Abstract
Organ regeneration in mammals is hypothesized to require a functional pool of stem or progenitor cells, but the role of these cells in lung regeneration is unknown. Whereas postnatal regeneration of alveolar tissue has been attributed to type II alveolar epithelial cells (AECII), we reasoned that bronchioalveolar stem cells (BASCs) have the potential to contribute substantially to this process. To test this hypothesis, unilateral pneumonectomy (PNX) was performed on adult female C57/BL6 mice to stimulate compensatory lung regrowth. The density of BASCs and AECII, and morphometric and physiologic measurements were recorded on days 1, 3, 7, 14, 28, and 45 days after surgery. Vital capacity was restored by day 7 after PNX. BASC numbers increased by day 3, peaked to 220% of controls (P<0.05) by day 14, then returned to baseline after active lung regrowth was complete, whereas AECII cell densities increased to 124% of baseline (N/S). Proliferation studies revealed significant BrdU uptake in BASCs and AECII within the first 7 days after PNX. Quantitative analysis using a systems biology model was used to evaluate the potential contribution of BASCs and AECII. The model demonstrated that BASC proliferation and differentiation contributes between 0 and 25% of compensatory alveolar epithelial (type I and II cell) regrowth, demonstrating that regeneration requires a substantial contribution from AECII. The observed cell kinetic profiles can be reconciled using a dual-compartment (BASC and AECII) proliferation model assuming a linear hierarchy of BASCs, AECII and AECI cells to achieve lung regrowth.
Keywords: regeneration, pneumonectomy, proliferation, stem cells, alveolar, bronchioalveolar
Introduction
The adult mouse exhibits a remarkable capacity for compensatory post-natal lung growth in comparison to larger mammals including humans (9, 14). Within two weeks after unilateral pneumonectomy, adult mice restore lung weight, volume, cellularity, DNA and protein content (4, 16, 30). While multiple cell lineages are implicated in compensatory lung regrowth (1, 8), it is unknown whether stem or progenitor cells play a role. Studies show that both AECII and Clara cells proliferate during compensatory lung regrowth following pneumonectomy (3, 15, 30); these cells may play an important role in restoration of alveolar and airway structures. Alternatively Clara cells and AECII may be replenished from a subset (i.e. variant) of these cells with unique proliferative potential, or from a distinctly different group of multi-potent or uni-potent stem cells.
Several candidate endogenous stem or progenitor cell populations have been identified in the lung, including Hoechst-dye-effluxing side population cells (17, 21, 26, 27), as well as variant Clara cells and bronchioalveolar stem cells (BASCs), which are located at the brochioalveolar duct junction (12) and show similar properties of naphthalene resistance and proliferation during lung injury. BASCs in particular may be pivotal for regeneration of epithelium since as they have been have been shown to proliferate in response to airway or alveolar injury in vivo (e.g. naphthalene, bleomycin), and cultured BASCs isolated by flow cytometry differentiate into Clara cells, AECI and AECII which defines these cells as multipotent (12). The present study was undertaken to determine if BASCs exhibit a proliferative response during compensatory lung regrowth, and to observe the kinetics of BASC in comparison to AECII in order in order to understand their relative quantitative contributions to alveolar regrowth following pneumonectomy. Physiology and cell kinetics were related using a three-compartment (BASC, AECII, and type 1 alveolar epithelial cell) systems biology model to assess whether BASC proliferation and differentiation could be sufficient to account for 100% of alveolar epithelial cell regrowth.
Materials and Methods
Study Design
The study involved 4 separate analyses: (1) characterization of the time course of changes in lung physiology and morphometry following pneumonectomy (2) characterization of the cellular kinetics of BASC and AECII based on immunofluorescence on tissue sections, (3) determination of the proliferation rate of BASC and AECII during the more rapid period of regrowth (days 0–7), and (4) systems biology analysis of cell kinetics and morphometry measurements to assess quantitatively the potential contributions of ACEII and BASCs to tissue regrowth. Mice received unilateral pneumonectomy (PNX) and underwent final measurements and euthanasia for tissue harvesting in groups of 5, on days 1, 3, 7, 14, 28, and 45. Five additional mice that did not receive any treatment (i.e. surgery) served as controls (day 0 control). Mice after PNX or sham surgery (thoracotomy without exteriorization or excision of the lung) were fed BrdU in drinking water (0.8 mg/mL) between days 0–3 or 4–7 to study time-specific proliferation in BASC and AECII.
Animals
Mice used for the pulmonary function, morphometry, and cell kinetics experiments were 10–12-week-old, ~20g, C57BL/6 females, and for the cell proliferation study 8-week-old C57BL/6 females obtained from Jackson Laboratories. All experiments were performed in accordance with NIH guidelines, as dictated by Institutional Animal Care and Use Committee at Tufts University. Mice were deemed free of infectious diseases by surveillance using sentinel animals, gross necropsy, and lung histology. Mice were anaesthetized by intraperitoneal injection of ketamine (50–75 mg/kg) and xylazine (5 mg/kg), and then received 2ml of warmed normal saline and 100mg/kg sodium ampicillin subcutaneously. Orotracheal intubation was performed under direct visualization using a 19-gauge steel-tipped endotracheal tube over a flexible stylette. Mice were secured in supine position, and mechanically ventilated (AUT6110, Buxco Electronics, Wilmington, NC) at 150–200 tidal breaths of 0.3ml of room air per minute, at positive end-expiratory pressure of 5 cm H2O during surgery and recovery.
Pneumonectomy procedure
After assessing adequate anesthetic depth via absence of response to toe-pinch, the left thoracic wall was clipped and disinfected. The skin, chest wall and pleura were incised at the 5th intercostal space, and the left lung was gently lifted through a ~7mm incision and ligated at the hilum with 4–0 silk. The lungs were then inflated to 30cmH20, and the chest wall closed during this inflation with a single interrupted suture. The skin was closed with 5–0 PDS in a simple interrupted pattern. Mice were extubated at the onset of vigorous spontaneous breathing. The mice recovered from surgery in a warmed cage, and post-operative pain was managed with buprenorphine subcutaneously (0.05mg/kg) as soon as mice showed conscious motor control, and every 12 hours thereafter as needed (<3 days). Chow, nutrient gel (on the cage floor), and water were provided ad libitum. Sham pneumonectomy animals underwent an identical procedure, except that after the thoracotomy, the chest was left open for 5 minutes to simulate the conditions of the pneumonectomy group, then closed as described.
Measurement of vital capacity and quasi-static lung compliance
On the final day of the experiment (1, 3, 7, 14, 28, or 45 days after PNX or no surgery as indicated by ‘day 0’ per protocol), vital capacity and respiratory system compliance were measured following ketamine/xylazine anesthesia. Pulmonary function testing was carried out using a whole body flow-type plethysmograph (PLY3111, Buxco Electronics, Wilmington, NC). Three volume history breaths were administered at 25 cmH20, and the lung re-inflated to 30cmH20 airway pressure. The breath was held for 0.4sec, then permitted to deflate passively to relaxation volume, when negative pressure was introduced to deflate the lung to residual volume (−30cmH20) (Forced Maneuvers System, AUT6110, Buxco Electronics). Tests were rejected if there was any spontaneous respiratory effort during the maneuver, and results from a minimum of 3 maneuvers were averaged for each data point. Vital capacity was measured as the volume of deflation from +30 cm H2O to −30 cm H2O. Respiratory system compliance (i.e. chord compliance) was determined as the linear slope of the quasi-static deflation pressure-volume between 10 and 0 cm H20 airway pressure.
