Abstract
It has long been known that the sympathetic innervation of the sweat glands is cholinergic in most mammalian species, and that during development, rodent sympathetic cholinergic sweat gland innervation transiently expresses noradrenergic traits. We show here that some noradrenergic traits persist in cholinergic sympathetic innervation of the sweat glands in rodents, but that lack of expression of the vesicular monoamine transporter renders these cells functionally non-noradrenergic. Adult human sweat gland innervation, however, is not only cholinergic, but co-expresses all of the proteins required for full noradrenergic function as well, including tyrosine hydroxylase, aromatic amino acid decarboxylase, dopamine ß-hydroxylase, and the vesicular monoamine transporter VMAT2. Thus, cholinergic/noradrenergic co-transmission is apparently a unique feature of the primate autonomic sympathetic nervous system. Furthermore, sympathetic neurons innervating specifically the cutaneous arteriovenous anastomoses (Hoyer Grosser organs) in humans also possess a full cholinergic/noradrenergic co-phenotype. Cholinergic/noradrenergic co-expression is absent from other portions of the human sympathetic nervous system, but is extended in the parasympathetic nervous system to the intrinsic neurons innervating the heart. These observations suggest a mode of autonomic regulation, based on co-release of norepinephrine and acetylcholine at parasympathocardiac, sudomotor, and selected vasomotor neuroeffector junctions, that is unique to the primate peripheral nervous system.
Keywords: co-transmission, vesicular neurotransmitter transporter, sympathetic, parasympathetic, skin nerves, cardiac innervation
Introduction
It is generally assumed that chemical coding of the autonomic nervous system is essentially similar in mammalian species, with exclusively acetylcholine released at post-ganglionic parasympathetic neuroeffector junctions, and exclusively norepinephrine released at post-ganglionic sympathetic neuroeffector junctions. The sole exception seems to be the expression of acetylcholine, but not norepinephrine, in sympathetic sudomotor neurons. A convergence of clinical data in human patients, anatomical and functional observations in primates, and increasingly precise delineation of the plasticity of expression of noradrenergic and cholinergic traits in the rodent, has led us to re-examine these assumptions. Thus, multiple clinical reports exist of noradrenergic sweating in human patients (Wolf and Maibach, 1974; Shields et al., 1987; Manusov and Nadeau, 1989), and examination of the perfused sweat glands of the rhesus macaque skin likewise strongly suggests some noradrenergic control (Sato and Sato, 1981a; Sato and Sato, 1981b; Sato et al., 1989b; Sato et al., 1989a). However, integration of these observations into a coherent mechanism for autonomic control has not occurred, due to a paucity of high-resolution primary neuroanatomical information in the human system, and numerous previous indications in better-studied rodent models, especially the rat, that noradrenergic/cholinergic plasticity in this system does not extend beyond early postnatal development (Landis and Keefe, 1983). The recent demonstration of the persistence of noradrenergic traits in the mouse sudomotor innervation however (Habecker et al., 2000; Habecker et al., 2002), as well as demonstration of transplantation-induced plasticity in rodent systems (Schotzinger et al., 1994), prompted us to examine whether a full complement of noradrenergic together with cholinergic traits might exist in any compartment of the adult mammalian sympathetic or parasympathetic autonomic nervous system, and in particular in the primate nervous system. Confocal microscopy in conjunction with the use of highly specific polyclonal antibodies against both biosynthetic enzymes and isovalent targets for cholinergic and noradrenergic transmission, namely the vesicular amine transporters, allowed us to examine this question at the level of individual cells, nerve fibers, and nerve terminals in the rodent and primate peripheral nervous system.
Materials and Methods
Tissue processing for immunocytochemistry
Fore- and hindpaws of adult male and female Wistar rats (n=6 for each sex) and adult male and female CD1 mice (n=10 for each sex), and various regions (hand, n=6, foot, n=2, axilla, n=7, and head, n=7) of human skin bearing eccrine sweat glands (obtained in accordance with Helsinki Principles, from patients undergoing plastic surgery after informed consent), and post-mortem human heart tissue (n=4) were fixed by immersion in Bouin Hollande or buffered 10% formalin. Rhesus monkey tissues (stellate and upper thoracic ganglia of the paravertebral sympathetic chain and heart) were obtained from buffered saline- followed by buffered 4% formalin-perfused animals (3 male and 3 female), and were immersion post-fixed in Bouin Hollande. Rat and mouse tissues were obtained in accordance with the German Law for animal protection and approved by the Regierungspäsidium Giessen. Experiments involving the use of rhesus macaques were approved by the Animal Care and Use Committee of Bioqual, Inc., an NIH-approved and Association for Assessment and Accreditation of Laboratory Animal Care-accredited research facility. All experiments were carried out using the ethical guidelines promulgated in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All tissues were processed for double immunofluorescence confocal microscopy on deparaffinized sections. Adjacent sections (7 μm thick) were cut and deparaffinized. Antigen retrieval to increase the sensitivity of immunodetection was performed by heating the sections at 92°-95° C for 15 min in 0.01 M citrate buffer (pH 6) according to the DAKO protocol (Hamburg, Germany). Nonspecific binding sites were blocked with 5% bovine serum albumin (BSA, Serva, Heidelberg, Germany) in PBS (pH 7.45) followed by an avidin-biotin blocking step (Avidin-biotin Blocking Kit, Boehringer Ingelheim, Germany).
