CA). The corresponding ABI TaqMan Assay-on-Demand probe/primer sets used were Mm00613618_m1 (Rnf38), Mm00446044_m1 (Xrcc3), Mm00448776_m1 (Smarcb1), Mm02527099_q1 (Hdac1), Mm00656735_q1 (Gapdh), Mm00724124_m1 (AW545966), Mm00457046_m1 (Tbpl1), Mm00516130_m1 (Khdrbs1), Mm00491841_q1 (Rbm8), Mm00488528_m1 (Refbp1), Mm00485509_m1 (Rad51ap1), Mm00456651_m1 (Eif1a), Mm00520817_m1 (Mlh3), Mm00432498_m1 (Chkl). The following custom TaqMan probe/primer sets were designed for
-
Mos (probe 5’CCGAGCCAAACCCTC3’,
forward primer 5’GGGAACAGGTATGTCTGATGCA3’,
reverse primer 5’CACCGTGGTAAGTAAGTGGCTTTATACA3’),
-
MUERV-L (probe 5’CCAGGAAAAGAGCCAAGACCTGCTGAT 3’,
forward primer 5’GGAATGAAGGTATGGGTCAATCC3’,
reverse primer 5’CCTTCACCTTCAGCCAGCAC3’),
-
Tead2 (probe 5’CGTGCGGCAGATCTACGACAAATTCC3’,
forward primer 5’TGGAGCACCACCCCTAGAGAG3’,
reverse primer 5’CCTCGGTCATACAGCTCGCG3’),
-
Msy2 (probe 5’CACCTCCCTCCCTAGTCAGGCTGAGAA 3’,
forward primer 5’CATCCTTATTGTTCCGAGGCA3’,
reverse primer 5’GGAGGTATGAGCTGGCTGGTT3’),
-
Pou5f1 (probe 5’CATGAAAGCCCTGCAGAAGGAGCTAGAAC 3’,
forward primer 5’CAACTCCCGAGGAGTCCCA3’,
reverse primer 5’GCTTCAGCAGCTTGGCAAAC 3’),
-
IAP (probe 5’CAGTTAGACAGGCTCGCCG3’, forward primer
5’TGCTAATTTTACCTTGGTGCAGTTA3’, reverse primer
5’GTTTGCCAGTCAGCAGTTA3’)
.
qRT-PCR amplification of human GAPDH was used as a control for normalization and no signal was observed in the minus RT control when assaying for Rnf38. Quantification was performed using the comparative CT method (ABI PRISM 7700 Sequence Detection System, user bulletin #2). The data were analyzed using Prism software (Graph Pad Software Inc., San Diego, CA).
Statistical analyses
Student’s t-test (paired analysis) was used to determine whether differences were statistically significant.
Results
MSY2 is required for mRNA stability during oocyte growth
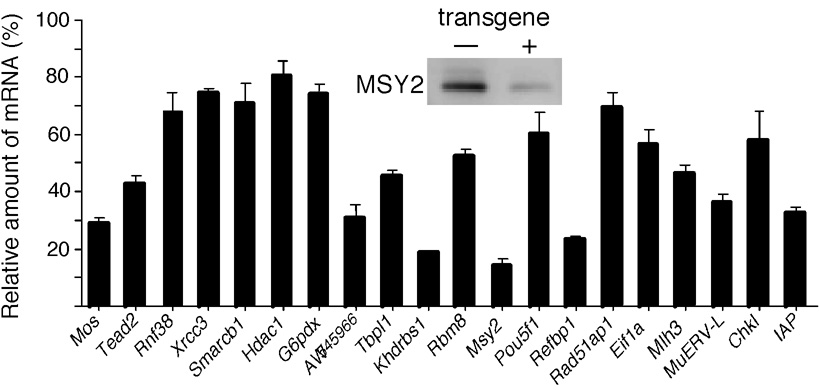
We previously proposed that MSY2 serves as a global regulator of mRNA stability and reported that reducing the amount of MSY2 protein in mouse oocytes by 50–70% using a transgenic RNAi approach results in a 20% decrease in the total amount of poly(A)-containing RNA in fully-grown oocytes (Yu et al., 2004). To assess whether the decrease in MSY2 results in a uniform or transcript-specific decrease in the mRNA population, we assayed by qRT-PCR the relative abundance of a variety of randomly selected transcripts in transgenic oocytes (Fig. 1). In every instance, a decrease in transcript abundance was observed, but the magnitude of the decrease, which ranged from ~20–80%, was variable; the decrease in Msy2 mRNA was due to targeting endogenous Msy2 mRNA by transgenic RNAi. None of the assayed transcripts were those that are unstable in fully-grown oocytes (Puschendorf et al., 2006) and only two transcripts (Rbm8 and Chk1) were previously shown to be selectively degraded during oocyte maturation (Su et al., 2007).
Figure 1.
Relative amounts of specific mRNAs in oocytes containing reduced amounts of MSY2. The relative amount of each transcript was determined by qRT-PCR and expressed relative to that in non-transgenic oocytes. The data are expressed as mean ± SEM, n=3. The insert shows an immunoblot demonstrating the reduced amount of MSY2 protein in transgenic oocytes. All of the differences are significant (p <0.001 except for Smarcb1 (p <0.01) and Hdac1 (p <0.05).