Tissue preparation and morphometry
After pulmonary function testing, the mice were euthanized via cervical dislocation. Following median sternotomy, right ventricular perfusion was performed with normal saline to clear the pulmonary vasculature of erythrocytes. The trachea was cannulated and the lungs removed en bloc. Tissue fixation was achieved with intra-tracheal 10% buffered formalin at 25cm H2O overnight. The trachea was then ligated, and the lung stored in 10% buffered formalin. In control mice the left lung was removed prior to embedding to avoid bias during cell counting on tissue sections. Mean linear intercept (Lm) values were calculated using an automated software system (SigmaScan Pro, Systat software, San Jose, CA) using standard methods (28). Five measurements were made per mouse, and the results averaged.
Tissue immunofluorescence for enumeration of BASC
Immunofluorescent staining (IF) was performed on formalin-fixed paraffin embedded sections (5 um). As primary antibodies, the polyclonal goat antibody anti-CC10 (Santa Cruz, dilution 1:200), the polyclonal rabbit antibody anti-proSPC (Chemicon AB3786, dilution 1:1000), and the monoclonal mouse antibody anti-BrdU (Santa Cruz, dilution 1:200) were used. Tissue sections were deparaffinized and hydrated using standard methods, and antigen retrieval was performed using a citrate buffer (pH6.0) and microwave heating (10 min). Tissues were washed (PBS with 0.1% Triton X-100) three times after antigen retrieval. BASCs were double-stained with CC10 and proSP-C. Detection was performed as follows: when triple staining for co-localization of proSP-C, CC10, and BrdU, donkey anti-rabbit Alexafluor 488 (green), donkey anti-goat Alexafluor 350 (blue), and donkey anti-mouse Alexafluor 594 (red); when staining for proSP-C and CC10, donkey anti-rabbit Alexafluor 488 and donkey anti-goat Alexafluor 594, respectively. The appropriate single-antibody and secondary-only controls were performed and no dual staining or relevant background staining was observed.
Between 16–30 bronchioalveolar duct junctions (BADJ) per mouse were photographed digitally (Nikon Eclipse E600, Spot cooled CCD camera and software) and the images merged in Adobe Photoshop 6.0. Bronchioalveolar stem cells (BASCs) were identified as those with a single nucleus (DAPI) and clear double staining for CC10 (rhodamine) and proSP-C (FITC), and were counted as number of BASC per BADJ. To ensure correct identification of BASCs, deconvolution microscopy was utilized where necessary to demonstrate that the CC10 and pro-SPC were associated with the cell cytoplasm in question (see supplementary figure 1 for deconvolution images). Type II alveolar epithelial cells (AECII) were identified as those with punctate staining with proSP-C. Total nucleated cells per HPF (i.e DAPI-stained nuclei) were counted, and the mean percentage of AECII cells/nucleated cells in a minimum of 15 hpf was obtained. The BASC/BADJ and AECII/nuclei were used as a monitor of cell densities over time.
Cell kinetic model
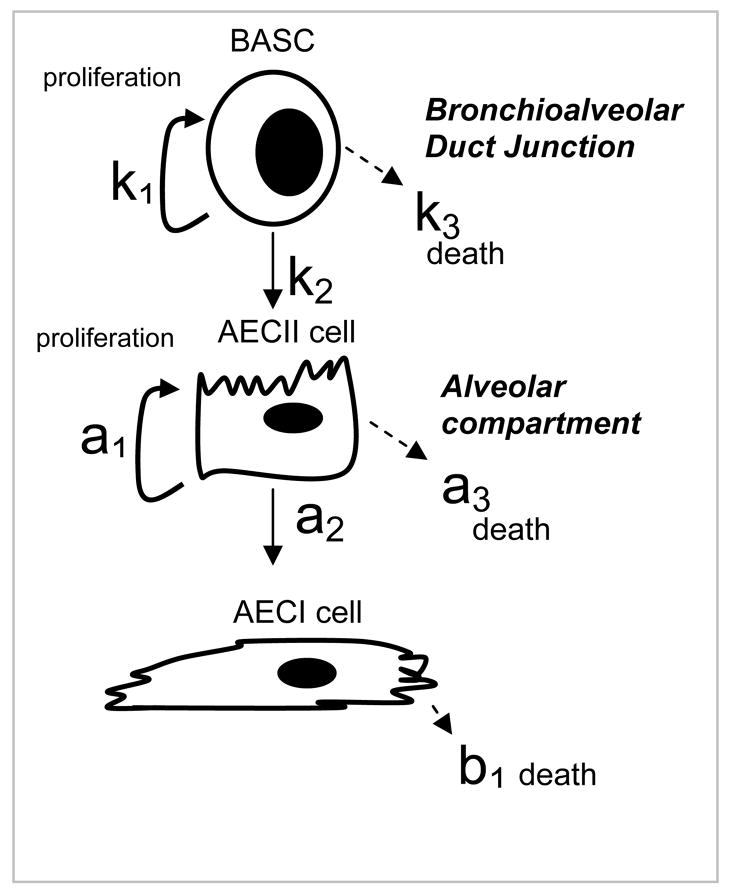
A three-compartment cell kinetics model (Figure 1) was developed to quantitatively characterize the relationship between BASC, AECII, and AECI cells during compensatory regrowth. Relative changes in lung surface area as a function of cell proliferation were calculated as a function of time by assuming that AECI cells contribute 90% of alveolar surface area and AECII cells 10% of alveolar surface area. The first differential equation describes BASC kinetics. BASCs are modeled as undergoing asymmetric division. BASCs have 3 potential fates: 1) proliferation with a rate constant k1; 2) differentiation into AECII cells with a rate constant k2; 3) cell death with a rate constant k3. The second differential equation describes AECII kinetics and assumes that AECII cells can arise either from BASCs (via rate constant k2) or via division from parent AECII cells. AECII cells have 3 potential fates: 1) proliferation into daughter AECII cells with a rate constant a1; 2) differentiation into AECI cells with a rate constant a2; 3) cell death with a rate constant a3. The third compartment describes AECI cell kinetics and assumes these cells are terminally differentiated, arising only from AECII cells. AECI cells have only one potential fate: cell death with a rate constant b1. The dynamics of lung regrowth can be described by simultaneous solution of these three equations:
Figure 1.
Lineage hierarchy and schematic representation of rate constants employed to model cellular contributions to alveogenesis during compensatory lung regrowth. The model tests the hypothesis that BASCs give rise to AECII which in turn produce AECI; BASCs and AECII have proliferative capacity whereas AECI are terminally differentiated.