Antibodies
As listed in Table 1, primary antibodies included rodent and primate-specific rabbit polyclonal antibodies against the vesicular acetycholine transporter (VAChT), a pan-species-specific goat polyclonal antiserum against VAChT, and polyclonal antisera against the vesicular monoamine transporter type 2 (VMAT2) that recognizes rat and primate VMAT2, or mouse VMAT2. These antisera were raised, fully characterized and applied in our laboratories, to characterize monoaminergic and cholinergic neuronal, neuroendocrine and immune phenotypes in rodent and primate species (Erickson et al., 1994; Schäfer et al., 1994; Weihe et al., 1994; Schäfer et al., 1995; Erickson et al., 1996; Weihe et al., 1996; Schäfer et al., 1997; Schäfer et al., 1998a; Schäfer et al., 1998b; Schütz et al., 1998; Eissele et al., 1999; Weihe and Eiden, 2000; Anlauf et al., 2003a; Anlauf et al., 2003b; Schütz et al., 2003; Anlauf et al., 2004; Eiden et al., 2004). They are available from Phoenix Pharmaceuticals, Belmont CA. In addition to these antisera, well-characterized commercially available rabbit and sheep polyclonal antibodies against tyrosine hydroxylase (TH), aromatic amino acid decarboxylase (AADC) also referred to as dopa-decarboxylase (DDC), and dopamine ß-hydroxylase (DßH) were used as listed in Table 1.
Table 1.
| Antigen/Species | Lab Code | Commercial Source/Code | Reference for IHC10 | Dilution | Donor Species |
|---|---|---|---|---|---|
| 1VAChT (human) | 80153 | Phoenix/ H-V005(2/95bleed) | Schäfer et al. 1995 | 1:600 | rabbit polyclonal |
| 2VAChT (common) | 1624 | Phoenix/ H-V007(bleed 12/99) | this report | 1:600 | goat polyclonal |
| 3VAChT (rat, mouse) | 80259 | Phoenix/ H-V006(2/95bleed) | Weihe et al. 1996 | 1:300 | rabbit polyclonal |
| 4VMAT2 (rat, human) | 80182 | Phoenix/ H-V003(finalbleed) | Erickson et al. 1996 | 1.200 | rabbit polyclonal |
| 5VMAT2 (rat) | 80214 | Phoenix/ H-V004(bleed 2/95) | Weihe et al 1995 | 1.200 | rabbit polyclonal |
| 6VMAT2 (mouse) | W1-2 | Phoenix/ H-V008(finalbleed) | this report | 1:200 | rabbit polyclonal |
| 7TH (primate, rat, mouse) | Chemicon/ AB1542 | Anlauf et al. 2003 | 1:80 | sheep polyclonal | |
| 8DßH (primate, rat, mouse) | Protos/ CA-301 bDBHrab | this report | 1:200 | sheep polyclonal | |
| 9AADC (primate, rat, mouse) | Chemicon/ AB1569 | this report | 1:200 | rabbit polyclonal |
1-6, Antigen epitope sequence (includes cysteine at N-terminus added when necessary for conjugation to KLH (keyhole limpet hemocyanin) through maleimide):
VAChT (human): CEDDYNYYYTRS
VAChT (common): CTRSRSERDVLLDEPPQGLYDAVRLRE
VAChT (rat, mouse): CEDDYNYYSRS
VMAT2 (rat, human): CTQNNIQSYPIGEDEESESD
VMAT2 (rat): CTQNNVQSYPIGDDEESESD
VMAT2 (mouse): CTQNNVQPYPVGDDEESESD
TH (native tyrosine hydroxylase purified from rat pheochromocytoma)
DßH (native dopamine ß-hydroxylase purified from bovine adrenal medulla)
AADC (recombinant bovine dopa decarboxylase expressed in E. coli and purified from inclusion bodies)
References for use of these antibodies immunohistochemistry (IHC) are to Materials and Methods section of this paper, or to previous reports, describing in detail the methods for use of these antibodies in immunohistochemical detection of antigen in mammalian tissues. For antibodies against TH, DßH, and AADC, the product sheets of the individual suppliers (TH and AADC, lots 24080597 and 23050121 respectively, Chemicon, Temecula, CA; DBH, Protos Biotech, New York; all other antibodies Phoenix Pharmaceuticals, Belmont CA). ‘Final bleed’ is the agreed-upon designation for the single existing bleed used by Weihe and Eiden laboratories and commercially available through Phoenix Pharmaceuticals. This applies to 80182, and W1-2.
Abbreviations: VAChT, vesicular acetycholine transporter; VMAT2, vesicular monoamine transporter type 2; TH, Tyrosine hydroxylase; AADC, aromatic amino acid decarboxylase also referred to as DDC, dopa-decarboxylase; DßH, dopamine ß-hydroxylase.
The specificities of the antisera against VAChT and VMAT2 from rodent and primate species have been well characterized in previous studies including homologous and heterologous preabsorption and the usage of cell lines transfected with species-specific VAChT and VMAT2 cDNAs (Erickson et al., 1994; Schäfer et al., 1994; Weihe et al., 1994; Schäfer et al., 1995; Erickson et al., 1996; Weihe et al., 1996; Schäfer et al., 1997; Schäfer et al., 1998a; Schütz et al., 1998). The specificity of the immunoreactions obtained in the present study using this panel of highly specific antisera was tested by preabsorption with excess appropriate antigens (50 μmol) against which the antisera were raised as detailed in Table 1. All immunoreactions for VAChT and VMAT2 described as specific in the present study have been found to be preabsorbable and were not seen after omission of the respective primary antisera. Omission of the commercially available primary antisera against catecholamine synthetic enzymes resulted in absence of specific immunoreactions. Antigens for preabsorption of these commercial antisera were unavailable to us.