CDC2A initiates MSY2 phosphorylation
We previously demonstrated that MSY2 is phosphorylated following GVBD (Yu et al., 2001). During maturation there is a dramatic increase in CDC2A activity that occurs concomitant with GVBD followed by activation of MAPK within an hour (Verlhac et al., 1994). Each of these kinases phosphorylates a S/T residue that is followed by a P residue and MSY2 contains three such potential phosphorylation sites. The Scansite algorithm (scansite.mit.edu) identifies T58, T67, and T78 as potential CDC2A phosphorylation sites using a low stringency filter; only T58 is identified when using a medium stringency filter. Examination of the time course of MSY2 phosphorylation and activation of CDC2A and MAPK revealed that phosphorylation, detected by the appearance of species of slower electrophoretic mobility, correlated with CDC2A activation and not MAPK activation (Fig. 2A); the multiple electrophoretic forms likely represent MSY2 that is phosphorylated at more than one site. Consistent with CDC2A, and not MAPK, triggering either direct phosphorylation of MSY2 (or indirectly by activating another protein kinase) is (1) the MEK inhibitor U0126, which ultimately inhibits MAPK (Phillips et al., 2002), did not prevent the maturation associated phosphorylation of MSY2, (2) treatment of oocytes that just underwent GVBD with the CDC2A inhibitor roscovitine (Phillips et al., 2002) blocked MSY2 phosphorylation, and (3) treatment of metaphase II-arrested eggs with roscovitine resulted in the conversion of the phosphorylated to the non-phosphorylated form (Fig. 2B). In addition, dephosphorylation of MSY2 that occurs following egg activation correlates with the decrease in CDC2A activity (Yu et al., 2001), which occurs prior to the decrease in MAPK activity (Moos et al., 1995).
Figure 2.
(A) Time course of MSY2 phosphorylation, and CDC2A and MAPK activation. The upper panel is an immunoblot of MSY2 showing the appearance of forms of slower electrophoretic mobility becoming more apparent following GVBD. The times from transfer to inhibitor-free medium are indicated above each lane and GVBD occurs between 60 and 75 min following transfer to inhibitor-free medium. The middle panel is CDC2A activity as assessed by histone H1 phosphorylation and the lower panel is MAPK activity as assessed by myelin basic protein phosphorylation. Duplicate samples for each time point are shown. (B) Immunoblots demonstrating effect of roscovitine and U0126 on MSY2 phosphorylation. GV, GV-intact oocyte; GVBD/R, roscovitine was added immediately after GVBD and the samples prepared 3 h later; MII, MII egg; MII/R, roscotivine added to MII eggs and the samples prepared 3 h later; GVBD/U, U0126 added immediately after GVBD and the samples prepared 3 h later; MII/U, U0126 added to MII eggs and the samples prepared 3 h later; MII, MII egg.
MSY2 phosphorylation correlates with changes in solubility and accessibility of endogenous mRNAs to exogenous RNase
The bulk of MSY2 is insoluble following Triton X-100 extraction, but RNase treatment of these preparations liberates MSY2 to the soluble fraction (Yu et al., 2003). Treating Triton X-100 preparations of GV-intact oocytes with increasing concentrations of KCl or RNase resulted in a concentration-dependent release from the insoluble fraction (Fig. 3A and Table 1). Significantly lower concentrations of KCl or RNase were required to liberate phosphorylated MSY2 present in MII eggs.
Figure 3.
Effect of MSY2 phosphorylation on MSY2 and mRNA solubility. (A) Effect of salt and RNase treatment on retention of MSY2 in the Triton X-100 insoluble fraction. Triton X- 100 insoluble fraction was prepared from oocytes and eggs and treated with increasing concentrations of KCl (left panel) for 10 min prior or RNase (right panel) for 15 min prior to preparing the samples for immunoblot analysis. The experiment was performed 3 times and shown is a representative immunoblot. (B) The relative amount of MSY2 present in GV-intact oocytes and MII eggs. Immunoblot analysis for MSY2 was performed on total oocyte and egg extracts and on the Triton X-100 insoluble material. Lane 1, Total amount of MSY2 in GV-intact oocyte; lane 2, amount of MSY2 in Triton X-100 insoluble material; lane 3, total amount of MSY2 in MII egg; lane 4, amount of MSY2 in Triton X-100 insoluble material in MII egg. Note that the majority of MSY2 remains associated with the Triton X-100 insoluble material. The histogram shows a decrease in the amount of MSY2 in eggs relative to that in GV-intact oocytes (first bar), whereas the second and third bars depict the amount of MSY2 present in the insoluble fraction relative to the amount present in untreated oocytes and MII eggs. The experiment was performed 3 times and the data are expressed as the mean ± SEM. The difference between GV insoluble/total and MII insoluble/total is significant (p<0.05, Student’s t-test). (C) Relative amount of different mRNAs present in the Triton X-100 insoluble fraction derived from oocytes (solid bars) and eggs (open bars). The experiment was performed three times and the data are are expressed as the mean ± SEM. The differences between GV and MII are significant (p <0.05) except for Hdac1, G6pdx, Khdrbs1, Rbm8, Rad51ap1, Mlh3, and MuERV-L.
Table 1.
Quantification of amount of MSY2 present in insoluble fraction following either KCl or RNase treatment
| Treatment | ||||||
|---|---|---|---|---|---|---|
| KCl [M] | RNase [ng/µl] | |||||
| 0.2 | 0.4 | 0.6 | 1 | 10 | 100 | |
| GV | 80±6 | 51±10 | 17±3 | 84±6 | 60±10 | 10±1 |
| MII | 46±10 | 33±8 | 4±1 | 43±8 | 17±2 | 3±1 |
The data are relative to extracted but untreated oocytes or MII eggs and are expressed as mean±SEM (n=3).
Note that extracted but untreated cells are exposed to 0.1 M KCl, which is present in the extraction buffer.
Consistent with the change in solubility properties of MSY2 associated with phosphorylation, the fraction of MSY2 in the insoluble fraction in MII-arrested eggs is less than the fraction in GV-intact oocytes (Fig. 3B). In addition, the bulk of mRNAs (typically >80%) present in GV-intact oocytes were associated with the Triton X-100 insoluble material but following maturation and arrest at MII, the fraction of mRNA present in this insoluble material was less (Fig. 3C). Taken together, these results suggest that MSY2 phosphorylation leads to a coordinate movement from the insoluble to soluble fraction of MSY2 and associated mRNA. Furthermore, these results suggest that MSY2 phosphorylation leads to changes in its ability to interact directly with mRNA or other proteins present in MSY2-containing ribonucleoprotein particles (RNPs). Such biochemical changes could make mRNAs present in MSY2-containing particles more susceptible to the degradation machinery, either directly or by being released into the soluble pool and hence made more accessible.