-
BASC compartment (B = BASC numbers)
-
AECII cell compartment (A2 = AECII numbers)
-
AECI cell compartment (Al = AECI numbers)
The time domain solution was derived using Laplace transforms (see supplementary data), and explicit expressions for B(t), A2(t), and A1(t) as functions of time and rate constants were obtained. Optimal values for the independent model parameter were derived by minimizing the root mean square model error equal to the difference between the sum of: 1) the numbers of cells predicted by the model and the actual numbers measured by immunohistochemistry at the various time points, and 2) the measured lung surface area and calculated surface area, estimated from the number of AECII cells at various time points. For simulation purposes, the study period was divided into seven intervals: Day 1–3, 4–6, 7–9, 10–12, 13–15, 16–18, and > 18 days, each with distinct kinetic parameters. The overall response was generated by solving the equations in a piecewise continuous fashion. Initial values for cell type were derived from published morphometry: the percentage of total lung cells that are BASC = 0.34%(12), AECI = 8%, and AECII = 16%(18). Normalized total surface area of the lung was determined as 0.9*AECI(t) + 0.1* AECII(t). AECI(t=0) and AECII(t=0) were both set equal 1, and values at all subsequent time points are expressed as a fraction of baseline. (18).
To assess the potential contribution of BASC differentiation to alveolar epithelial cell regrowth following pneumonectomy, the percent differentiation of BASCs into AECII cells was systematically increased from 0 to 50%, and population doubling times then determined by iteration to minimize modeling error. Model error is reported as the ratio of the root mean square error (which is equal to Σ [(measured value − predicted value)2]1/2, where the summation is performed for BASC numbers, AECII cell numbers, and lung surface area at each time) to the root mean square signal (which is equal to (Σ [(measured value)2]1/2, where the summation is performed for BASC numbers, AECII cell numbers, and lung surface area at each time). Initial values for BASC doubling time used in the minimization analysis were 8–24 hrs based on Kim (12) and a doubling time of 85–270 hrs for AECII (29). Minimum error values on the order of 10% were achieved using realistic ranges for population doubling times of BASC (19–23 hours) and AECII cells (38–51 hours). Only the initial values used in the iterative calculations for error minimization were selected from the literature; the actual final values were determined from the error minimization process.
Statistics
Measures of lung function and mean linear intercept were analyzed by repeated measures ANOVA with a two-way Dunnett’s post hoc test. Changes in BASC and AECII numbers and proliferation were analyzed using a mixed effect model, admitting mouse as a random effect. The ratio of AECII to total parenchymal nuclei was determined and the distribution tested for normality using the Shapiro Wilks test (25). The ratio was examined, using mixed effects modeling with inter-subject variability as a random effect, to see if there was an association between ratio and observation point (day). Mixed effects analysis under these situations is routinely used to admit the subject-specific aspect of variation into the analysis. Failure to do this leads to excessive type 1 errors (i.e. smaller than appropriate estimates of errors in the regression coefficients). To explore observation point (day) association with the BASC values we used Cuzick’s trend test (5) as there were discrete, as opposed to continuous values (or levels) for BASC. A P-value of 0.05 was considered significant.
RESULTS
Physiology and morphometry
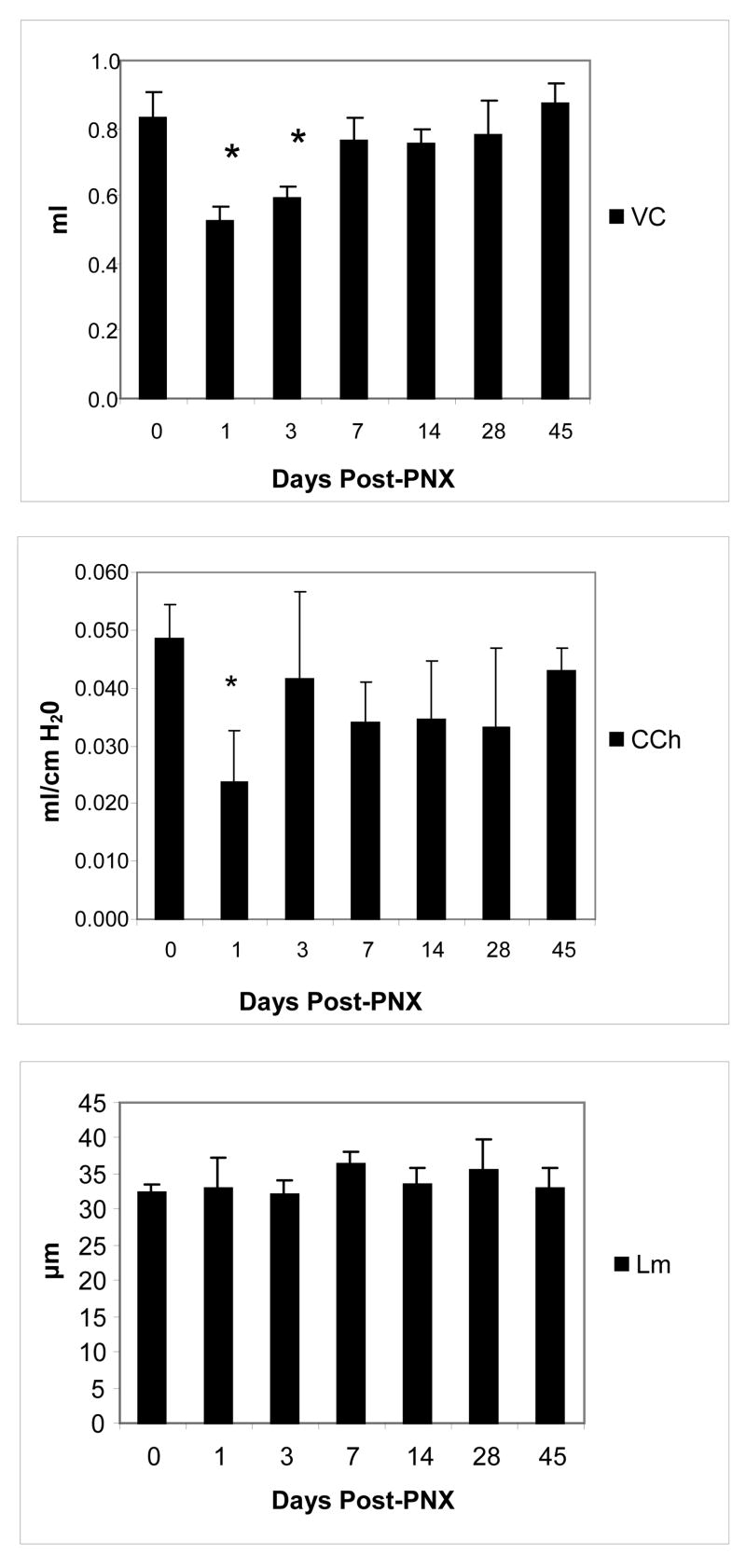
We measured lung function at various time points after partial pneumonectomy. Pneumonectomy resulted in a drop in vital capacity (VC) at day 1 (P<0.001) consistent with the volume of the excised left lung. Evidence of regrowth was evident on day 3 (figure 2). By day 7, regrowth measured in terms of VC was essentially complete, with no significant differences between PNX and control mice. Chord compliance was significantly lower (P=0.001) on day 1 post-operatively than baseline but returned to control values by day 3 and thereafter. Baseline Lm of control mice was 32.3um +/− 0.105. No significant differences in alveolar dimension were observed between control and PNX mice at any time point.
