Abstract
Background
Cryptococcal meningitis (CM) is the proximate cause of death in 20–30% of persons with AIDS in Africa.
Methods
Two prospective observational cohorts enrolled HIV-infected, antiretroviral-naïve persons with CM in Kampala, Uganda; the first in 2001–02 (n=92) prior to HAART availability; the second in 2006–07 (n=44) with HAART available.
Results
Ugandans presented with prolonged CM symptoms (median 14, IQR: 7 to 21 days). The 14-day survival was 49% (45/92) in 2001–02 and 80% (35/44) in 2006 (P<.001). HAART was started 35 ± 13 days from CM diagnosis and does not explain the improved 14-day survival in 2006. In 2006–07, survival continued to decline after hospitalization with only 57% (25/44) alive before initiating HAART. Probable cryptococcal-related immune reconstitution inflammatory syndrome (IRIS) occurred in 42% (10/24) with 4 deaths. At 6 months after CM diagnosis, 18 persons (41%) were alive and on HAART in 2007.
The median CSF opening pressure was 330mm H2O with 81% having elevated pressure (≥200mm). Only five patients consented to therapeutic lumbar punctures. Pressures >250mm trended towards higher mortality (Odds Ratio (OR)= 2.1; 95% CI: 0.9 to 5.2; P=.09). Initial CSF WBC <5 cells/mL was associated with failure of CSF sterilization (OR=17.3; 95% CI: 3.1 to 94.3; P<.001), and protein level <35mg/dL was associated with higher mortality (OR=2.0; 95% CI: 1.2 to 3.3; P=.007).
Conclusions
Significant CM mortality persists despite amphotericin and HIV therapy due to high mortality prior to HAART and to IRIS-related complications after HAART initiation. Approaches to increase acceptance of therapeutic lumbar punctures are needed.
MeSH Keywords: HIV, AIDS, Cryptococcosis, Cryptococcal Meningitis, Amphotericin B, Sub-Saharan Africa, immune reconstitution inflammatory syndrome
INTRODUCTION
Cryptococcal meningitis (CM) is the most common fatal central nervous system infection in patients with AIDS in Sub-Saharan Africa [1], where patients often present with advanced HIV disease, and CM is often the initial AIDS-defining illness [2]. The rate of cryptococcal infection in Uganda (40.4/1000 person-years) is double that reported in HIV-patients in North America (17–20/1000 person-years) prior to highly active anti-retroviral therapy (HAART) and exponentially higher than during the HAART era (1.5/1000 person-years) [3,4,5]. In two cohorts from Uganda, 20–30% of those with advanced HIV died from CM with a median survival of 26 days [4,6]. Even though antiretroviral access is rapidly expanding in Africa, management of opportunistic infections (OIs) remains a major challenge of HIV/AIDS care.
In North America, the cryptococcal mortality rate is <10% with the combination of amphotericin B given 0.7–1.0 mg/kg/day, flucytosine (5-FC), and aggressive management of raised intracranial pressure (ICP) [7]. Mortality at 14 days for HIV-associated CM in Sub-Saharan Africa following amphotericin ranges from 17% to 36% with the median survival of approximately one month [8,9,10,11,12]. Risk factors for mortality include delay in diagnosis, lack of amphotericin, lack of HAART, and greater fungal burdens [11]. A prospective study in Thailand using amphotericin 0.7mg/kg/day reported a 14 day CM mortality of 16% [13]. In clinical practice in Africa, fluconazole alone is routinely used due to affordability and ease of use [14,15,16]. With fluconazole and without HAART, the survival of HIV-associated CM in an African setting at 6 months is dismal with <5% surviving [14,15,16,17,18].
We conducted two prospective observational studies, before and after HAART availability, to determine the clinical presentation and mortality rate of CM among HIV-infected adults receiving amphotericin.
METHODS
Study Design
Two prospective, observational studies of HIV-infected adults diagnosed with CM were conducted in Kampala, Uganda at Mulago Hospital, the national tertiary referral and teaching hospital. The first observational period was from November 2001 through March 2002, prior to the availability of HAART. All patients presenting with headache, neck stiffness, photophobia, fever, and/or mental status change were screened for study eligibility in the emergency ward. Patients were enrolled if they were ≥18 years of age, HIV seropositive by ELISA, not receiving HAART, and had qualitative CSF culture with C. neoformans. Exclusion criteria were: hemoglobin <5.0 g/dL, creatinine >3.0 mg/dL, or being comatose and thus unable to consent.
The second study period was July 2006 through December 2006, after the availability of HAART in Uganda. Screening and inclusion criteria were identical, except CM status was considered positive if two of three of the following CSF tests were positive: India ink, cryptococcal antigen (CRAG), or C. neoformans quantitative culture.
Written informed consent was obtained from each subject. Study protocols were approved by the University of Minnesota, Makerere University, and Ugandan National committees.