We had previously demonstrated that RNase treatment (50 µg/ml) of TX-100 insoluble material present in oocytes resulted in total degradation of most mRNAs and release of virtually all of the MSY2 to the soluble fraction (Yu et al., 2003). Using conditions for RNase treatment (1 ng/ml) that resulted in little release of MSY2 from the insoluble fraction (Fig 3A), we then assayed a battery of randomly selected transcripts present in the insoluble fractions in GV-intact and MII eggs and found that the transcripts were more readily digested in eggs, where MSY2 is phosphorylated, than in oocytes, where the bulk of MSY2 is not phosphorylated (Fig. 4). These results suggest the MSY2 phosphorylation leads to mRNAs present in MSY2-containing RNPs being more susceptible to RNase.
Figure 4.
Effect of exogenous RNase on mRNA degradation in Triton X-100 preparations made from oocytes (solid bars) or MII eggs (open bars). The samples were incubated with RNase (1 ng/ml) for 15 min at room temperature and then processed for qRT-PCR. The data are expressed relative to that in untreated samples. The experiment was performed 3 times and the data are expressed as the mean ± SEM. All differences are significant (p<0.01).
MSY2 phosphorylation correlates with mRNA degradation during oocyte maturation
Maturation initiates degradation of maternal mRNAs that persists into the 2-cell stage, such that for many mRNAs greater than 90% of the maternal transcript is degraded by this time (Schultz, 1993). Transcripts that are common to the oocyte and embryo, e.g., actin, are replaced by zygotic expression that is clearly evident by the 2-cell stage (Latham et al., 1991; Hamatani et al., 2004; Wang et al., 2004; Zeng et al., 2004; Zeng and Schultz, 2005). We monitored degradation of the selected transcripts by measuring the relative amount of each transcript at MI and MII compared to that in the GV-intact oocyte (Fig. 5A). These experiments demonstrated that each mRNA was degraded during maturation, but the kinetics and relative stability varied for each mRNA. For example, some mRNAs show little degradation by MI (Rnf38), whereas others exhibit significant degradation by this time (Eif1a). Most mRNAs displayed a further decrease between MI and MII such that by MII the relative amount of the transcript ranged between ~80% (Mos) to ~10% (Chk1) of that present in the GV-intact oocyte. Of note is that repetitive sequences, e.g., IAP, seemed most susceptible to degradation, which is consistent with a previous study that noted such sequences were very unstable in oocytes (Puschendorf et al., 2006).
Figure 5.
Maturation-associated decrease in degradation of specific mRNAs. (A) qRT-PCR analysis of relative abundance of randomly selected transcripts expressed in oocytes at MI (solid bars) and MII (open bars) relative to the amount present in GV-intact oocytes. The experiment was performed three times and the data are expressed as the mean ± SEM. For MI, all differences are significant (p<0.05, Student’s t-test) except for Mos, Tead2, and Rnf38, which are not. For MII, all differences are significant (p<0.05). (B) Relative abundance of mRNAs in milrinone-treated oocytes injected with Cdc2a mRNA. qRT-PCR analysis was conducted at times that correspond to MI (solid bars) and MII (open bars) and the data are expressed relative to the amount present in GV-intact oocytes; top panel control and bottom panel Cdc2a mRNA-injected oocytes. The experiment was performed three times and the data are expressed as the mean ± SEM. All differences in the control and experimental MI and MII groups are significant (p<0.05) except for Rnf38 in MI and MII eggs, which is not. The insert is an immunoblot of MSY2 in GV-intact oocytes (—) and oocytes injected with Cdc2a mRNA (+) that underwent GVBD and demonstrates that MSY2 is phosphorylated in these oocytes. (C) Continuous phosphorylation of MSY2 is required for degradation of most mRNAs. Roscovitine was added shortly after GVBD and relative amounts of the different mRNA were determined at times that corresponded to MI (solid bars) and MII (open bars); top panel control and bottom panel roscovitine-treated oocytes. The experiment was performed three times and the data are expressed as the mean ± SEM. All differences in the control MI and MII groups are significant (p<0.05, Student’s t-test) except for Rnf38, which is not for MI. For the roscotivine-treated group, the only differences that are significant are for Rnf38 in MII and Rad51ap1 in both MI and MII. Under these conditions, MSY2 returns to the form of greatest electrophoretic mobility within 60 min following roscovitine addition (Insert).
To test the hypothesis that CDC2A could serve as the primary trigger to induce mRNA degradation, Cdc2a mRNA was injected into oocytes inhibited from undergoing spontaneous maturation in vitro by the PDE3 inhibitor milrinone. Such injected oocytes underwent GVBD as previously described (de Vantéry et al., 1997) and the extent of both MSY2 phosphorylation and mRNA degradation was similar to that observed during the course of normal maturation (Fig. 5B). Likewise, induction of GVBD in meiotically incompetent oocytes required injection of both Cdc2a and Ccnb1 mRNA as previously described (de Vantéry et al., 1997) and induced MSY2 phosphorylation and mRNA degradation (data not shown).
To determine if continuous phosphorylation of MSY2 was required for mRNA degradation, we established conditions in which roscovitine was added immediately after GVBD, at which time MSY2 becomes phosphorylated, and the time course for dephosphorylation. These experiments demonstrated that 60 µM roscovitine resulted in conversion of phosphorylated MSY2 to the non-phosphorylated form within 60 min (Fig. 5C insert). Examining degradation of a selected sub-set of mRNAs in these treated oocytes revealed that the extent of degradation observed at times that corresponded to MI and MII was less than that which occurred during maturation (Fig. 5C). In addition, when oocytes were matured to MII, mRNA degradation continued in the MII-arrested eggs that were cultured for an additional 6 h (data not shown); virtually all of the MSY2 is phosphorylated in MII-arrested eggs.
Dominant-negative forms of MSY2 inhibit and constitutively-active forms promote degradation of maternal mRNAs
The tight correlation between MSY2 phosphorylation and mRNA degradation suggests MSY2 phosphorylation triggers the transition from mRNA stability to mRNA instability, i.e., mRNA degradation. If a causal relationship exists, non-phosphorylatable forms of MSY2 should inhibit the maturation-associated decrease in maternal mRNAs, whereas constitutively active forms should lead to mRNA degradation in the absence of maturation and CDC2A activation.