Figure 2.
Measurements of vital capacity and quasistatic lung compliance following left-sided pneumonectomy in adult mice. Vital capacity (top) was significantly lower (P=<0.001) at day 1 and 3 post-PNX. Chord compliance (center) was significantly lower (P=0.001) on day 1 only. There were no significant changes from baseline in mean linear intercept (Lm, bottom); n=5 mice/time point. Data are mean values +/− SEM.
BASC and AECII density in tissue following pneumonectomy
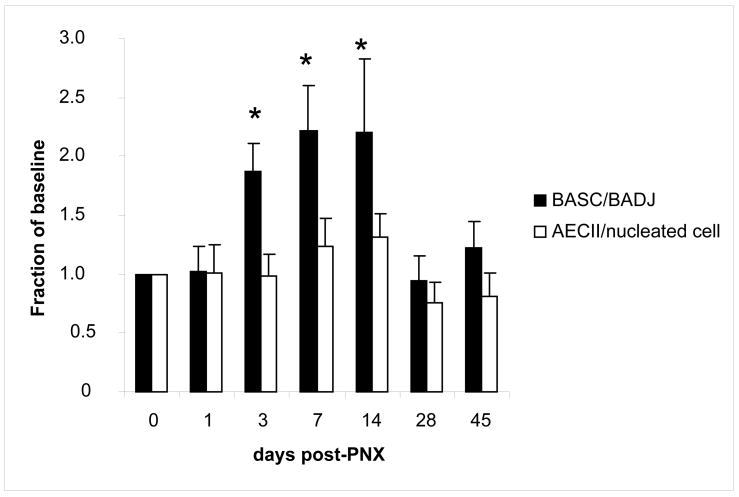
The relative numbers of BASCs and AECIIs were measured at various time points after pneumonectomy using immunofluorescence. In unoperated control mice, 32% (+/− 6% SEM) of bronchioalveolar duct junctions (BADJ) showed BASC. A significant (P<0.01) increase in BASCs per terminal bronchiole in PNX versus unoperated control mice was first detected on day 3 (187% control value), followed by further increases on day 7 (221% control, P=0.006) and 14 (220% control, P=0.007). After a period of active lung regrowth (days 3–14), BASC numbers dropped back to baseline levels by day 28, remaining so on day 45 (figure 3). Interestingly, BASCs were rarely (<1%) observed outside the bronchioalveolar duct junction (BADJ) in lungs from mice that underwent pneumonectomy, but not in sham lungs; BASCs were identified in peribronchiolar regions and alveolar spaces near BADJs (figure 4). On days 7 and 14, mean AECII counts increased to 122 and 124% of control values, respectively, but these differences failed to reach statistical significance (P=0.55 and 0.14 respectively) (Figure 3).
Figure 3.
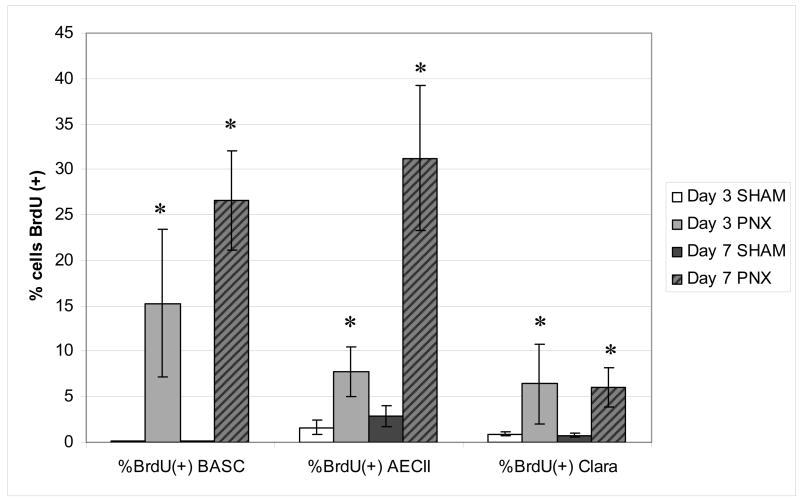
Analysis of cell density after pneumonectomy. Number of BASC per bronchioalveolar junction (BADJ) show 2.2 fold increase from controls (0 days post-PNX) by day 7 post-PNX (n=5/group), while the number of AECII per nucleated cell show a trend towards increase in cell density (1.3 fold) on days 7–14. Data are expressed as mean +/− SEM, * demonstrates significant increases over baseline (P<0.05).
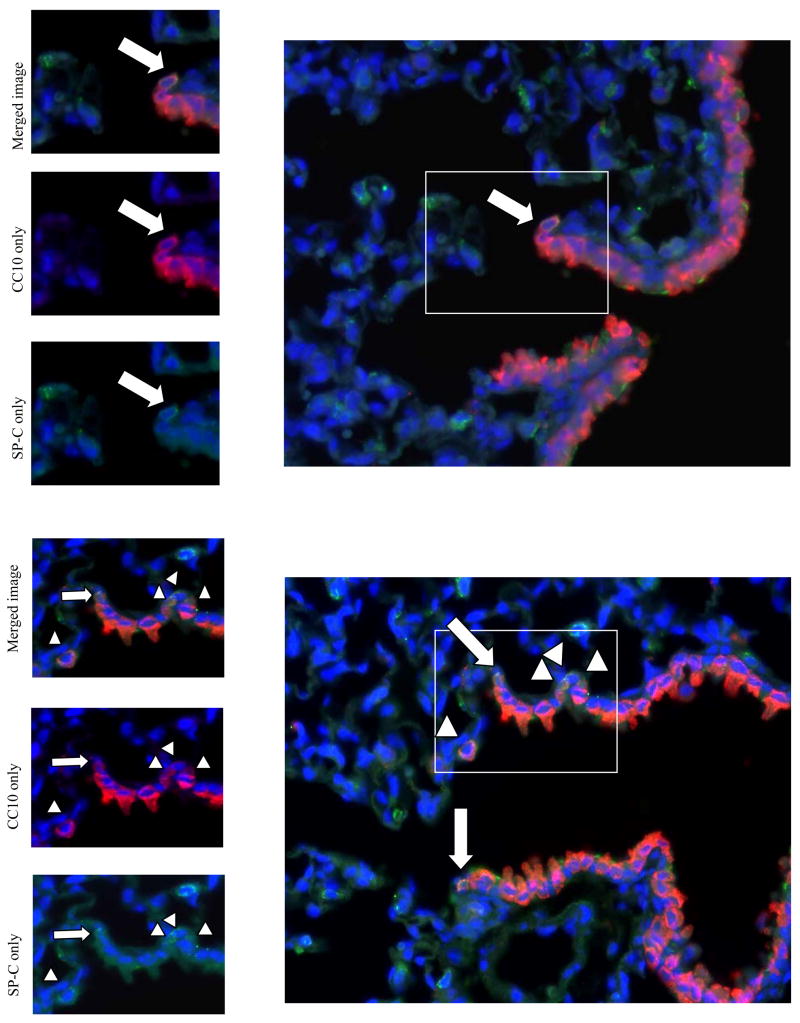
Figure 4.