Treatment
Amphotericin B deoxycholate was given for 14 consecutive days at a dose of 0.7 mg/kg/day. Intravenous fluid of 1L of normal saline (NS) daily was recommended in 2001 and provided in 2006. Renal function was monitored in 2001 at baseline, day 7, and day 14 versus baseline then three times weekly in 2006. Those developing acute renal dysfunction (creatinine >3.0 mg/dL) received: ≥2 L NS daily, alternate day amphotericin, and daily creatinine measurements. To manage elevated ICP (>200 mm H2O), CSF was drained until the pressure was reduced by 50% and/or pressure was <200 mm H2O, and then daily LPs were recommended; however subjects often refused subsequent therapeutic LPs [19]. Upon hospital discharge, patients received consolidation therapy with fluconazole 400mg/day for eight weeks at the Infectious Diseases Institute (IDI) clinic. Thereafter, maintenance therapy with fluconazole 200mg/day for secondary prophylaxis was given indefinitely.
In 2006, patients were seen in the IDI clinic within one week of hospital discharge for HAART counseling and began HAART 1–2 weeks thereafter. Initial HAART regimens were zidovudine (AZT), lamivudine (3TC), and efavirenz; or stavudine (D4T), 3TC, and nevirapine.
Data Collection
Medical history, physical examination, neurological assessment, and lumbar puncture were performed, and CSF opening pressure (up to 560 mm H2O) was measured. CSF was analyzed for protein, glucose, cell count with differential, Indian ink stain, Gram’s stain, Ziehl-Neelsen stain for mycobacteria, CSF CRAG by latex agglutination titer (Murex Diagnostics, Norcross, GA), and C. neoformans culture on Sabouraud dextrose agar. Quantitative CSF cultures were performed in 2006 using a calibrated loop with 10μL of CSF supernatant cultured on Sabouraud and Chocolate agars. Visible colonies were counted on each agar plate by two different technicians with the average recorded. CSF was re-cultured on day 14. Study personnel visited subjects daily and repeated neurologic and laboratory evaluations on days 7 and 14.
After initiation of HAART, patients were evaluated for evidence of immune reconstitution inflammatory syndrome (IRIS) defined as an atypical or exaggerated infectious or inflammatory condition temporally related to the initiation of HAART whereby the symptoms cannot be explained by: an alternate infection, malignancy, treatment failure of the OI, adverse drug reaction, or complete antiretroviral non-compliance. When CM-IRIS was suspected, subjects had LPs performed with India ink, qualitative CRAG testing, and repeat quantitative cultures to exclude CM relapse. The common triad for CM-IRIS is 1) treated CM, 2) recently started HAART, and 3) new aseptic meningitis.
Statistical Analysis