Confocal double-immunofluorescence microscopy
Appropriate combinations of two primary antibodies raised in different donor species were coapplied in appropriate dilutions (Table 1) in 1% BSA/50 mM PBS at pH 7.4 and incubation was carried out overnight at 16 °C followed by 2 h at 37°C. After extensive washing with 1% BSA/PBS over 1 hour, immunoreactions were visualized by appropriate species-specific secondary antibodies labeled with Alexa Fluor® 488, or Alexa Fluor® 647 (both MoBiTec, Göttingen, Germany; dilution 1:200 in 1% BSA/PBS), and by species-specific biotinylated secondary antisera (Dianaova, Hamburg, Germany; diluted 1:200 in 1% BSA/PBS) followed by streptavidin conjugated with Alexa Fluor® 488 or Alexa Fluor® 647 (diluted 1:200 in PBS). Biotinylated or fluorochrome-labeled secondary antibodies and streptavidin conjugated with flurorochromes were applied for 2 hours at 37°C. Sections were extensively washed in PBS followed by distilled water for 1 hour before they were coverslipped with FluorSave™ reagent (Calbiochem, Merck Biosciences, Schwalbach, Germany). Immunofluorescence staining was documented as digitized false-color images (8 bit Tiff format) obtained with an Olympus BX50WI laser scanning microscope (Olympus Optical, Hamburg, Germany) and the Olympus Fluoview 2.1 software. Adobe Photoshop 7.0 was used to compose and label the plates from the respective single Tiff images without manipulations of contrast or brightness.
Results
Cutaneous innervation
The presence of cholinergic sympathetic innervation of sweat glands in the rat has previously been ascribed to a conversion or developmental switch from a fully functional noradrenergic phenotype to a cholinergic phenotype under the influence of sweat gland (target-derived) growth factors (Landis, 1990; Landis, 1994). At least some, if not most cholinergic sympathetic neurons however, possess a cholinergic phenotype long before target innervation (Schäfer et al., 1997; Stanke et al., 2000). Recently, Hiltunen and Airaksinen have demonstrated that neurturin, through its receptor GFRalpha2, mediates cholinergic cell soma size and nerve terminal development, but not phenotype, in the rodent sympathetic nervous system (Hiltunen and Airaksinen, 2004). These observations are consistent with a previous suggestion that the ‘cholinergic switch’ occurs not at the level of the transcriptome, but at the level of transport to the nerve terminal of proteins enabling cholinergic neurotransmission (Weihe and Eiden, 2000). Landis and co-workers have demonstrated in addition that in the mouse, the noradrenergic phenotype persists into adulthood, allowing the possibility of full noradrenergic functionality in cholinergic neurons of the mammalian sympathetic nervous system. However, Habecker et al. (2000) have reported loss of GTP-cyclohydrolase from adult mouse sudomotor innervation, indicating these neurons are unlikely to be functionally noradrenergic. We have therefore examined adult mammalian sweat glands from several species, for the possible expression of the four major protein components of a fully functional noradrenergic neurotransmission phenotype, including tyrosine hydroxylase (TH), aromatic amino acid decarboxylase (AADC), dopamine ß-hydroxylase (DßH), and the vesicular monoamine transporter (VMAT2).
As depicted in Figure 1, TH is abundantly expressed within cholinergic (VAChT-expressing) nerve terminals innervating the sweat glands of the adult mouse forepaw. However, TH expression is unaccompanied by co-expression of the vesicular monoamine transporter VMAT2, although this protein is easily visualized in vascular innervation within the same field, directly adjacent to forepaw sweat gland innervation. Similar examination of the rat forepaw sweat gland innervation revealed a similar pattern, but without expression of either TH or VMAT2 in the adult animal (summarized in Table 2).
Fig. 1. Catecholaminergic traits in mouse cholinergic sweat gland innervation.