Prior to undertaking these studies, we first needed to identify the phosphorylated residues in MSY2. The limited amounts of material that can be isolated, however, precluded using mass spectroscopy to identify these sites. To circumvent this problem we focused on the three potential phosphorylation sites T58, 67, and 78 and used in vitro mutagenesis to convert these T residues to A residues. The mRNA to be injected was a MSY2 fused in frame to GFP. This permitted us to resolve and quantify the exogenously expressed MSY2 from endogenous MSY2 using our affinity-purified MSY2 antibody. Moreover, we could ascertain whether the mutated forms underwent an electrophoretic shift diagnostic for phosphorylation.
As anticipated the non-mutated form displayed an electrophoretic shift but the triple mutant did not (Fig. 6A). The T78A form exhibited a shift similar to that for WT, whereas the T58A form displayed a smaller shift, suggesting that both T58 and T67 are phosphorylated. The T67A form, however, didn’t manifest any apparent shift. Although this could suggest that T67 phosphorylation is prerequisite for T58 phosphorylation, as described below, a putative constitutively-active form T58D didn’t reveal an electrophoretic shift whereas the T67D form did (Fig. 6C). Thus, T58 phosphorylation may not lead to an electrophoretic shift.
Figure 6.
Effect of putative dominant-negative and constitutively active forms of MSY2 on degradation of maternal mRNAs. For dominant-negative forms, oocytes were injected with the indicated Msy2-Egfp chimeric mRNAs and following incubation to allow expression, a fraction of the sample was removed for both immunoblot and qRT-PCR analyses. The remainder of the sample was matured in vitro and following maturation both immunoblotting and qRT-PCR analyses were performed. (A) Immunoblot analysis. Shown are the regions containing the EGFP-MSY2 fusion protein and endogeous MSY2. (B) qRT-PCR analysis in which the amount of mRNA in MII eggs is expressed relative to that in the GV-intact oocyte. Data are expressed as mean ± SEM. a, P<0.05; b, P<0.01; c, P<0.001. In both cases, the experiment was performed three times and similar results were obtained in each case; shown is a representative example for the immunoblotting analysis. For constitutively active forms, oocytes were injected with the indicated Egfp-chimeric mRNAs and following injection, a fraction of the sample was removed for qRT-PCR analyses. The remainder of the sample cultured in vitro under conditions that inhibit maturation and following incubation both immunoblotting and qRT-PCR analyses were performed. (C) Immunoblot analysis. Shown are the regions containing the EGFP-MSY2 fusion protein and endogeous MSY2. (D) qRT-PCR analysis in which the amount of transcript in oocytes that had been microinjected is expressed relative to that in un-injected oocytes. Data are expressed as mean ± SEM. a, P<0.05; b, P<0.01; c, P<0.001.In both cases, the experiment was performed three times and similar results were obtained in each case; shown is a representative example for the immunoblotting analysis.
Expression of the exogenous MSY2 dominant-negative forms expanded the endogenous pool by ~26% as assessed by quantifying the immunoblots. It should be noted that MSY2 is an abundant protein in oocytes, constituting ~2% of total protein (Yu et al., 2001). Thus, a modest expansion of the endogenous pool of MSY2 was expected and consequently, any inhibitory effects of the putative dominant-negative forms on mRNA degradation would not be anticipated to be dramatic. In addition, the magnitude of any effect would depend on the ability of the exogenously expressed MSY2 to be incorporated into MSY2-containing RNPs. Consistent with MSY2 phosphorylation leading to a transition from mRNA stability to instability is the statistically significant effect of the mutant forms, and in particular the T67A form, to inhibit the maturationassociated decrease in maternal mRNAs relative to oocytes expressing the non-mutated form (Fig. 6B). The effects of the individual mutant forms can be compared to each other with a fair degree of confidence because the overall level of expression for each form was similar.
As anticipated, expressing putative constitutively active forms of MSY2, in which the T residue was mutated to a D residue, in oocytes inhibited from resuming meiosis by incubating them in the presence of IBMX resulted in mRNA degradation (Fig. 6D). Again, because the endogenous MSY2 pool was only moderately expanded (~42%) by expressing the putative constitutively active forms of MSY2 (Fig. 6C), the stimulatory effect was expected to be modest as was observed. Moreover, the T67D form seemed more effective, consistent with the T67A form often being a more potent inhibitor of maturation-associated mRNA degradation. Last, expressing constitutively active forms of MSY2 in oocytes also resulted in an increased susceptibility of endogenous mRNAs to exogenous RNase treatment (Fig. 7).
Figure 7.
Effect of putative constitutively active forms of MSY2 on susceptibility of endogenous mRNAs to exogenously added RNase. Following injection and incubation under conditions that inhibit maturation, the samples were extracted with Triton X-100 and either treated or not treated with RNase, and qRT-PCR was then performed. The data are expressed as the amount of transcript in RNase-treated preparations relative to that in untreated ones. The experiment was performed three times and the data are expressed as mean ± SEM. a, P<0.05; b, P<0.01.
When taken together, results of these experiments provide strong evidence that MSY2 phosphorylation triggers the transition from mRNA stability to instability that occurs shortly after GVBD.
The amount of MSY2 protein decreases during oocyte maturation
The amount of MSY2 decreases about 30% during maturation (Fig. 3B). This decrease could be a consequence of the maturation-associated decrease in Msy2 mRNA (Fig. 5A). To test this hypothesis, oocytes were injected with Msy2 dsRNA and incubated in medium containing milrinone to inhibit maturation. After 18 h in culture, the same amount of time used to determine the amount of MSY2 following maturation in vitro, the amount of MSY2 present relative to the amount in uninjected oocytes was determined by immunoblotting. In addition the relative amount of Msy2 mRNA was quantified by qRT- PCR. Results of these experiments, in which MSY2 was not phosphorylated, indicated that 57% ± 2% (mean ± SEM, n=3) of the MSY2 protein and 21% ±3% (mean ± SEM, n=3) of the Msy2 mRNA remained when compared to controls, i.e., there was ~40% and 80% reduction in the amount of protein and mRNA, respectively. Thus, the maturation-associated decrease in MSY2 protein is likely due to the maturation-associated decrease in Msy2 mRNA. This decrease is proportionate to the 20–30% decrease in the amount of MSY2 protein and ~50% decrease in Msy2 mRNA that occurs during maturation.