Data were analyzed using SPSS 15.0 (Chicago, IL). The log rank test identified categorical factors associated with risk of death. Logistic regression was used to assess the risk of microbiologic treatment failure and death during the first 2 weeks of amphotericin therapy. Normally distributed continuous variables are described by mean ±SD and compared by t-test. Non-normally distributed variables are described by median and interquartile range (IQR) and compared by the Mann-Whitney U. Categorical differences between time periods are compared by Chi-square, and survival analysis by Cox-proportional hazards regression. Statistical significance is P<.05.
RESULTS
Baseline characteristics
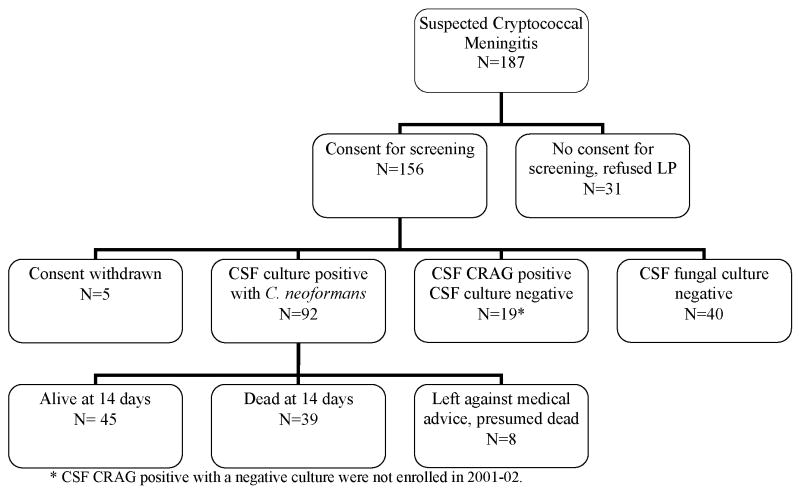
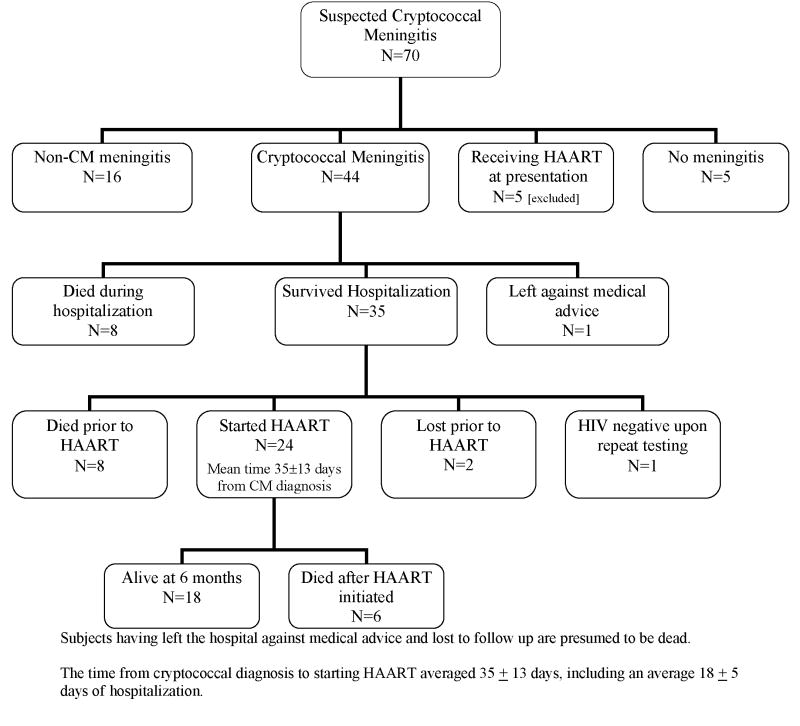
Among the 226 persons screened with symptoms and signs suggestive of meningitis, 136 persons had CM (92 in 2001–02, 44 in 2006, (Figure 1). The median antecedent headache duration was 14 days (IQR: 7 to 21 days), and 38% presented with altered mental status. CSF cryptococcal cultures were positive in 89% (122/136) with a mean colony count of 5×104 colonies/mL (range 5×102 – 7.5 ×105), and of these, the CSF CRAG and India ink stain were positive in 100% and 97%, respectively. The median CSF CRAG titer was 1:2048 (IQR 1:640 to 1:4096). The serum CRAG was positive in 97% (57/59). The mean CSF opening pressure was 350 ±144 mm H2O (median 330, IQR: 240 to 476) with opening pressures greater than the maximum measurable (≥560 mm H2O) present in 20%. Papilledema, present in 37%, was associated with increasing opening pressure. Each 100 mm increase in CSF pressure increased the odds of papilledema (OR=2.3; 95% CI: 1.7 to 3.2; P<.001). Comparing 2001 to 2006, there were no significant differences in patient demographics, duration of illness, clinical symptoms, and CSF parameters including opening pressure.
Figure 1.
Figure 1a. Study Profile Pre-HAART Era, 2001–2002.
Figure 1b. Study Profile in the HAART Era, 2006.
Outcomes
Pre-HAART Era (2001)
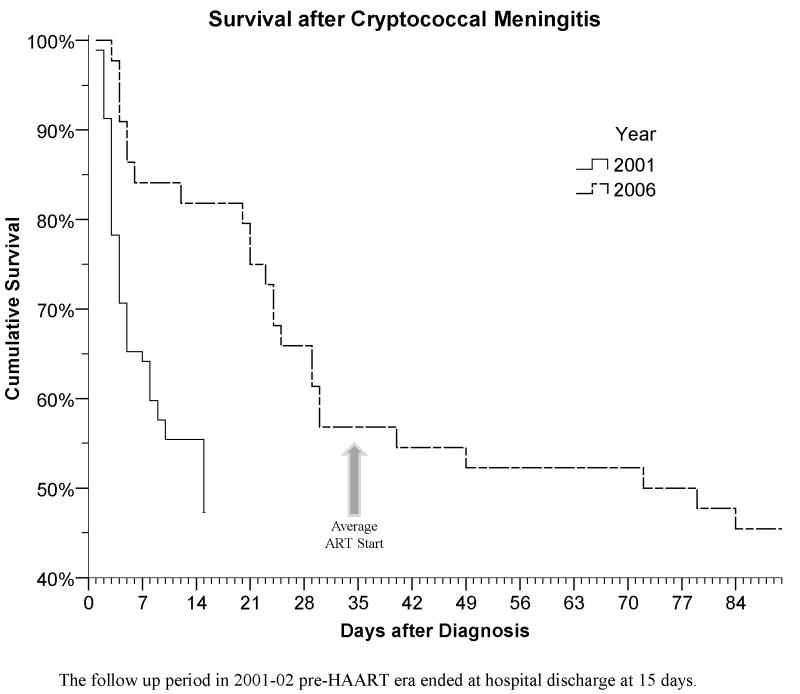
CM mortality was high with 41% (39/92) dying within the first two weeks. The median time of death was day 4 (Figure 2). Eight patients (9%) prematurely left the hospital against medical advice, thus only 49% (45/92) were known to be alive at 14 days. Resolution of headache by day 7 of anti-fungal therapy occurred in 32% and by day 14 in 69%. Of the 45 survivors completing 2 weeks of amphotericin, the median CRAG titer was 1:512 (IQR 1:128 to 1:1024), and 25 subjects (56%) remained culture positive for C. neoformans. In 2001, long term follow up was not performed as HAART was unavailable in Uganda. However, nearly all CM patients reported in the literature died by 6 months without HAART [14,15,16,17,18].