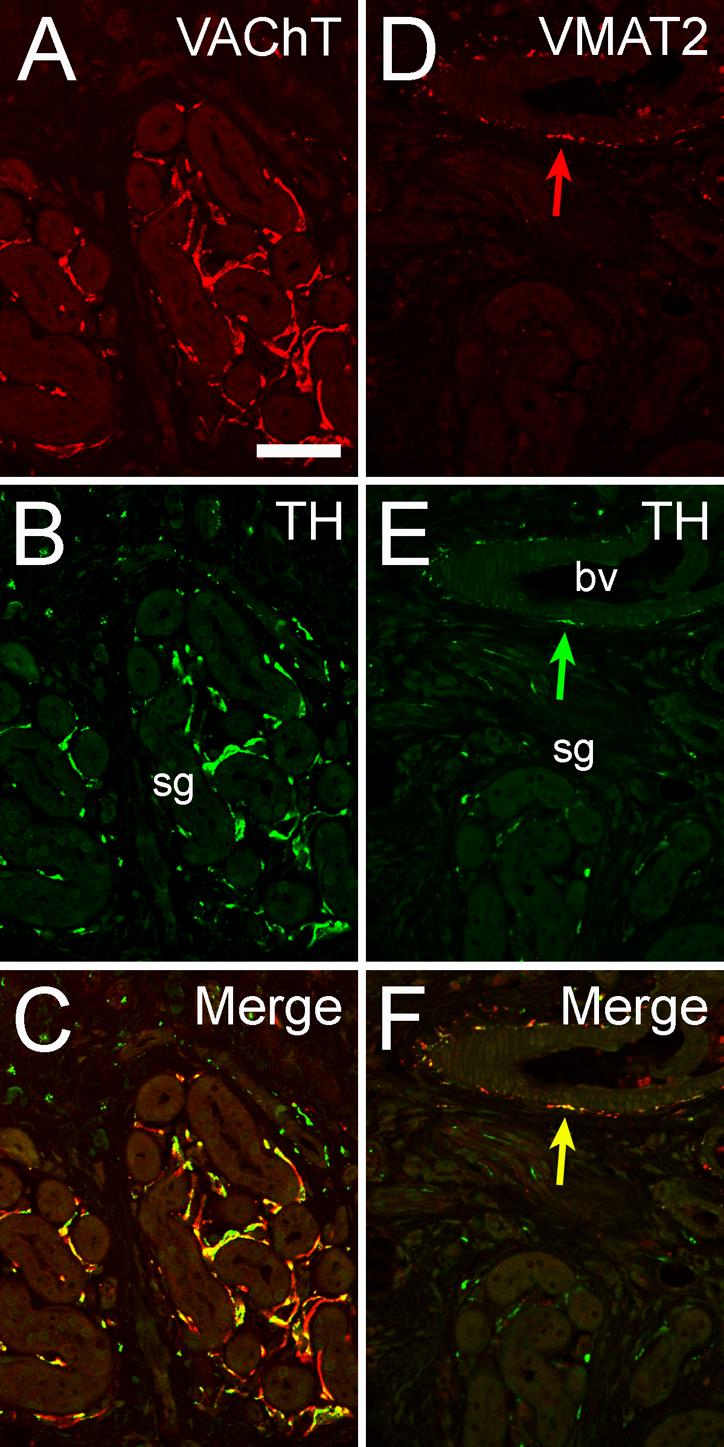
Confocal images of double immunofluorescence illustrate co-staining for VAChT and TH (A-C) in sudomotor nerves targeting mouse eccrine sweat glands (sg). Note presence of VMAT2 in TH-positive fibers (arrow in D-F) supplying blood vessels (bv), but total absence of VMAT2 staining from sweat gland innervation (D). Size bar 50 μm (A-F).
Table 2.
Aminergic and cholinergic co-phenotypes in rodent and human sudomotor innervation
| Rat | Mouse | Human | |
|---|---|---|---|
| TH | − | + | + |
| AADC | + | + | + |
| DßH | + | + | + |
| VMAT2 | − | − | + |
| VAChT | + | + | + |
+, present; −, absent. Abbreviations: TH, tyrosine hydroxylase; AADC, aromatic amino acid decarboxylase; DßH, dopamine ß-hydroxlyase; VMAT2, vesicular monoamine transporter type 2; VAChT, vesicular acetylcholine transporter.
The adult rodent species variability in noradrenergic trait expression prompted us to examine carefully adult primate sweat gland innervation as well. There is a well-documented primate literature indicating that functional noradrenergic innervation of the sweat glands exists, albeit it has previously been interpreted chemoanatomically as a parallel innervation to that by cholinergic sympathetic neurons (Uno and Montagna, 1975; Uno, 1977; Sato and Sato, 1981b; Shields et al., 1987; Sato et al., 1989b; Sato et al., 1989a).
While VMAT2 is absent from the rodent adult cholinergic sweat gland innervation, it is however present in human eccrine sweat gland innervation of both the extremities and the head. As shown in Figure 2, eccrine sweat glands in the human axilla are innervated by TH and VAChT positive nerve terminals (Fig. 2A-C), in which VMAT2 is co-expressed (Figure 2D-F). Note that at the level of resolution obtained in Figure 2, VAChT and TH immunoreactivity (compare Figure 2A, 2B, 2C), although contained in the same fibers, is partially spatially resolved due to the presence of TH in cytoplasm and VAChT in small synaptic vesicles in adjacent regions of co-immunopositive fibers. The observation that TH positive fibers occasionally lack VAChT (Fig. 2C) is due to the presence of TH in terminal varicose fibers, intervaricose segments and upstream to terminal arborizations while VAChT is concentrated in terminal varicose compartments. Some of the TH+ but VAChT negative fibers seen in Fig 2C (labeled by arrow head) can represent tangentionally sectioned purely noradrenergic vascular innervation. These terminals also express AADC and DßH, i.e. all of the biosynthetic and transport proteins required for full noradrenergic function (Figure 2G-L). Cholinergic and noradrenergic co-expression was also observed in arteriovenous anastomoses (Hoyer-Grosser organs) of the human skin (Fig. 3), a special region of the vasculature of digital, palmar and plantar skin intimately involved, like the eccrine sweat glands, in thermoregulation. We confirmed using primate stellate ganglion tissue that TH and VAChT co-expression occurs in a corresponding population of principal ganglion cells (Fig. 4), i.e. from the sympathetic paravertebral chain ganglia, at the level that supply upper extremities in which sweat glands (axillary region) and AV anastomoses exhibit cholinergic/catecholaminergic co-phenotypes. In fact co-existence of VAChT and TH was not only seen in stellate but also in the adjacent upper thoracic sympathetic ganglia that also project to extremities. Unlike in rodent, where VAChT-positive, VMAT2-negative, as well as TH-positive, VMAT2-negative cell bodies can be seen, our unpublished observations based on nickle-enhanced immunostaining indicate that all TH positive postganglionic sympathetic neurons in paravertebral ganglia are positive for VMAT2 as well, implying that the TH/VAChT co-existence also extends to VMAT2/VAChT co-existence.
Fig 2. Catecholaminergic and cholinergic co-phenotypes in nerves supplying eccrine sweat glands of human axilla.