Discussion
Results described here provide the first evidence that phosphorylation of MSY2 constitutes a molecular switch in which non-phosphorylated MSY2 stabilizes maternal mRNAs whereas phosphorylation of MSY2, as a consequence of CDC2A activation, triggers the maturation-associated degradation of maternal mRNAs. Because MSY2 is a germ cell-specific RNA-binding protein, MSY2 is ideally suited to serve as such a switch to promote degradation of maternal mRNAs. If an RNA-binding protein that is expressed in both oocytes and somatic cells served as the switch, i.e., exhibited the proposed change in function following CDC2A-mediated phosphorylation, most somatic mRNAs would likely become susceptible to degradation during M-phase of each cell cycle. In fact, most mRNAs appear to become more stable during M-phase (Ross, 1997), which may represent the outcome of selective pressures to ensure that the daughter cells inherit an essentially full complement of mRNAs. It should be noted that mouse oocytes also express MSY4 (Davies et al., 2000); a role for MSY4, if any, in the transition from maternal mRNA stability to instability remains unknown.
Based on the time-course for MSY2 phosphorylation and the ability of roscovitine, but not U0126, to inhibit that phosphorylation, the results of experiments described here suggest that CDC2A triggers phosphorylation of MSY2, which contains consensus CDC2A phosphorylation sites. There are three consensus CDC2A phosphorylation sites—recombinant CDC2A-CCNB1 stoichiometically converts MSY2 to the phosphorylated form in vitro (Medvedev, unpublished observations)—and it is interesting that early in the course of maturation we detect more than one form of slower electrophoretic mobility. CDC2A would be an ideal candidate to initiate MSY2 phosphorylation, because it would ensure that nuclear and cytoplasmic maturation are coordinated, and thereby maximize the likelihood of producing an egg capable of being fertilized and supporting development to term. We cannot exclude, however, that MSY2 is phosphorylated by a protein kinase that is activated by CDC2A. For example, the Xenopus ortholog of MSY2, FRGY2 is phosphorylated by casein kinase II (Deschamps et al., 1997), but there is no evidence that CKII is activated by CDC2A during the course of oocyte maturation. In mouse male germ cells, a kinase that co-immunoprecipitates with MSY2 can phosphorylate MSY2 in vitro, but the kinase is not CKII (Herbert and Hecht, 1999).
The Scansite algorithm identifies an AKT phosphorylation site on S139 of MSY using a low stringency filter. Recently, AKT was implicated in phosphorylating the Ybox protein, YB-1 (Evdokimova et al., 2006). Although AKT is activated during oocyte maturation, its activation precedes CDC2A activation. Moreover, its activation is transient and virtually no activity is detected shortly after GVBD (Kalous et al., 2006). These findings minimize the likelihood that AKT is the responsible kinase for MSY2 phosphorylation. Of interest, however, is that YB-1 preferentially interacts with the 5’ cap and displaces the cap-binding protein eIF-4A that is essential for translation (Evdokimova et al., 2006). In addition, AKT-mediated phosphorylation of YB-1 on S102, e.g., in response to IGF1 stimulation, decreases the ability of YB-1 to interact with the 5’ cap and displace eIF-4A; the Scansite algorithm detects this site using a low stringency filter. Thus, AKT-mediated phosphorylation of YB-1 could promote relief of translational repression of mRNAs in YB-1 containing RNPs. It is tempting to speculate that MSY2 phosphorylation leads to similar changes that could ultimately lead to decreased mRNA stability that is coupled to translation.
We noted a tight correlation between MSY2 phosphorylation and mRNA degradation and that mRNA degradation required continuous phosphorylation of MSY2. The spectrum of differences in the temporal pattern of degradation of specific mRNAs likely reflects another level of regulation beyond a function for MSY2. Thus, if MSY2 confers mRNA stability and phosphorylation relieves that protection, the stability of each mRNA may then be dictated by combination of regulatory sequences in that mRNA and the protein composition of the MSY2-containing RNP. At present, we have no information regarding the composition of MSY2-containing RNPs in mouse oocytes.
Following the transition from mRNA stability to instability, the machinery responsible for degradation of maternal mRNAs has not been described in mouse. In zebrafish a single miRNA (Mir-430) that is expressed when the embryonic genome is activated and responsible for targeting degrading of several hundred maternal mRNAs (Giraldez et al., 2006). In mouse the bulk of maternal mRNAs appear degraded prior to genome activation. Of those maternal mRNAs degraded during maturation, only a small fraction contained seed sequences for miRNAs (Murchison et al., 2007) suggesting that miRNAs do not constitute the major pathway for clearing maternal mRNAs. Rather a large fraction of the degraded maternal mRNAs were enriched in AU-rich elements and sequences derived from repetitive elements. The presence of repetitive element-derived sequences could target these mRNAs by an RNAi pathway using siRNAs derived from repetitive sequences. In Drosophila the CCR4/POP2/NOT deadenylase catalyzes the first steps in degradation of maternal mRNAs (Semotok et al., 2005). The role of this deadenylase, or that of either decapping enzyme DCP2 or the 5’-exonuclease XRN1 (Eulalio et al., 2007), in degradation of maternal mouse mRNAs is not known.