Figure 2.
Kaplan-Meier survival curves stratified by time period
HAART Era (2006)
In 2006, of the 44 antiretroviral naive patients with CM treated with amphotericin, 35 survived hospitalization (80%). Survival at 14-days was greater during the HAART era (absolute risk reduction (ARR) 31%; 95% CI: 15% to 46%; P<.001). The 2006 survival benefit persisted when excluding those leaving against medical advice and who were presumed dead (ARR: 28%; P<.001).
After hospital discharge, eight persons died before starting HAART, two did not return for clinic enrollment and were lost to follow up despite repeated attempts to reach them by mobile phone (presumed dead), and one upon repeat testing by ELISA was HIV seronegative. Only 24 (56%) started HAART at a mean time from CM diagnosis of 35 ±13 days (range 17–78 days). The median CD4 count among survivors at the time of HAART initiation was 20 (IQR: 7 to 47, maximum 77) cells/μL. By 3 months of HAART, the median CD4 had increased to 76 cells/tL (IQR: 33 to 153) and by 6 months to 66 cells/tL (IQR: 55 to 109) cells/μL. Plasma HIV RNA was undetectable (<400 copies/mL) in 44% (8/18) at 3 months and 83% (15/18) at 6 months. Only 18 persons (41%) were alive after 6 months of HAART, and each survived >12 months (median ART of 16 months).
Predictors of Survival
Lack of CSF inflammation correlated with poor outcome. An initial CSF WBC count of <5 cells/mL was independently associated with a positive CSF C. neoformans culture after 14 days (23/31 (74%) vs. 2/14 (14%); OR=17.3: 95% CI: 3.1 to 94.3; P<.001). A normal CSF protein level (<35mg/dL) was associated with higher 14-day mortality (24/48 (50%) vs. 17/88 (19%); adjusted OR=2.0; 95% CI: 1.2 to 3.3; P=.007).
More advanced disease, as evidenced by abnormal mental status (MMSE ≤25) at presentation, was associated with increased mortality (19/27 (70%) vs. 20/57 (35%); adjusted OR=6.5; 95% CI: 2.1 to 19.7; P=.001). Higher quantitative colony counts (>5 × 104/mL) trended toward higher mortality (P=.054). In contrast, the initial CSF CRAG titer was not associated with mortality (P=.7) or mycologic failure at 14 days (P=.4). Other baseline clinical and CSF parameters, including opening pressure, were not helpful in predicting survival.
By multivariate Cox-regression, the only clinical and laboratory parameters statistically associated with more rapid death were MMSE scores ≤25 (Hazard Ratio (HR) = 3.0; 95% CI: 1.6 to 5.8; P=.001), CSF protein level <35 mg/dL (HR = 2.1; 95% CI: 1.1 to 4.0; P=.03), or body weight <55 kg (HR = 2.0; 95% CI: 1.1 to 3.9; P=.04).
Immune Reconstitution Inflammatory Syndrome (IRIS)
Of the 24 CM patients who started antiretroviral therapy, 7 subsequently developed cryptococcal-related IRIS manifest as aseptic meningitis (n=5), generalized lymphadenopathy (n=1), and severe pneumonitis with respiratory failure (n=1) within 2–33 weeks from HAART initiation. Among the 5 subjects with aseptic meningitis, presenting features were severe headache, vomiting, negative CSF cultures, and high opening pressures (range: 280–320 mm H2O). One subject experienced sudden onset of transient, bilateral blindness twice after 20 and 25 weeks of HAART in conjunction with aseptic meningitis and a normal ophthalmologic exam without papilledema. Another three subjects developed possible cryptococcal-related IRIS events including chorioretinitis, phlyctenular conjunctivitis, and lobar pneumonitis. IRIS also presented as unmasking of pulmonary TB in one patient and varicella zoster in another. Thus, suspected IRIS events occurred in 50% of CM patients (95% CI: 29% to 71%). Six persons subsequently died after starting HAART, four from cryptococcal-related IRIS, one from possible CM-IRIS after 12 days of HAART, and one from profound anemia (hemoglobin 1.4 g/dL) with lactic acidosis after 10 weeks of an AZT-3TC-efavirenz regimen.