Confocal images of double immunofluorescence illustrate co-staining for VAChT and TH (A-C), for VAChT and VMAT2 (D-F), for TH and AADC (G-I), and for TH and DßH (J-L) in sudomotor nerves targeting eccrine sweat glands. Note that the majority of nerve fibers co-stain for TH and VAChT (arrow in C), but that some nerve fibers that stain for TH lack VAChT (arrow head in C). Note VMAT2 positive mast cells (arrows in E). Note that VAChT and TH immunoreactivity although contained in the same fibers (A, B), is partially spatially resolved due to the presence of TH in cytoplasm and VAChT in small synaptic vesicles in adjacent regions of co-immunopositive fibers. Size bar 50 μm (A-L).
Fig. 3. Catecholaminergic and cholinergic co-phenotypes in nerves supplying human arteriovenous anastomoses.

Confocal images of double immunofluorescence illustrate co-expression of VAChT and VMAT2 (A-C) and of TH and VAChT (D-F) in vasomotor nerves targeting an AV anastomosis of a digit, shown on two consecutive sections. Size bar 50 μm (A-F).
Fig. 4. Co-localization of VAChT and TH in principal ganglion cells in rhesus monkey stellate ganglion.

Cell bodies of postganglionic sympathetic neurons co-staining for VAChT and TH are labelled by arrows. Note VAChT-positive pre-ganglionic terminals negative for TH. Size bar 50 μm.
Cardiac innervation
Cholinergic/noradrenergic co-expression, demonstrated by co-existence of TH or VMAT2 with VAChT, was also observed in a major subpopulation (40-50%) of intrinsic cardiac ganglion cells of the rhesus monkey (Fig. 5) and human heart, extending both to atrial and ventricular nerve endings (Fig 5G-I). These intrisic cardiac neurons and terminals also expressed AADC and DßH (data not shown). This demonstrates the presence of all noradrenergic proteins required for full noradrenergic functionality in these intrinsic cardiac cholinergic neurons, in addition to the extrinsic noradrenergic sympathetic innervation of the primate heart. Staining for VAChT, as a marker for expression from the cholinergic gene locus required for cholinergic function, not only establishes the duality of neurotransmitter expression in parasympathetic ganglia, but that markers for both cholinergic and noradrenergic functional neurotransmission are co-localized at the various cardiac neuroeffector junctions throughout the heart. In contrast, mouse intrinsic cardiac neurons, albeit expressing TH in about 40-50 % of the VAChT positive intrinsic cardiac ganglionic cells, totally lack VMAT2 expression (Fig. 7) and therefore, cannot exert full noradrenergic function.
Fig. 5. Catecholaminergic and cholinergic co-phenotypes in primate intrinsic cardiac innervation.

Confocal images of double immunofluorescence illustrate that TH (B, C) and VMAT2 (E, F) are present in a major subpopulation of the VAChT-positive neuronal cell bodies of an intracardiac parasympathetic ganglion. Note adjacent TH-positive, VAChT-negative extrinsic catecholaminergic nerve fibers (asterisks in A and B). A subpopulation of TH-positive catecholaminergic nerves in the left ventricle also stains for VAChT (arrows in G, H, I). Note discrepancies between varicose fiber space staining for VAChT and TH in G-I which are due to the pan-cytoplasmic varicose and non-varicose localization of the enzyme TH in contrast to the concentration of VAChT to areas of the nerve fiber with local enrichment of cholinergic small clear vesicles. Arrow heads in G-I mark TH+ but VAChT negative fiber. Size bars 50 μm (A-C; D-I).
Fig. 7. Catecholaminergic traits in cholinergic ganglionic cells of intrinsic mouse cardiac ganglia.
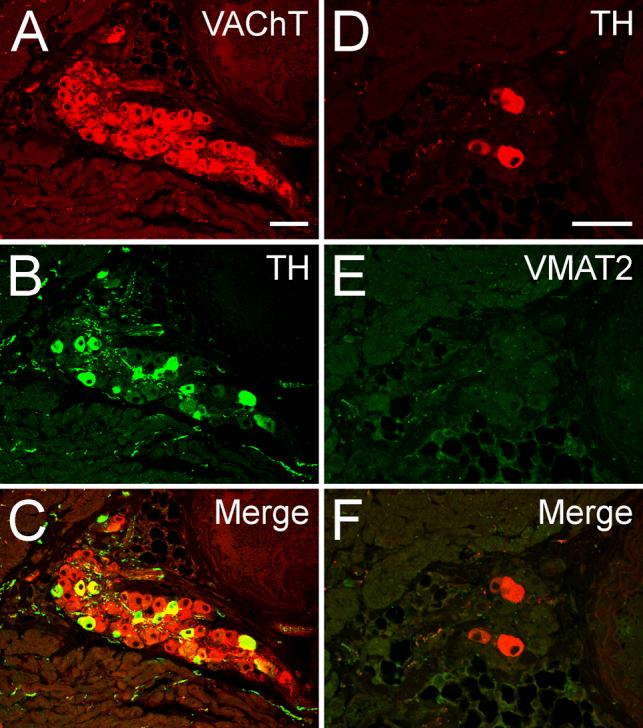
Confocal images of double immunofluorescence illustrate that TH is present in a major subpopulation of the VAChT positive neuronal cell bodies (A-C). Note that expression of TH is not accompanied by expression of VMAT2 (D-F). Size bars 50 μm.
Discussion
Co-transmission of classical and non-classical neurotransmitters has important pharmacological and functional implications for mammalian neurotransmission (Lundberg and Hokfelt, 1986). Our observations establish for the first time the neurochemical basis for functional cholinergic and noradrenergic neurotransmission in mammalian neurons. These results imply that co-transmission of the two principal classical transmitters acetylcholine and norepinephrine, historically considered to be expressed in a mutually exclusive manner (Dale, 1934), may occur at selected sites, as originally postulated for the sweat gland innervation of some mammals based on pharmacological and physiological considerations (Burnstock, 1978). The possibility for functional co-transmission appears to be limited to a few specialized targets. All other areas of the human autonomic nervous system we have examined so far with the same antisera and under the same conditions, including in greatest detail the enteric nervous system (Anlauf et al., 2003b), appear to express noradrenergic and cholinergic traits in a classical, mutually exclusive fashion (data not shown).