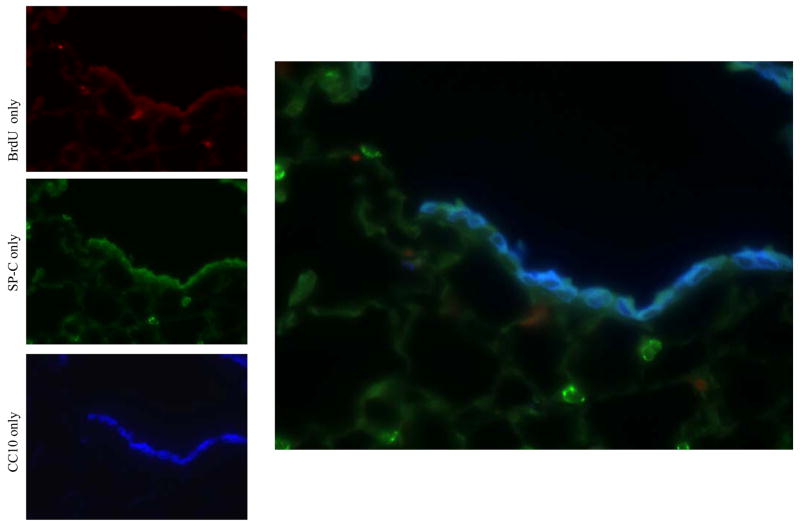
Immunofluorescence analysis of lung cells after pneumonectomy. (Top) Day 3 post-pneumonectomy. 1 normal BASC (arrow) found at the bronchio-alveolar junction. (Bottom) Day 14 post-pneumonectomy. Terminal bronchiole with 6 BASCs. In contrast to controls, BASCs were increased in number at the bronchioalveolar junction (arrows) and found in ectopic locations, including the alveolar space and sub-bronchiolar regions (arrowheads) red=CC10; green=proSP-C ; blue=DAPI.
Proliferation in BASCs, AECII and Clara cells following pneumonectomy
In order to confirm that increases in tissue cell density were associated with increases in cellular proliferation, we examined BrdU uptake at 2 different periods during early regrowth, namely post-operative days 0–3 and 4–7; shams for each group were similarly given BrdU during these periods (Figure 5). Notably, none of the BASCs from the sham-operated group demonstrated BrdU uptake (Figure 5A), revealing that these cells are quiescent in the absence of PNX, but show a significant increase in DNA synthesis during compensatory growth (Figure 5B). There were significant increases in BrdU uptake in PNX versus SHAM in BASCs, Clara cells, and AECIIs over the 7 day period (P=0.001 - 0.003) as well as at both individual time periods (days 0–3 and 4–7; P=0.014 – 0.047) (Figure 5C). The composite proliferative indices in AECII and Clara cells over 7 days were 31% and 12.4% respectively in the pneumonectomy group and 5.2% and 1.6% in the sham-operated group, compared to 41 and 0% in BASC, respectively. In summary, at baseline BASCs were quiescent, while a constant low background level of cell cycling was detected in AECII and Clara cells in sham operated mice. Following pneumonectomy, all three cell types demonstrated significant increases in proliferative index, with BASCs demonstrating the greatest surge in BrdU uptake.
Figure 5.
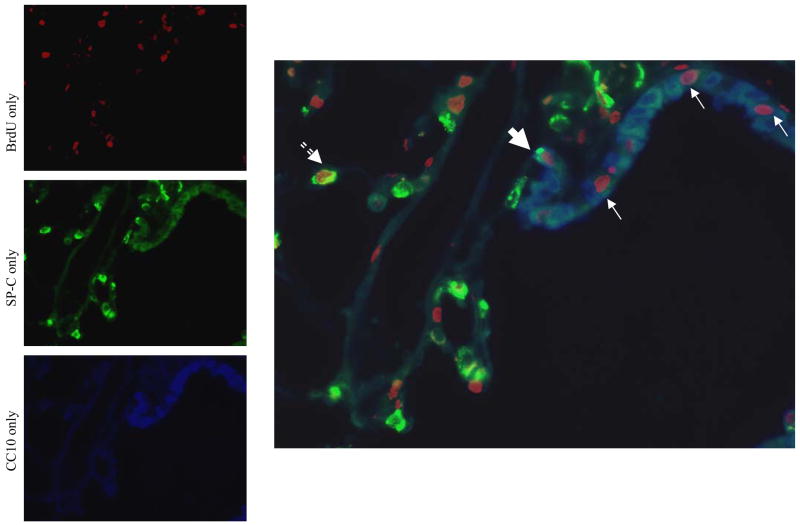
Analysis of BrdU incorporation after pneumonectomy. (A) Photomicrograph (400x mag) of bronchioalveolar duct junction in SHAM operated mouse (day 7) with nuclear BrdU (red), cytoplasmic immunostaining for Clara cells (blue), AECII (punctate green). Note the relative absence of BrdU-positive cells in the airways and parenchyma compared to PNX image; (B) PNX operated mouse (day 7) with a BrdU-positive BASC (large arrow), BrdU-positive Clara cells (small arrow) and a BrdU-positive AECII (dashed arrow). C) The percentage of BASCs, AECII and Clara cells identified by immunofluorescence that incorporated BrdU label administered in drinking water on days 0–3 or 4–7 post-pneumonectomy. Note that BASCs show no BrdU uptake after the sham procedure, but proliferate during lung regrowth. AECII also proliferate after PNX. Clara cells show a muted mitotic response to PNX that is stable between days 0–3 and 4–7. Data are mean values +/− SEM; * indicates P<0.05 between PNX and SHAM.
Cell kinetic model
To assess the potential contribution of BASC and AECII differentiation to alveolar epithelial cell regrowth following pneumonectomy, we used a three-compartment model (Figure 1, Methods and Supplementary Data). First, to test the hypothesis that BASCs were able to repopulate the parenchyma independently of AECII contribution, AECII doubling times were initially set at zero (i.e. could the observed cell numbers and lung volumes be matched with the model in the absence of AECII proliferation). As shown in supplementary figure 2 (panel G), model simulations with 0% contribution from AECII did not fit the data; BASC proliferation and differentiation in the absence of AECII proliferation could not account for the observed kinetics and morphometry.