The median time between CM diagnosis and initiating HAART was 34 days (IQR: 27 to 41) for IRIS cases and 37 days (IQR: 29 to 42) for those not experiencing IRIS (P=.7). The onset of IRIS averaged 12.6 ± 8.6 weeks (median 12, range 2–33 weeks) of HAART.
Management of elevated ICP
Initial opening pressure was measured in 92% (126/136) of CM subjects, of whom 81% had opening pressures ≥200mm H2O (Figure 3) and 69% met the criterion for therapeutic CSF drainage (>250 mm H2O) per IDSA guidelines [19]. However, only five eligible patients consented for therapeutic LPs. There is a strong cultural bias against LPs in Uganda. Considerable efforts to convince patients and their caregivers of the necessity of therapeutic LPs were unsuccessful. Surprisingly, the initial opening pressure was unassociated with mortality by logistic regression (P=.7), even comparing those with markedly increased (>560 mm) versus normal CSF pressure (OR=1.9; 95% CI: 0.6 to 6.1; P=.3) at CM diagnosis. Patients who met the IDSA criterion for therapeutic CSF drainage (>250 mm H2O) trended toward a higher 14-day mortality (OR=2.1; 95% CI: 0.9 to 5.2; P=.09) compared with those with lower CSF pressures.
Figure 3.
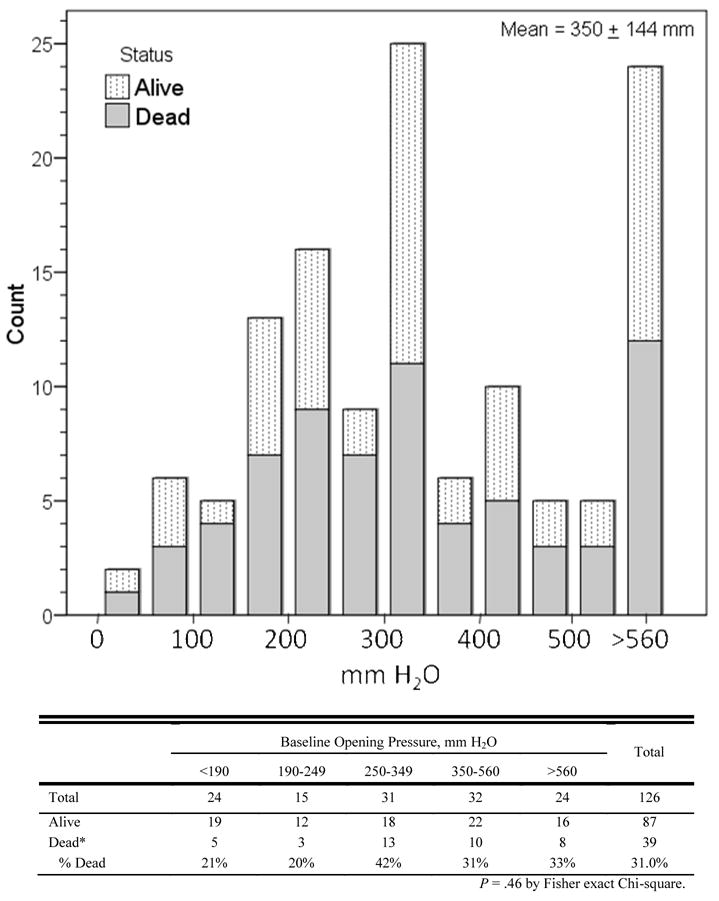
Distribution of CSF Opening Pressure in 126 HIV-infected Patients with Cryptococcal Meningitis (2001 and 2006)
Toxicity
Nausea and vomiting with amphotericin occurred in 17%, and 10% experienced marked rigors with amphotericin. Renal toxicity from amphotericin was uncommon; only one person (1%) stopped amphotericin on day 7 due to a creatinine >3 mg/dL and only seven (9%) showed creatinine levels ≥3.0 mg/dL at day 14. The median creatinine rise in day 14 survivors was 0.6 mg/dL (IQR: 0 to 1.5 mg/dL), but a survival bias may be present. Neither creatinine at presentation nor change by day 7 was associated with mortality (P > .9).
In 2006, renal function and electrolytes (Na, K) were measured three times weekly. An unintended result was that 66% of survivors had amphotericin held temporarily for ≥1 day and 33% for ≥2 days because of concerns for renal toxicity. Although these patients missed doses, all but two patients completed 14 doses of amphotericin over 14–18 days.