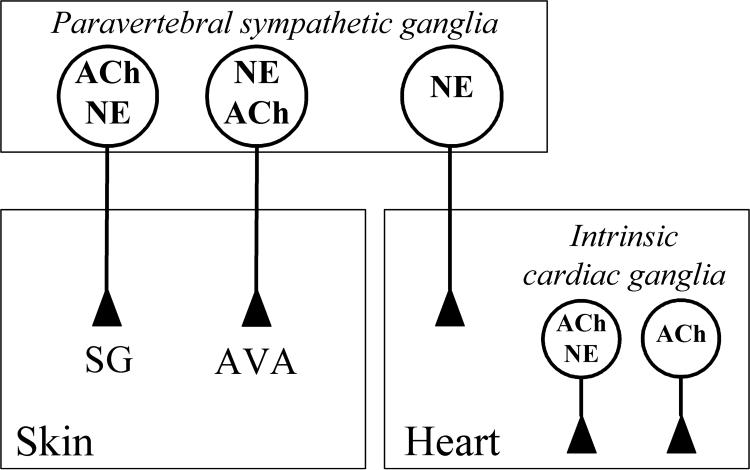
Figure 6 depicts the three locations at which cholinergic/noradrenergic co-transmission might occur: sympathetic innervation of sweat glands, sympathetic innervation of cutaneous arteriovenous anastomoses (Hoyer-Grosser organs), and intrinsic parasympathetic innervation of the heart. These findings should afford a more complete interpretation of the physiology and pharmacology of human autonomic function than available previously, and point to a physiological requirement specific to primates for fine-tuning of chemically-coded neurotransmission at these sites.
Fig. 6. Schematic diagram summarizing the concept of noradrenergic/cholinergic co-phenotypes in the human autonomic nervous system revealed here.
Postganglionic cholinergic sympathetic neurons of the paravertebral sympathetic chain innervating sweat glands (SG) are also noradrenergic (NE). Noradrenergic postganglionic sympathetic vasomotor innervation of cutaneous arteriovenous anastomoses (AVA) is also cholinergic (ACh). Other cutaneous vasomotor nerves are essentially noradrenergic (not shown). A subpopulation of 40-50% of cholinergic neurons of intrinsic (parasympathetic) cardiac ganglia supplying atria and ventricles are also noradrenergic (NE). Thus, the heart receives two populations of noradrenergic innervation, an intrinsic mixed cholinergic/noradrenergic innervation from parasympathetic cardiac ganglia and an extrinsic noradrenergic innervation from the paravertebral sympathtetic ganglia. Preganglionic cholinergic sympathetic and parasympathetic input is not shown.
For example, the phenomenon of ‘noradrenergic sweating’ in humans, with alpha-adrenergic blockade of both eccrine and apocrine sweating in hyperhidrosis, full secretory responses to both cholinergic and catecholaminergic stimulation in isolated primate sweat glands, and visualization of catecholamine fluorescence in the vicinity of sweat glands in the skin of the rhesus macaque, has been known for decades (Sato and Sato, 1981b; Sato et al., 1989b; Sato et al., 1989a). The chemical coding of primate sweat gland innervation has thus been a subject of interest and controversy for as long, and noradrenergic effects have been previously ascribed as humoral effects and/or due to the release of catecholamines from sparse exclusively catecholaminergic innervation in the neighborhood of cholinergic sudomotor neuroeffector junctions (Shields et al., 1987), and references therein. As we now report here, using confocal microscopy and antibodies for human VAChT and the full panoply of biosynthetic enzymes and the vesicular transporter for norepinephrine as unequivocal markers for functional cholinergic and noradrenergic nerve terminals in situ, the eccrine sweat glands of human skin possess a single type of sympathetic innervation which is both cholinergic and noradrenergic. Noradrenergic sweating, including so-called nonthermogenic or psychogenic sweating, may now be regarded in terms of coordinated differential catecholamine and acetylcholine secretion at both sudomotor and vasomotor neuroeffector junctions in the skin under sympathetic control.
Noradrenergic and cholinergic regulation of heart rate and force of contraction, as well as the regulation of the coronary circulation, might similarly be under the control of the intrinsic parasympathetic innervation of the heart, in concert with the heart's noradrenergic/non-cholinergic extrinsic sympathetic innervation. It remains to be established whether adrenoceptors associated with post-ganglionic parasympathetic neuroeffector junctions are pre- or post-synaptic, and thus whether norepinephrine functions principally as a neurotransmitter, as is apparently the case at the sweat gland, or as a neuromodulator governing the duration and intensity of cholinergic neurotransmission at these junctions. In any event, the potential effects of norepinephrine and acetylcholine co-release at the same neuroeffector junction must now be integrated into a complete clinical understanding of the effects of cholinergic and noradrenergic agents, and changes in vagal and sympathetic tone, especially on human ventricular function in vivo (Freis, 1989).