In GV-intact oocytes >80% of the mRNAs analyzed are present in insoluble material following Triton X-100 extraction. This finding could account for the observed mRNA stability during oocyte growth, because mRNAs present in this compartment may not be translated and hence less susceptible to degradation. In addition, these mRNAs may also be shielded from the RNA degradation machinery. Messenger RNAs present in the soluble fraction would provide the source of transcripts that support protein synthesis during oocyte growth; these transcripts would be in a dynamic equilibrium with their counterparts in the insoluble fraction. Phosphorylation changes the biochemical properties of MSY2, e.g., it is released from the insoluble fraction at lower salt concentrations than non-phosphorylated MSY2. Phosphorylation-induced changes in MSY2 could also underlie the dramatic increase in susceptibility of endogenous mRNAs to exogenous RNase in MII eggs, suggesting that phosphorylation of MSY2 increases accessibility of mRNAs present in RNPs to enzymes involved in mRNA degradation.
The most compelling case for a direct linkage that MSY2 phosphorylation triggers the transition from mRNA stability to instability is that expressing putative dominant-negative forms of MSY2 inhibit the maturation-associated decrease in endogenous mRNAs, whereas expressing putative constitutively-active forms results in a decrease in these mRNAs in the absence of maturation. Consistent with the increased susceptibility of endogenous mRNAs in MII eggs, in which MSY2 is phosphorylated, to exogenous RNase is the observation that expressing putative constitutively-active forms of MSY2 in oocytes also increases the susceptibility of endogenous mRNAs to exogenous RNase. This finding is consistent with the increased sensitivity of endogenous mRNAs to degradation by exogenously added RNase in MII eggs in which the bulk of MSY2 is phosphorylated when compared to their GV-intact counterparts.
Selex analysis reveals that FRGY2 exhibits preferential binding for AACAUC sequence (Bouvet et al., 1995) and a similar sequence has been determined for MSY2 (Hecht, unpublished results). In male germ cells MSY2 preferentially associated with a population of mRNAs (Yang et al., 2005a). MSY2 can also serve as a transcription factor, and ChIP assays revealed that most mRNAs that preferentially bind MSY2 are transcribed from genes that contain a Y-box DNA-binding in their promoters (Yang et al., 2005a). Whether MSY2 preferentially binds to a sub-population of mRNAs in oocytes is not known. Analysis of microarray data of transcript abundance of oocytespecific mRNAs during oocyte maturation and early embryogenesis reveals waves of degradation of these mRNAs with some being degraded by MII, and others being degraded following fertilization or after the 2-cell stage (P. Svoboda, personnel communication). How these mRNAs evade being degraded during maturation is not known (nor what promotes their degradation at later times); it will be of great interest to ascertain whether MSY2 is bound to them or not.
We propose that MSY2 is a major factor responsible for mRNA stability during oocyte growth and that its phosphorylation, which initiates at the onset of oocyte maturation and is mediated by CDC2A, results in a transition from a state of mRNA stability to mRNA instability. Maturation is also associated with a decrease in the amount of MSY2 protein, with the decrease in Msy2 mRNA likely contributing to this decrease. This finding suggests a positive-feedback loop in which MSY2 phosphorylation leads to degradation of Msy2 mRNA (among many mRNAs) that in turn leads to a decrease in MSY2 protein, the net effect being a further decrease in global mRNA stability that is triggered by CDC2A-mediated phosphorylation of MSY2. The ability to inhibit mRNA degradation by over-expressing dominant-negative MSY2 permits an experimental approach to assess the requirement for degradation of maternal mRNAs in the maternal-to-zygotic transition in mouse. Whether a similar role for MSY2 and its phosphorylation in the transition from mRNA stability to instability exists in other mammals remains to be determined.
Acknowledgements
This research was supported by grants from the NIH (HD044449 to NBH and RMS, and HD22681 to RMS). We thank Junying Yu for conducting the experiments that resulted in the data shown in Fig. 2A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bachvarova R, DeLeon V. Polyadenylated RNA of mouse ova and loss of maternal RNA in early development. Dev.Biol. 1980;74:1–8. doi: 10.1016/0012-1606(80)90048-2. [DOI] [PubMed] [Google Scholar]
- Bouvet P, Matsumoto K, Wolffe AP. Sequence-specific RNA recognition by the Xenopus Y-box proteins. An essential role for the cold shock domain. J. Biol. Chem. 1995;270:28297–28303. doi: 10.1074/jbc.270.47.28297. [DOI] [PubMed] [Google Scholar]
- Brower PT, Gizang E, Boreen SM, Schultz RM. Biochemical studies of mammalian oogenesis: synthesis and stability of various classes of RNA during growth of the mouse oocyte in vitro. Dev. Biol. 1981;86:373–383. doi: 10.1016/0012-1606(81)90195-0. [DOI] [PubMed] [Google Scholar]
- Chapman DL, Wolgemuth DJ. Identification of a mouse B-type cyclin which exhibits developmentally regulated expression in the germ line. Mol. Reprod. Dev. 1992;33:259–269. doi: 10.1002/mrd.1080330305. [DOI] [PubMed] [Google Scholar]
- Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J. Reprod. Fertil. 1989;86:679–688. doi: 10.1530/jrf.0.0860679. [DOI] [PubMed] [Google Scholar]