DISCUSSION
Cryptococcal infection remains a significant problem in patients with AIDS in Sub-Saharan Africa. We report 20–42% two-week mortality with HIV-associated CM despite amphotericin therapy. This mortality is much higher than the 5.5%–15% mortality reported in North American studies [3,7], but is comparable to that reported elsewhere in Sub-Saharan Africa [1,8,9]. The two-week mortality rate has improved between 2001 to 2006 likely due to increased institutional expertise and better intravenous fluid support. Nevertheless, even with the availability of HAART, the 6-month survival after CM is only 41%. The one year survival in Uganda with HAART remains four-fold worse than CM outcomes in France and approximately two-fold worse than in Thailand [20,21].
CM mortality in Africa may remain a problem because late presentation with advanced CM is common. In this study, the median duration of symptoms was 2 weeks. A significant proportion had altered mental status (38%), similar to the experience in France (33%), but much higher than the 10–12% in U.S. reports [7,22]. The median CSF opening pressure in this study was also higher than the NIAID Mycosis Study Group (330 vs. 250mm H2O; P<.001), as was the proportion of patients with CSF opening pressures ≥250mm H2O, meeting the recommended criterion for therapeutic LPs (70% vs. 50%) [23].
As highlighted by Pappas [24], management of increased ICP is critical, yet only 6% of patients in our study with an ICP ≥250mm consented for therapeutic LPs. There is a strong cultural bias against lumbar punctures, reinforced by the high mortality of meningitis and inconsistent availability of local anesthetic for the procedure. In our experience, patients and their families consented to diagnostic LPs when near death and/or near-comatose, yet they would refuse later therapeutic LPs. An additional 24% (36/151) refused consent for the initial diagnostic LP in 2001, were never included into this study.
Surprisingly, we did not find a correlation between CSF pressure and mortality. Perhaps this is because most patients presented late with advanced disease and a high proportion had elevated pressure. We found that lack of CSF inflammation was associated with worse outcome. For example, low-normal protein (<35 mg/dL) was associated with increased acute mortality, and low-normal CSF WBC counts (<5 cells/mL) were associated with culture positivity at day 14 which is associated with delayed mortality; in agreement with other observations [13,25].
IRIS is also an emerging problem in Africa, the magnitude of which is poorly characterized. Unlike the cases of CM-IRIS from North America which reported no mortality [26] in this study, four of ten persons with cryptococcal IRIS died, similar to French (3/10; 30%) and South African (6/9; 66%) reports [27,28]. Yet, delaying HAART as a strategy to prevent IRIS in resource-limited areas is not likely the answer, as mortality continues to accrue between hospital discharge and starting HAART among those with very advanced HIV/AIDS. In our 2006–2007 cohort, the 21% mortality between 14 to 28 days equaled the initial 14-day mortality (20%). Since there was also mortality with IRIS after HAART initiation, the optimal timing for initiation of HAART remains unknown, and randomized trials are needed.
Given the high mortality of CM in Africa, alternative and complimentary approaches should be considered. Adjunctive, flucytosine (5-FC) is unavailable and cost prohibitive (>$120/day). From a public health perspective, scaling up routine HIV counseling and testing offer the potential to intervene with HAART before AIDS-related OIs occur. Early HIV care offers the potential of timely screening with a serum CRAG as cryptococcal antigenemia precedes CM symptoms by a median of 22 days [4,29]. Those asymptomatic with cryptococcal antigenemia have a 7-fold higher odds of death after starting HAART with an 18% attributable risk [30]. Unfortunately, the CRAG’s current cost of $15-$25 is prohibitive. Another potential cost-effective approach is primary fluconazole prophylaxis in populations with a high incidence of CM and antiretroviral unavailability [31].
The limitations of this work include unclear reasons for improvement in survival in 2006. Although the amount of intravenous fluid administered was not quantified, in 2001–02 fluid use was intermittent due to frequent shortages, but in 2006, fluid use was universal. Basic intravenous fluid management to limit amphotericin toxicity should not be neglected, and we hypothesize that improved fluid management may improve mortality in resource-limited regions. This requires further study.
In summary, our study demonstrates a high cryptococcal mortality despite amphotericin therapy and the availability of HAART. Although use of HAART and early detection of HIV may eventually lead to a reduction in OIs, including CM, management of OIs remains a key facet of AIDS care in Africa. Trials to delineate the optimal timing of HAART initiation and treatment for cryptococcal-IRIS are needed, as are studies to understand the pathophysiology of IRIS.
Supplementary Material
Acknowledgments
We wish to thank Darlisha Williams MPH, Sarah J. Lee M.D, MPH, (University of Minnesota) and Vivian Cox M.D. (University of Colorado) for assistance with data collection; Emmanuel Okiring and Dr. Henry Kajumbula for laboratory support in 2006; Henry Wamala MBChB (Makerere University) for assistance with data collection in 2000–2001. We wish to thank Keith McAdam, MB BChir, FRCP, FWACP for institutional support.