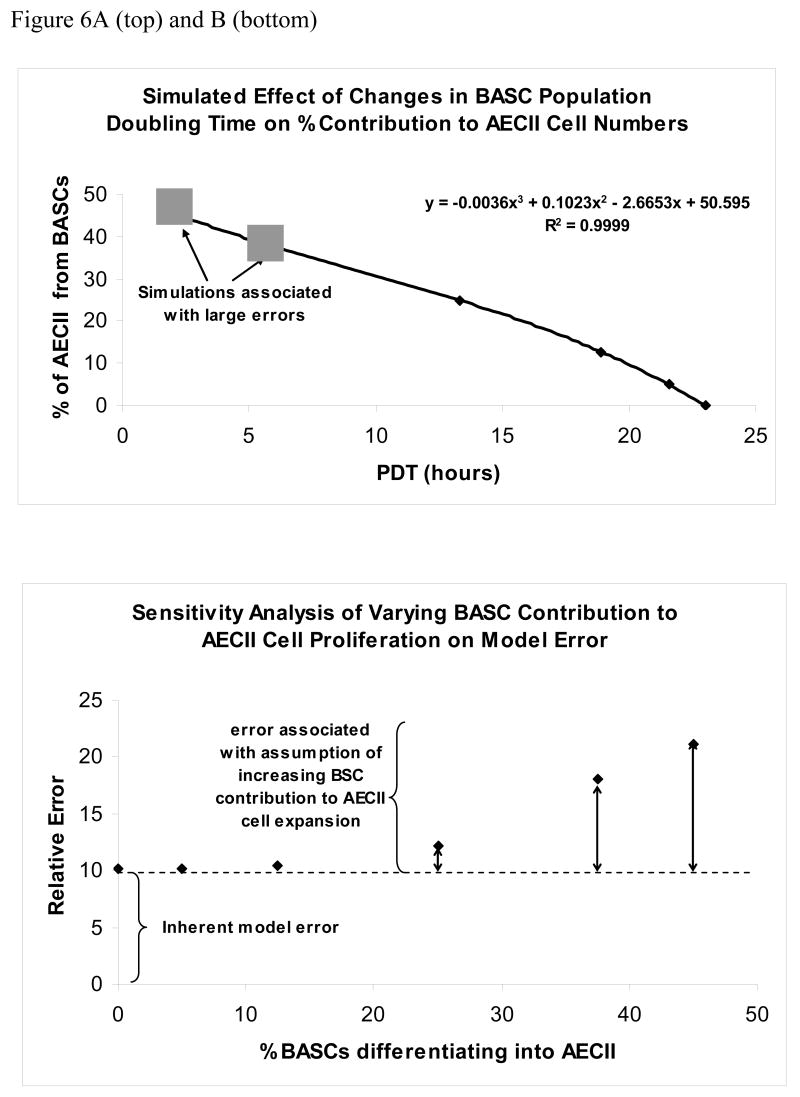
Model predictions closely matched measured cell numbers and lung volumes for simulations in which BASCs contributed between 0 to 25% of ACEII during lung regrowth (supplementary figure 2, panels A-D). Simulations indicate that as percentage differentiation of BASCs into AECII cells increased above this level, the BASC population doubling times required to approximate the results exceeded physiologic limits, i.e. <5hrs (Figure 6A). The model cannot match the data using similar population doubling times for BASCs and AECIIs, indicating that it is very unlikely that population doubling times for BASCS and AECII cells in vivo are in fact the same. Furthermore, simulation errors were stable (around 10%) between 0–12.5% contribution of BASC to AECII, then increased abruptly at ≥25% contribution of BASC to AECII suggesting that model fit deteriorated above this level (Figure 6B). These simulations indicate that the model cannot quantitatively match the morphometry and physiology of post-PNX lung re growth if ≥ 25% of BASCs are required to differentiate into AECII cells during growth.
Figure 6.
The range of PDTs that accommodate 0 to 45% BASC differentiation into AECII cells, and a sensitivity analysis showing the ability of the cell kinetics model to match experimental data over this range of % BASC differentiation into AECII cells are presented. Panel A (top) shows that BASC PDTs of 18 to 23 hours can allow up to a 12.5% differentiation rate of BASCs into AECII cells. Larger % differentiation rates are associated with PDTs that are not likely achievable in vivo. Panel B (bottom) shows modeling errors increase steadily as with simulated increases in the % BASC differentiation into AECII cells, even when high PDTs are used.
Discussion
This study is the first to describe the cellular dynamics of a putative multipotential stem cell during post-natal lung regrowth following pneumonectomy. Our data show that pneumonectomy is a strong stimulus for proliferation of BASCs, which exhibit a temporal response that parallels compensatory lung regrowth, i.e. BASC proliferated during regrowth and declined as growth subsided. The BASC population is quiescent at baseline, and the transition from quiescence (BrdU = 0% BASC in controls) to a high rate of proliferation suggests that BASCs play a role in supporting some aspect of compensatory lung regrowth. This study also confirms that AECII are necessary to compensatory lung regrowth. While AECII are necessary and BASCs alone are insufficient to repopulate the alveoli during regrowth, these data suggest that both populations (BASC and AECII) should be included in studies that address the biologic mechanisms of regrowth.
Pulmonary function testing was performed in concert with Lm measurements to define the pattern of regrowth, discriminate regrowth from alveolar enlargement, and determine how changes in morphology correspond to changes in pulmonary function (4, 20, 22, 30). In this experiment, there was no change in Lm at point after surgery, confirming previous studies in mice at days 4, 8, 10, 12, or 21 post-PNX that changes in lung volumes are associated with an increase in alveolar septation rather than simple alveolar distension (7, 22, 30). According to Fehrenbach (7) compensatory lung regrowth in the mouse is characterized stereologically by an increase in alveolar numbers (neoalveolarization), the bulk of which is established by 6 days after pneumonectomy. Thus regrowth in the mouse can be reasonably defined as a form of tissue ‘regeneration’ akin to the return of functional mass after excision of liver or pancreatic tissue. Vital capacity was chosen as a robust physiological measurement with stringently standardized starting conditions, namely the deflation from a standard airway pressure with careful attention to volume history. In measurements taken the day following PNX, vital capacity decreased by 34%, in agreement with past studies showing the loss of 31–34% lung volume from excision of the left lung in the mouse (4, 30). By day 7, values for vital capacity returned to control values. These data are consistent with previous descriptions of murine compensatory lung growth (4, 22, 30). The mechanism that stimulates compensatory lung regrowth and BASC proliferation was not established in this study, but prior investigations have focused on mechanical stress as the principal factor that initiates lung regeneration (2, 10, 11, 19, 24, 31). The measurements of lung physiology are useful for comparison with cell kinetics measurements, and represent a novel approach to integrate stem cell numbers with functional outcome in vivo.
Compensatory lung regrowth is traditionally thought to be achieved by differentiation of AECII to AECI, and supported further by AECII proliferation. Our model simulations support the potential role of AECII to restore lung surface area through differentiation into AECI. AECII are known to proliferate after lung injury, and it has been hypothesized that this response modulates lung regrowth and repletion of the AECI population (6). We noted an increase in BrdU uptake in AECII during regrowth and increased lung volume. Our quantitative modeling analysis confirms that the observed AECII density was sufficient to account for either all or a large part of alveolar regrowth. For example, an AECII population doubling time of 38 hours was adequate to ensure matching of both the cell numbers and lung volume changes. Based on our previous in vitro studies showing that BASCs are multipotent for AECII and Clara cells, BASCs may play an important role in repopulation of the lung during compensatory lung regrowth. As the contribution of BASCs to AECII repopulation is increased from 0 to 12.5% in the model, AECII doubling times which matched the experimental data increase, but remain physiologic. Together these results are consistent with prior studies demonstrating a critical role for the AECII cell in alveolar compartment re-expansion and healing, and suggest that AECII cells (or a subpopulation of AECII) exists that have reparative progenitor cell function.