- Davies HG, Giorgini F, Fajardo MA, Braun RE. A sequence-specific RNA binding complex expressed in murine germ cells contains MSY2 and MSY4. Dev. Biol. 2000;221:87–100. doi: 10.1006/dbio.2000.9658. [DOI] [PubMed] [Google Scholar]
- de Vantéry C, Stutz A, Vassalli JD, Schorderet-Slatkine S. Acquisition of meiotic competence in growing mouse oocytes is controlled at both translational and posttranslational levels. Dev.Biol. 1997;187:43–54. doi: 10.1006/dbio.1997.8599. [DOI] [PubMed] [Google Scholar]
- DeRenzo C, Seydoux G. A clean start: degradation of maternal proteins at the oocyte-to-embryo transition. Trends Cell Biol. 2004;14:420–426. doi: 10.1016/j.tcb.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Deschamps S, Jacquemin-Sablon H, Triqueneaux G, Mulner-Lorillon O, Potier M, Le Caer JP, Dautry F, le Maire M. mRNP3 and mRNP4 are phosphorylatable by casein kinase II in Xenopus oocytes, but phosphorylation does not modify RNA-binding affinity. FEBS Lett. 1997;412:495–500. doi: 10.1016/s0014-5793(97)00833-8. [DOI] [PubMed] [Google Scholar]
- Eulalio A, Behm-Ansmant I, Izaurralde E. P bodies: at the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell Biol. 2007;8:9–22. doi: 10.1038/nrm2080. [DOI] [PubMed] [Google Scholar]
- Evdokimova V, Ruzanov P, Anglesio MS, Sorokin AV, Ovchinnikov LP, Buckley J, Triche TJ, Sonenberg N, Sorensen PH. Akt-mediated YB-1 phosphorylation activates translation of silent mRNA species. Mol. Cell. Biol. 2006;26:277–292. doi: 10.1128/MCB.26.1.277-292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldez AJ, Mishima Y, Rihel J, Grocock RJ, Van Dongen S, Inoue K, Enright AJ, Schier AF. Zebrafish MiR-430 Promotes Deadenylation and Clearance of Maternal mRNAs. Science. 2006;312:75–79. doi: 10.1126/science.1122689. [DOI] [PubMed] [Google Scholar]
- Gu W, Tekur S, Reinbold R, Eppig JJ, Choi YC, Zheng JZ, Murray MT, Hecht NB. Mammalian male and female germ cells express a germ cell-specific Y-box protein, MSY2. Biol. Reprod. 1998;59:1266–1274. doi: 10.1095/biolreprod59.5.1266. [DOI] [PubMed] [Google Scholar]
- Hamatani T, Carter MG, Sharov AA, Ko MS. Dynamics of global gene expression changes during mouse preimplantation development. Dev. Cell. 2004;6:117–131. doi: 10.1016/s1534-5807(03)00373-3. [DOI] [PubMed] [Google Scholar]
- Herbert TP, Hecht NB. The mouse Y-box protein, MSY2, is associated with a kinase on non-polysomal mouse testicular mRNAs. Nucleic Acids Res. 1999;27:1747–1753. doi: 10.1093/nar/27.7.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Hou Y, Inouye M. CspA, the major cold-shock protein of Escherichia coli, is an RNA chaperone. J. Biol. Chem. 1997;272:196–202. doi: 10.1074/jbc.272.1.196. [DOI] [PubMed] [Google Scholar]
- Kalous J, Solc P, Baran V, Kubelka M, Schultz RM, Motlik J. PKB/AKT is involved in resumption of meiosis in mouse oocytes. Biol. Cell. 2006;98:111–123. doi: 10.1042/BC20050020. [DOI] [PubMed] [Google Scholar]
- Latham KE, Garrels JI, Chang C, Solter D. Quantitative analysis of protein synthesis in mouse embryos. I. Extensive reprogramming at the one- and two-cell stages. Development. 1991;112:921–932. doi: 10.1242/dev.112.4.921. [DOI] [PubMed] [Google Scholar]
- Moore GPM. The RNA polymerase activity of the preimplantation mouse embryo. J.Embryol.exp.Morph. 1975;34:291–298. [PubMed] [Google Scholar]
- Moore GPM, Lintern-Moore S. Transcription of the mouse oocyte genome. Biol.Reprod. 1978;17:865–870. doi: 10.1095/biolreprod18.5.865. [DOI] [PubMed] [Google Scholar]
- Moos J, Visconti PE, Moore GD, Schultz RM, Kopf GS. Potential role of mitogen-activated protein kinase in pronuclear envelope assembly and disassembly following fertilization of mouse eggs. Biol. Reprod. 1995;53:692–699. doi: 10.1095/biolreprod53.3.692. [DOI] [PubMed] [Google Scholar]
- Murchison EP, Stein P, Xuan Z, Pan H, Zhang MQ, Schultz RM, Hannon GJ. Critical roles for Dicer in the female germline. Genes Dev. 2007;21:682–693. doi: 10.1101/gad.1521307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KP, Petrunewich MA, Collins JL, Booth RA, Liu XJ, Baltz JM. Inhibition of MEK or cdc2 kinase parthenogenetically activates mouse eggs and yields the same phenotypes as Mos(−/−) parthenogenotes. Dev. Biol. 2002;247:210–223. doi: 10.1006/dbio.2002.0680. [DOI] [PubMed] [Google Scholar]
- Puschendorf M, Stein P, Oakeley EJ, Schultz RM, Peters AH, Svoboda P. Abundant transcripts from retrotransposons are unstable in fully grown mouse oocytes. Biochem. Biophys. Res. Commun. 2006;347:36–43. doi: 10.1016/j.bbrc.2006.06.106. [DOI] [PubMed] [Google Scholar]
- Ross J. A hypothesis to explain why translation inhibitors stabilize mRNAs in mammalian cells: mRNA stability and mitosis. BioEssays. 1997;19:527–529. doi: 10.1002/bies.950190612. [DOI] [PubMed] [Google Scholar]
- Salvetti A, Batistoni R, Deri P, Rossi L, Sommerville J. Expression of DjY1, a protein containing a cold shock domain and RG repeat motifs, is targeted to sites of regeneration in planarians. Dev. Biol. 1998;201:217–229. doi: 10.1006/dbio.1998.8996. [DOI] [PubMed] [Google Scholar]
- Schindelin H, Jiang W, Inouye M, Heinemann U. Crystal structure of CspA, the major cold shock protein of Escherichia coli. Proc. Natl. Acad. Sci. USA. 1994;91:5119–5123. doi: 10.1073/pnas.91.11.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RM. Regulation of zygotic gene activation in the mouse. BioEssays. 1993;15:531–538. doi: 10.1002/bies.950150806. [DOI] [PubMed] [Google Scholar]