Financial Support: This work was supported in part by the Academic Alliance Foundation (AK), University of Minnesota Academic Health Center (PRB,DBM), NIH Fogarty International Center: AIDS International Training and Research Program (AITRP) at Case Western Reserve University (TW000011: AK), National Institute of Allergy and Infectious Diseases (T32AI055433; L30AI066779; K12RR023247: DRB), Minnesota Medical Foundation (PRB,DRB,DBM), University of Minnesota Office of International Programs (DRB), the Colorado Center for AIDS Research (NIAID P30AI054907: ENJ) and the Veterans Affairs Research Service (ENJ).
Footnotes
Conflicts of Interest: No conflicts of interest exist.
References
- 1.Hakim JG, Gangaidzo IT, Heyderman RS, et al. Impact of HIV infection on meningitis in Harare, Zimbabwe: a prospective study of 406 predominantly adult patients. AIDS. 2000;14:1401–7. doi: 10.1097/00002030-200007070-00013. [DOI] [PubMed] [Google Scholar]
- 2.“3 by 5” Progress Report: December 2003-June 2004, July 2004 WHO Publication
- 3.Chuck SL, Sande MA. Infection with Cryptococcus neoformans in the Acquired Immunodeficiency Syndrome. N Engl J Med. 1989;231:794–9. doi: 10.1056/NEJM198909213211205. [DOI] [PubMed] [Google Scholar]
- 4.French N, Gray K, Watera C, et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS. 2002;16:1031–8. doi: 10.1097/00002030-200205030-00009. [DOI] [PubMed] [Google Scholar]
- 5.Sacktor N, Lyles RH, Skolasky MA, et al. HIV-associated neurologic diseases incidence changes: Multicenter AIDS cohort Study, 1990–1998. Neurology. 2001;56:257–260. doi: 10.1212/wnl.56.2.257. [DOI] [PubMed] [Google Scholar]
- 6.Lara-Peredo O, Cuevas LE, French N, Bailey JW, Smith DH. Cryptococcal infection in an HIV-positive Ugandan population. J Infect. 2000;41:195. doi: 10.1053/jinf.2000.0697. [DOI] [PubMed] [Google Scholar]
- 7.van der Horst CM, Saag MS, Cloud GA, et al. Treatment of Cryptococcal meningitis associated with the Acquired Immunodeficiency Syndrome. N Engl J Med. 1997;337:15–21. doi: 10.1056/NEJM199707033370103. [DOI] [PubMed] [Google Scholar]
- 8.Schutte CM, Van der Meyden CH, Magazi DS. The impact of HIV on meningitis as seen at a South African Academic Hospital (1994 to 1998) Infection. 2000;28:3–7. doi: 10.1007/pl00012241. [DOI] [PubMed] [Google Scholar]
- 9.Heyderman RS, Gangaidzo IT, Hakim JG, Mielke J, Taziwa A, Musvaire P, Robertson VJ, Mason PR. Cryptococcal meningitis in human immunodeficiency virus-infected patients in Harare, Zimbabwe. Clin Infect Dis. 1998;26:284–9. doi: 10.1086/516298. [DOI] [PubMed] [Google Scholar]
- 10.McCarthy KM, Morgan J, Wannemuehler KA, et al. Population-based surveillance for cryptococcosis in an antiretroviral-naive South African province with a high HIV seroprevalence. AIDS. 2006;20:2199–206. doi: 10.1097/QAD.0b013e3280106d6a. [DOI] [PubMed] [Google Scholar]
- 11.Bicanic T, Meintjes G, Wood R, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis. 2007;45:76–80. doi: 10.1086/518607. [DOI] [PubMed] [Google Scholar]
- 12.Millogo A, Ki-Zerbo GA, Andonaba JB, et al. Cryptococcal meningitis in HIV-infected patients at Bobo-Dioulasso hospital (Burkina Faso) Bull Soc Pathol Exot. 2004;97:119–21. [PubMed] [Google Scholar]
- 13.Pitisuttithum P, Tansuphasawadikul S, Simpson AJ, Howe PA, White NJ. A prospective study of AIDS associated cryptococcal meningitis in Thailand treated with high-dose amphotericin B. J Infect. 2002;43:226–33. doi: 10.1053/jinf.2001.0916. [DOI] [PubMed] [Google Scholar]
- 14.Collett G, Parrish A. Fluconazole donation and outcomes assessment in cryptococcal meningitis. S Afr Med J. 2007;97:175–6. [PubMed] [Google Scholar]