Our observations should prompt a comprehensive re-interpretation of human autonomic pharmacology and therapeutics, and human dysautonomic disease and its treatment. These several examples of classical neurotransmitter co-expression in the adult human autonomic nervous system are likely to reflect widespread functional chemical co-transmission homeostatically regulated not only by the cholinergic/noradrenergic balance inherent in target innervation by anatomically distinct cholinergic and noradrenergic innervation, but by the local fine-tuning of cholinergic/noradrenergic balance within neuroanatomically identical nerve terminals in heart and skin. Recently, rapid reciprocal induction of co-expression of cholinergic and noradrenergic transmission phenotypes in rodent neonatal neurons co-cultured with cardiac myocytes under neurotrophin control has been reported (Yang et al., 2002). Thus, modulation of autonomic function by reciprocal regulation of acetylcholine and norepinephrine release from anatomically distinct neuronal projections, may be importantly augmented by reciprocal regulation of the release of these two classical neurotransmitters from the same nerve terminal under different physiological conditions.
Dysautonomic and thermodysregulatory syndromes, cardiovascular effects of ganglionic blocking agents, and local and systemic effects of noradrenergic blocking agents and agonists in hyperhidrotic and anhidrotic syndromes may all yield to better understanding based on this new neuroanatomical information. In particular, clinical conditions characterized by sudomotor and/or vasomotor dysfunction such as familial and diabetic dysautonomia, thermodysregulatory syndromes, complex regional pain syndromes, Parkinson's disease and hyperhidrosis may be approachable by selective cholinergic and adrenergic drugs, or agents that may regulate cholinergic/noradrenergic balance in sweat gland and AVA nerve terminals, applied locally to the skin (Manusov and Nadeau, 1989). Likewise, cholinergic/adrenergic co-transmission in intrinsic cardiac innervation may play as yet unrecognized roles in the balanced neuronal regulation of cardiac blood flow, pacemaker activity and cardiac force with possible relevance for coronary heart disease, arrhythmia, post-infarct syndromes, and heart transplantation. Further understanding of cardiac pathogenesis and therapy may also be accompanied by better interpretation of cardiac imaging involving either VAChT or VMAT2-dependent specific radioligand uptake.
Acknowledgements
We thank Marion Zibuschka, Petra Sack, Petra Lattermann, Heidemarie Schneider, Michael Schneider, and Elke-Rodenberg-Frank for technical assistance.
*Supported by a grant of the Volkswagen-Stiftung (VW-I-75184) to EW and LEE.
Footnotes
Associate Editor: Paul E. Sawchenko
Literature cited
- Anlauf M, Eissele R, Schafer MK, Eiden LE, Arnold R, Pauser U, Kloppel G, Weihe E. Expression of the two isoforms of the vesicular monoamine transporter (VMAT1 and VMAT2) in the endocrine pancreas and pancreatic endocrine tumors. J. Histochem. Cytochem. 2003a;51:1027–1040. doi: 10.1177/002215540305100806. [DOI] [PubMed] [Google Scholar]
- Anlauf M, Schafer MK, Depboylu C, Hartschuh W, Eiden LE, Kloppel G, Weihe E. The vesicular monoamine transporter 2 (VMAT2) is expressed by normal and tumor cutaneous mast cells and Langerhans cells of the skin but is absent from Langerhans cell histiocytosis. J. Histochem. Cytochem. 2004;52:779–788. doi: 10.1369/jhc.4A6264.2004. [DOI] [PubMed] [Google Scholar]
- Anlauf M, Schäfer MK-H, Eiden LE, Weihe E. Chemical coding of the human gastrointestinal nervous system: Cholinergic, VIPergic and catecholaminergic phenotypes. J. Comp. Neurol. 2003b;459:90–111. doi: 10.1002/cne.10599. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Do some sympathetic neurones synthesize and release both nordrenaline and acetylcholine? Prog. Neurobiol. 1978;11:205–222. doi: 10.1016/0301-0082(78)90013-8. [DOI] [PubMed] [Google Scholar]
- Dale HH. Chemical transmission of the effects of nerve impulses. Br. Med. J. 1934;1:835–841. doi: 10.1136/bmj.1.3827.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden LE, Schafer MK, Weihe E, Schutz B. The vesicular amine transporter family (SLC18): amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine. Pflugers Arch. 2004;447:636–640. doi: 10.1007/s00424-003-1100-5. [DOI] [PubMed] [Google Scholar]
- Eissele R, Anlauf M, Schäfer MK-H, Eiden LE, Arnold R, Weihe E. Expression of vesicular monoamine transporters in endocrine hyperplasia and endocrine tumors of the oxyntic stomach. Digestion. 1999;60:428–439. doi: 10.1159/000007688. [DOI] [PubMed] [Google Scholar]
- Erickson JD, Schäfer MK-H, Bonner TI, Eiden LE, Weihe E. Distinct pharmacological properties and distribution in neurons and endocrine cells of two isoforms of the human vesicular monoamine transporter. Proc. Natl. Acad. Sci. USA. 1996;93:5166–5171. doi: 10.1073/pnas.93.10.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JD, Varoqui H, Schäfer M, Diebler M-F, Weihe E, Modi W, Rand J, Eiden LE, Bonner TI, Usdin T. Functional characterization of the mammalian vesicular acetylcholine transporter and its expression from a ‘cholinergic’ gene locus. J. Biol. Chem. 1994;269:21929–21932. [PubMed] [Google Scholar]