The present analysis precludes the definition of the precise contribution of BASCs to postnatal lung regrowth following pneumonectomy. BASCs may be an important modulator of lung regrowth and repair, since they proliferate at appropriate times in relation to lung regrowth. Our observed BASC proliferation, which we propose occurred de novo, could potentially be confused with cells (e.g. Clara cells or AECII) that gain expression of CC10 and/or proSP-C at the bronchioalveolar duct junction as a consequence of pneumonectomy; this study does not formally rule out the possibility that Clara cells or AECII give rise to new BASCs in this setting. Arguing against this possibility, BASCs do not derive from AECII cells in our previous culture studies. In addition, our data do not rule out the possibility that an undefined precursor cell gives rise to BASCs.
Importantly, the fact that BASCs are the first cells to significantly expand in number, are the most highly proliferative subpopulation based upon the fold change in BrdU incorporation compared to sham-treated animals, and continue to proliferate for an extended period suggests that BASCs likely play an important role in lung regrowth. Potential roles for the BASCs that are consistent with our data set include the following: 1) a stem cell that gives rise to a subset of AECII cells which subsequently undergo rapid expansion and/or differentiation to produce AECI cells for regeneration, 2) a stem cell for Clara cells that contributes to regrowth of the small airways during expansion of the alveolar compartment following PNX, and thereby contributing only to local bronchiolar growth rather than distal AECI expansion, 3) a critical modulatory cell that regulates AECII and Clara cell regrowth through paracrine mechanisms, and 4) a synthetic cell that secretes extracellular matrix components to ensure structural integrity of the BADJ. Importantly, these roles for BASCs are not necessarily mutually exclusive, and large numbers of BASC are not required to assume these roles. Given the combination of our cell kinetic measurements and model simulations, our data support a dual compartment model in which both BASCs and AECII proliferate in response to pneumonectomy and AECII act as the major progenitor cell for AECI cells. Future analysis using lineage tagging will better define the quantitative role of BASC vs. AECII in cell regrowth. As additional cell kinetic data becomes available the current model can be used to study this data.
Our current hypothesis places BASCs at the top of a hierarchy (Figure 1) and AECII cells as transit amplifying cells between BASCs and AECI. While there is no data to support the role of Clara cell or AECII to produce BASC, this theory can not be excluded. Several additional limitations of the model exist that may affect our interpretation of the role of BASCs in lung regeneration. The assumption that BASCs represent an independent self-renewing pool, rather than a cell derived from an as yet unidentified precursor may be incorrect, although there is no data to the contrary. Given our earlier findings that BASC cultures give rise to Clara, AECII and AECI cells in culture (12), the assumption in our model that AECI arise only from AECII may underestimate the contribution of BASCs to lung regeneration. Furthermore, differences in how BASC versus AECII density were quantified for entry into the 3 compartment model introduce a potential impediment to comparing the kinetics of both groups. Specifically BASC were measured within an anatomically restricted zone (the BADJ) and the cell density of non-BASC in this region were not considered in the denominator. Hence, BASC per BADJ unlike AECII per nuclei did not represent the relative density of BASC compared to other cells at the BADJ that may have proliferated. However, quantifying the cell density of non-BASC which proliferate along the axis of the airways was beyond the scope of this study. While the measurement of ‘BASC per BADJ’ differed qualitatively from ‘AECII per nuclei’ in this respect, the number of BASC per BADJ should accurately reflect proliferation of this cell at this anatomically restricted site. The measurement of AECII per nuclei on the other hand may underestimate the proliferative potential of AECII since multiple cell types in that compartment were likely proliferating concomitantly (i.e. contributing to a larger denominator). Hence the studies employing BrDU incorporation were used to compliment the interpretations of these data. Finally, in the current model, the death rate of lung cells during rapid regrowth is assumed to be negligible. This assumption, supported by previous data (13) may be incorrect, since previous studies show significant apoptosis of endothelial and epithelial cells after the initial surge of compensatory lung regrowth in mice (23) Further investigation will need to be performed to demonstrate if the return of BASC from a state of proliferation back to quiescence is mediated by differentiation or cell death.
In summary, we found that pneumonectomy caused a transition from quiescence to proliferation that is temporally regulated in lung epithelia. Three cell types were observed to proliferate: 1) the Clara cell; 2) the AECII and 3) the BASC. Cell cycle kinetics for these three cells differed substantially. BASCs expanded in number soon after PNX, had the highest fold proliferative index, and remained in increased numbers out to 14 days. Proliferation of all three cell types was transient, returning to baseline after the completion of lung regrowth. Kinetic modeling and quantification of BASCs and AECII suggests that BASCs and AECII may both contribute to lung regrowth following pneumonectomy in the mouse, with AECII proliferation dominating the proliferative landscape. Specific lineage tracing will be necessary to identify the exact contribution of BASCs and AECII cells to alveoli and airways during lung regeneration.
Supplementary Material
Acknowledgments
This work was supported by NHLBI 072780-02 (E.P.L) and grant 5-UO1-CA84306-06 from the National Institutes of Health and partially by Cancer Center Support (core) grant P30-CA14O51 from the National Cancer Institute (T.J.). The project described was partially supported by Grant Number R01HL090136 from the National Heart, Lung, And Blood Institute (C.F.K.). T.J. is a Howard Hughes Investigator and a Daniel K. Ludwig Scholar. C.F.K. was a Merck Fellow of The Jane Coffin Childs Memorial Fund for Medical Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, the National Heart, Lung, And Blood Institute, or the National Institutes of Health.