- 15.Schaars CF, Meintjes GA, Morroni C, Post FA, Maartens G. Outcome of AIDS-associated cryptococcal meningitis initially treated with 200 mg/day or 400 mg/day of fluconazole. BMC Infect Dis. 2006;6:118. doi: 10.1186/1471-2334-6-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mwaba P, Mwansa J, Chintu C, et al. Clinical presentation, natural history, and cumulative death rates of 230 adults with primary cryptococcal meningitis in Zambian AIDS patients treated under local conditions. Postgrad Med J. 2001;77:769–73. doi: 10.1136/pmj.77.914.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayanja-Kizza H, Oishi K, Mitarai S, et al. Combination therapy with fluconazole and flucytosine for cryptococcal meningitis in Ugandan patients with AIDS. Clin Infect Dis. 1998;26:1362–6. doi: 10.1086/516372. [DOI] [PubMed] [Google Scholar]
- 18.Maher D, Mwandumba H. Cryptococcal meningitis in Lilongwe and Blantyre, Malawi. J Infect. 1994;28:59–64. doi: 10.1016/s0163-4453(94)94161-0. [DOI] [PubMed] [Google Scholar]
- 19.Saag MS, Graybill RJ, Larsen RA, et al. Practice guidelines for the management of cryptococcal disease. Clinl Infect Dis. 2000;30:710–8. doi: 10.1086/313757. [DOI] [PubMed] [Google Scholar]
- 20.Lortholary O, Poizat G, Zeller V, et al. Long-term outcome of AIDS-associated cryptococcosis in the era of combination antiretroviral therapy. AIDS. 2006;20:2183–91. doi: 10.1097/01.aids.0000252060.80704.68. [DOI] [PubMed] [Google Scholar]
- 21.Jongwutiwes U, Kiertiburanakul S, Sungkanuparph S. Impact of antiretroviral therapy on the relapse of cryptococcosis and survival of HIV-infected patients with cryptococcal infection. Curr HIV Res. 2007;5:355–60. doi: 10.2174/157016207780636551. [DOI] [PubMed] [Google Scholar]
- 22.Dromer F, Mathoulin-Pélissier S, Launay O, Lortholary O French Cryptococcosis Study Group. Determinants of disease presentation and outcome during cryptococcosis: the CryptoA/D study. PLoS Med. 2007;4:e21. doi: 10.1371/journal.pmed.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graybill JR, Sobel J, Saag M, et al. Diagnosis and management of increased intracranial pressure in patients with AIDS and cryptococcal meningitis. The NIAID Mycoses Study Group and AIDS Cooperative Treatment Groups. Clin Infect Dis. 2000;30:47–54. doi: 10.1086/313603. [DOI] [PubMed] [Google Scholar]
- 24.Pappas PG. Managing cryptococcal meningitis is about handling the pressure. Clin Infect Dis. 2005;40:480–2. doi: 10.1086/427222. [DOI] [PubMed] [Google Scholar]
- 25.Siddiqui AA, Brouwer AE, Wuthiekanun V, et al. IFN-gamma at the site of infection determines rate of clearance of infection in cryptococcal meningitis. J Immunol. 2005;174:1746–50. doi: 10.4049/jimmunol.174.3.1746. [DOI] [PubMed] [Google Scholar]
- 26.Shelburne SA, 3rd, Darcourt J, White AC, Jr, et al. The role of Immune Reconstitution Inflammatory Syndrome in AIDS-related Cryptococcus neoformans disease in the era of highly active antiretroviral therapy. Clin Inf Dis. 2005;40:1049–53. doi: 10.1086/428618. [DOI] [PubMed] [Google Scholar]
- 27.Lortholary O, Fontanet A, Memain N, et al. Incidence and risk factors of immune reconstitution inflammatory syndrome complicating HIV-associated cryptococcosis in France. AIDS. 2005;19:1043–1049. doi: 10.1097/01.aids.0000174450.70874.30. [DOI] [PubMed] [Google Scholar]
- 28.Lawn SD, Bekker LG, Myer L, Orrell C, Wood R. Cryptococcocal immune reconstitution disease: a major cause of early mortality in a South African antiretroviral programme. AIDS. 2005;19:2050–2. doi: 10.1097/01.aids.0000191232.16111.f9. [DOI] [PubMed] [Google Scholar]
- 29.Micol R, Lortholary O, Sar B, et al. Prevalence, determinants of positivity, and clinical utility of cryptococcal antigenemia in Cambodian HIV-infected patients. J Acquir Immune Defic Syndr. 2007;45:555–9. doi: 10.1097/QAI.0b013e31811ed32c. [DOI] [PubMed] [Google Scholar]
- 30.Liechty CA, Solberg P, Were W, et al. Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Trop Med Int Health. 2007;12:929–35. doi: 10.1111/j.1365-3156.2007.01874.x. [DOI] [PubMed] [Google Scholar]
- 31.Chetchotisakd P, Sungkanuparph S, Thinkhamrop B, Mootsikapun P, Boonyaprawit P. A multicentre, randomized, double-blind, placebo-controlled trial of primary cryptococcal meningitis prophylaxis in HIV-infected patients with severe immune deficiency. HIV Med. 2004;5:140–3. doi: 10.1111/j.1468-1293.2004.00201.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.