- Schultz RM, Wassarman PM. Biochemical studies of mammalian oogenesis: Protein synthesis during oocyte growth and meiotic maturation. J. Cell Sci. 1977;24:167–194. doi: 10.1242/jcs.24.1.167. [DOI] [PubMed] [Google Scholar]
- Semotok JL, Cooperstock RL, Pinder BD, Vari HK, Lipshitz HD, Smibert CA. Smaug recruits the CCR4/POP2/NOT deadenylase complex to trigger maternal transcript localization in the early Drosophila embryo. Curr. Biol. 2005;15:284–294. doi: 10.1016/j.cub.2005.01.048. [DOI] [PubMed] [Google Scholar]
- Sommerville J, Ladomery M. Masking of mRNA by Y-box proteins. Faseb J. 1996;10:435–443. doi: 10.1096/fasebj.10.4.8647342. [DOI] [PubMed] [Google Scholar]
- Stitzel ML, Seydoux G. Regulation of the oocyte-to-zygote transition. Science. 2007;316:407–408. doi: 10.1126/science.1138236. [DOI] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Woo Y, Wigglesworth K, Kamdar S, Affourtit J, Eppig JJ. Selective degradation of transcripts during meiotic maturation of mouse oocytes. Dev. Biol. 2007;302:104–117. doi: 10.1016/j.ydbio.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda P, Stein P, Hayashi H, Schultz RM. Selective reduction of dormant maternal mRNAs in mouse oocytes by RNA interference. Development. 2000;127:4147–4156. doi: 10.1242/dev.127.19.4147. [DOI] [PubMed] [Google Scholar]
- Swamynathan SK, Nambiar A, Guntaka RV. Role of single-stranded DNA regions and Y-box proteins in transcriptional regulation of viral and cellular genes. Faseb J. 1998;12:515–522. doi: 10.1096/fasebj.12.7.515. [DOI] [PubMed] [Google Scholar]
- Tafuri SR, Wolffe AP. Xenopus Y-box transcription factors: molecular cloning, functional analysis and developmental regulation. Proc. Natl. Acad. Sci. USA. 1990;87:9028–9032. doi: 10.1073/pnas.87.22.9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekur S, Pawlak A, Guellaen G, Hecht NB. Contrin, the human homologue of a germ-cell Y-box-binding protein: cloning, expression, and chromosomal localization. J. Androl. 1999;20:135–144. [PubMed] [Google Scholar]
- Thieringer HA, Singh K, Trivedi H, Inouye M. Identification and developmental characterization of a novel Y-box protein from Drosophila melanogaster. Nucleic Acids Res. 1997;25:4764–4770. doi: 10.1093/nar/25.23.4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlhac MH, Kubiak JZ, Clarke HJ, Maro B. Microtubule and chromatin behavior follow MAP kinase activity but not MPF activity during meiosis in mouse oocytes. Development. 1994;120:1017–1025. doi: 10.1242/dev.120.4.1017. [DOI] [PubMed] [Google Scholar]
- Wang QT, Piotrowska K, Ciemerych MA, Milenkovic L, Scott MP, Davis RW, Zernicka-Goetz M. A genome-wide study of gene activity reveals developmental signaling pathways in the preimplantation mouse embryo. Dev. Cell. 2004;6:133–144. doi: 10.1016/s1534-5807(03)00404-0. [DOI] [PubMed] [Google Scholar]
- Wiersma A, Hirsch B, Tsafriri A, Hanssen RG, Van de Kant M, Kloosterboer HJ, Conti M, Hsueh AJ. Phosphodiesterase 3 inhibitors suppress oocyte maturation and consequent pregnancy without affecting ovulation and cyclicity in rodents. J. Clin. Invest. 1998;102:532–537. doi: 10.1172/JCI2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe AP. Structural and functional properties of the evolutionarily ancient Y-box family of nucleic acid binding proteins. Bioessays. 1994;16:245–251. doi: 10.1002/bies.950160407. [DOI] [PubMed] [Google Scholar]
- Wolffe AP, Tafuri S, Ranjan M, Familari M. The Y-box factors: a family of nucleic acid binding proteins conserved from Escherichia coli to man. New Biol. 1992;4:290–298. [PubMed] [Google Scholar]
- Yang J, Medvedev S, Reddi PP, Schultz RM, Hecht NB. The DNA/RNA-binding protein MSY2 marks specific transcripts for cytoplasmic storage in mouse male germ cells. Proc. Natl. Acad. Sci. USA. 2005a;102:1513–1518. doi: 10.1073/pnas.0404685102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Medvedev S, Yu J, Tang LC, Agno JE, Matzuk MM, Schultz RM, Hecht NB. Absence of the DNA-/RNA-binding protein MSY2 results in male and female infertility. Proc. Natl. Acad. Sci. USA. 2005b;102:5755–5760. doi: 10.1073/pnas.0408718102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Deng M, Medvedev S, Yang J, Hecht NB, Schultz RM. Transgenic RNAi-mediated reduction of MSY2 in mouse oocytes results in reduced fertility. Dev. Biol. 2004;268:195–206. doi: 10.1016/j.ydbio.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Yu J, Hecht NB, Schultz RM. Expression of MSY2 in mouse oocytes and preimplantation embryos. Biol. Reprod. 2001;65:1260–1270. doi: 10.1095/biolreprod65.4.1260. [DOI] [PubMed] [Google Scholar]
- Yu J, Hecht NB, Schultz RM. RNA-binding properties and translation repression in vitro by germ cell-specific MSY2 protein. Biol. Reprod. 2002;67:1093–1098. doi: 10.1095/biolreprod67.4.1093. [DOI] [PubMed] [Google Scholar]
- Yu J, Hecht NB, Schultz RM. Requirement for RNA-binding activity of MSY2 for cytoplasmic localization and retention in mouse oocytes. Dev. Biol. 2003;255:249–262. doi: 10.1016/s0012-1606(02)00094-5. [DOI] [PubMed] [Google Scholar]
- Zeng F, Baldwin DA, Schultz RM. Transcript profiling during preimplantation mouse development. Dev. Biol. 2004;272:483–496. doi: 10.1016/j.ydbio.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Zeng F, Schultz RM. RNA transcript profiling during zygotic gene activation in the preimplantation mouse embryo. Dev. Biol. 2005;283:40–57. doi: 10.1016/j.ydbio.2005.03.038. [DOI] [PubMed] [Google Scholar]