References
- 1.Adamson IY, Bowden DH. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest. 1974;30(1):35–42. [PubMed] [Google Scholar]
- 2.Brody JS. Time course of and stimuli to compensatory growth of the lung after pneumonectomy. J Clin Invest. 1975;56(4):897–904. doi: 10.1172/JCI108169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brody JS, Burki R, Kaplan N. Deoxyribonucleic acid synthesis in lung cells during compensatory lung growth after pneumonectomy. Am Rev Respir Dis. 1978;117(2):307–16. doi: 10.1164/arrd.1978.117.2.307. [DOI] [PubMed] [Google Scholar]
- 4.Brown LM, Malkinson AM, Rannels DE, Rannels SR. Compensatory lung growth after partial pneumonectomy enhances lung tumorigenesis induced by 3-methylcholanthrene. Cancer Res. 1999;59(20):5089–92. [PubMed] [Google Scholar]
- 5.Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4(1):87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 6.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Renewal of alveolar epithelium in the rat following exposure to NO2. Am J Pathol. 1973;70(2):175–98. [PMC free article] [PubMed] [Google Scholar]
- 7.Fehrenbach H, Voswinckel R, Michl V, Mehling T, Fehrenbach A, Seeger W, Nyengaard JR. Neoalveolarization contributes to compensatory lung growth following pneumonectomy in mice. Eur Respir J. 2007 doi: 10.1183/09031936.00109407. [DOI] [PubMed] [Google Scholar]
- 8.Halatek T, Opalska B, Rydzynski K, Bernard A. Pulmonary response to methylcyclopentadienyl manganese tricarbonyl treatment in rats: injury and repair evaluation. Histol Histopathol. 2006;21(11):1181–1192. doi: 10.14670/HH-21.1181. [DOI] [PubMed] [Google Scholar]
- 9.Hsia CC. Signals and mechanisms of compensatory lung growth. J Appl Physiol. 2004;97(5):1992–8. doi: 10.1152/japplphysiol.00530.2004. [DOI] [PubMed] [Google Scholar]
- 10.Hsia CC, Wu EY, Wagner E, Weibel ER. Preventing mediastinal shift after pneumonectomy impairs regenerative alveolar tissue growth. Am J Physiol Lung Cell Mol Physiol. 2001;281(5):L1279–87. doi: 10.1152/ajplung.2001.281.5.L1279. [DOI] [PubMed] [Google Scholar]
- 11.Karl HW, Wolpert EB, Rannels DE. Minimizing perioperative hypoxemia does not affect postpneumonectomy lung growth. Am J Physiol. 1988;255(1 Pt 1):E65–9. doi: 10.1152/ajpendo.1988.255.1.E65. [DOI] [PubMed] [Google Scholar]
- 12.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121(6):823–35. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 13.Landesberg LJ, Ramalingam R, Lee K, Rosengart TK, Crystal RG. Upregulation of transcription factors in lung in the early phase of postpneumonectomy lung growth. Am J Physiol Lung Cell Mol Physiol. 2001;281(5):L1138–49. doi: 10.1152/ajplung.2001.281.5.L1138. [DOI] [PubMed] [Google Scholar]
- 14.Laros CD, Westermann CJ. Dilatation, compensatory growth, or both after pneumonectomy during childhood and adolescence. A thirty-year follow-up study. J Thorac Cardiovasc Surg. 1987;93(4):570–6. [PubMed] [Google Scholar]
- 15.Leuwerke SM, Kaza AK, Tribble CG, Kron IL, Laubach VE. Inhibition of compensatory lung growth in endothelial nitric oxide synthase-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2002;282(6):L1272–8. doi: 10.1152/ajplung.00490.2001. [DOI] [PubMed] [Google Scholar]
- 16.Li D, Fernandez LG, Dodd-o J, Langer J, Wang D, Laubach VE. Upregulation of hypoxia-induced mitogenic factor in compensatory lung growth after pneumonectomy. Am J Respir Cell Mol Biol. 2005;32(3):185–91. doi: 10.1165/rcmb.2004-0325OC. [DOI] [PubMed] [Google Scholar]
- 17.Majka SM, Beutz MA, Hagen M, Izzo AA, Voelkel N, Helm KM. Identification of novel resident pulmonary stem cells: form and function of the lung side population. Stem Cells. 2005;23(8):1073–81. doi: 10.1634/stemcells.2005-0039. [DOI] [PubMed] [Google Scholar]
- 18.Mason RJ, Williams MC. Alveolar Type II cells. In: Crystal RG, West JB, editors. Lung: Scientific Foundations. New York; Raven Press Ltd: 1991. p. 235. chapt. 3.1.9. [Google Scholar]
- 19.Olson LE, Hoffman EA. Lung volumes and distribution of regional air content determined by cine X-ray CT of pneumonectomized rabbits. J Appl Physiol. 1994;76(4):1774–85. doi: 10.1152/jappl.1994.76.4.1774. [DOI] [PubMed] [Google Scholar]
- 20.Rannels DE, Rannels SR. Compensatory growth of the lung following partial pneumonectomy. Exp Lung Res. 1988;14(2):157–82. doi: 10.3109/01902148809115122. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds SD, Giangreco A, Power JH, Stripp BR. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol. 2000;156(1):269–78. doi: 10.1016/S0002-9440(10)64727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakurai MK, Greene AK, Wilson J, Fauza D, Puder M. Pneumonectomy in the mouse: technique and perioperative management. J Invest Surg. 2005;18(4):201–5. doi: 10.1080/08941930591004485. [DOI] [PubMed] [Google Scholar]
- 23.Sakurai MK, Lee S, Arsenault DA, Nose V, Wilson JM, Heymach JV, Puder M. Vascular Endothelial Growth Factor (VEGF) Accelerates Compensatory Lung Growth After Unilateral Pneumonectomy. Am J Physiol Lung Cell Mol Physiol. 2006 doi: 10.1152/ajplung.00064.2006. [DOI] [PubMed] [Google Scholar]
- 24.Sekhon HS, Smith C, Thurlbeck WM. Effect of hypoxia and hyperoxia on postpneumonectomy compensatory lung growth. Exp Lung Res. 1993;19(5):519–32. doi: 10.3109/01902149309031725. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro SS, Wilks MB. An analysis of variance test for normality. Biometrika. 1965;52(3–4):591–611. [Google Scholar]
- 26.Summer R, Fitzsimmons K, Dwyer D, Murphy J, Fine A. Isolation of an Adult Mouse Lung Mesenchymal Progenitor Cell Population. Am J Respir Cell Mol Biol. 2007 doi: 10.1165/rcmb.2006-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Summer R, Kotton DN, Sun X, Ma B, Fitzsimmons K, Fine A. Side population cells and Bcrpl expression in lung. Am J Physiol Lung Cell Mol Physiol. 2003;285(1):L97–104. doi: 10.1152/ajplung.00009.2003. [DOI] [PubMed] [Google Scholar]
- 28.Thurlbeck WM. Measurement of pulmonary emphysema. Am Rev Respir Dis. 1967;95(5):752–764. doi: 10.1164/arrd.1967.95.5.752. [DOI] [PubMed] [Google Scholar]
- 29.Uhal BD. Flow cytometric study of the type II pneumocyte cell cycle in vivo and in vitro. Cytometry. 1994;15(1):46–52. doi: 10.1002/cyto.990150108. [DOI] [PubMed] [Google Scholar]
- 30.Voswinckel R, Motejl V, Fehrenbach A, Wegmann M, Mehling T, Fehrenbach H, Seeger W. Characterisation of post-pneumonectomy lung growth in adult mice. Eur Respir J. 2004;24(4):524–32. doi: 10.1183/09031936.04.10004904. [DOI] [PubMed] [Google Scholar]
- 31.Wu EY, Hsia CC, Estrera AS, Epstein RH, Ramanathan M, L JR., Jr Preventing mediastinal shift after pneumonectomy does not abolish physiological compensation. J Appl Physiol. 2000;89(1):182–91. doi: 10.1152/jappl.2000.89.1.182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.