- Freis ED. Hexamethonium, a forgotten drug in relation to “new” concepts in the management of heart failure. Am. Heart J. 1989;118:426–427. doi: 10.1016/0002-8703(89)90213-5. [DOI] [PubMed] [Google Scholar]
- Habecker BA, Klein MG, Cox BC, Packard BA. Norepinephrine transporter expression in cholinergic sympathetic neurons: Differential regulation of membrane and vesicular transporters. Dev. Biol. 2000;220:85–96. doi: 10.1006/dbio.2000.9631. [DOI] [PubMed] [Google Scholar]
- Habecker BA, Klein MG, Sundgren NC, Li W, Woodward WR. Developmental regulation of neurotransmitter phenotype through tetrahydrobiopterin. J. Neurosci. 2002;22:9445–9452. doi: 10.1523/JNEUROSCI.22-21-09445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltunen PH, Airaksinen MS. Sympathetic cholinergic target innervation requires GDNF family receptor GFR alpha 2. Mol. Cell. Neurosci. 2004;26:450–457. doi: 10.1016/j.mcn.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Landis SC. Target regulation of neurotransmitter phenotype. TINS. 1990;13:344–350. doi: 10.1016/0166-2236(90)90147-3. [DOI] [PubMed] [Google Scholar]
- Landis SC. Development of sympathetic neurons: neurotransmitter plasticity and differentiation factors. Prog. Brain Res. 1994;100:19–23. doi: 10.1016/s0079-6123(08)60763-3. [DOI] [PubMed] [Google Scholar]
- Landis SC, Keefe D. Evidence for neurotransmitter plasticity in vivo: Developmental changes in the properties of cholinergic sympathetic neurons. Dev. Biol. 1983;98:349–372. doi: 10.1016/0012-1606(83)90365-2. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Hokfelt T. Multiple co-existence of peptides and classical transmitters in peripheral autonomic and sensory neurons--functional and pharmacological implications. Prog. Brain Res. 1986;68:241–262. doi: 10.1016/s0079-6123(08)60242-3. [DOI] [PubMed] [Google Scholar]
- Manusov EG, Nadeau MT. Hyperhidrosis: a management dilemma. J. Fam. Pract. 1989;28:412–415. [PubMed] [Google Scholar]
- Sato K, Kang WH, Saga K, Sato KT. Biology of sweat glands and their disorders. I. Normal sweat gland function. J. Am. Acad. Dermatol. 1989a;20:537–563. doi: 10.1016/s0190-9622(89)70063-3. [DOI] [PubMed] [Google Scholar]
- Sato K, Kang WH, Saga K, Sato KT. Biology of sweat glands and their disorders. II. Disorders of sweat gland function. J. Am. Acad. Dermatol. 1989b;20:713–726. doi: 10.1016/s0190-9622(89)70081-5. [DOI] [PubMed] [Google Scholar]
- Sato K, Sato F. Cyclic AMP accumulation in the beta adrenergic mechanism of eccrine sweat secretion. Pflugers Arch. 1981a;390:49–53. doi: 10.1007/BF00582710. [DOI] [PubMed] [Google Scholar]
- Sato K, Sato F. Pharmacologic responsiveness of isolated single eccrine sweat glands. Am. J. Physiol. 1981b;240:R44–51. doi: 10.1152/ajpregu.1981.240.1.R44. [DOI] [PubMed] [Google Scholar]
- Schäfer MK-H, Eiden LE, Weihe E. Cholinergic neurons and terminal fields revealed by immunohistochemistry for VAChT, the vesicular acetylcholine transporter I. Central nervous system. Neuroscience. 1998a;84:331–359. doi: 10.1016/s0306-4522(97)00516-2. [DOI] [PubMed] [Google Scholar]
- Schäfer MK-H, Eiden LE, Weihe E. Cholinergic neurons and terminal fields revealed by immunohistochemistry for VAChT, the vesicular acetylcholine transporter II. Peripheral nervous system. Neuroscience. 1998b;84:361–376. doi: 10.1016/s0306-4522(97)80196-0. [DOI] [PubMed] [Google Scholar]
- Schäfer MK-H, Schütz B, Weihe E, Eiden LE. Target-independent cholinergic differentiation in the rat sympathetic nervous system. Proc. Natl. Acad. Sci. USA. 1997;94:4149–4154. doi: 10.1073/pnas.94.8.4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer MK-H, Weihe E, Erickson JD, Eiden LE. Human and monkey cholinergic neurons visualized in paraffin-embedded tissues by immunoreactivity for VAChT, the vesicular acetylcholine transporter. J. Mol. Neurosci. 1995;6:225–235. doi: 10.1007/BF02736782. [DOI] [PubMed] [Google Scholar]
- Schäfer MK-H, Weihe E, Varoqui H, Eiden LE, Erickson JD. Distribution of the vesicular acetylcholine transporter (VAChT) in the central and peripheral nervous systems of the rat. J. Mol. Neurosci. 1994;5:1–18. doi: 10.1007/BF02736691. [DOI] [PubMed] [Google Scholar]
- Schotzinger R, Yin X, Landis S. Target determination of neurotransmitter phenotypes in sympathetic neurons. J. Neurobiol. 1994;25:620–639. doi: 10.1002/neu.480250605. [DOI] [PubMed] [Google Scholar]
- Schütz B, Damadzic R, Weihe E, Eiden LE. Identification of a region from the human cholinergic gene locus that targets expression of the vesicular acetylcholine transporter to a subset of neurons in the medial habenular nucleus in transgenic mice. J Neurochem. 2003;87:1174–1183. doi: 10.1046/j.1471-4159.2003.02095.x. [DOI] [PubMed] [Google Scholar]
- Schütz B, Schäfer MK-H, Eiden LE, Weihe E. Vesicular amine transporter expression and isoform selection in developing brain, peripheral nervous system and gut. Dev. Brain Res. 1998;106:181–204. doi: 10.1016/s0165-3806(97)00196-x. [DOI] [PubMed] [Google Scholar]
- Shields SA, MacDowell KA, Fairchild SB, Campbell ML. Is mediation of sweating cholinergic, adrenergic, or both-A comment on the literature. Psychophysiology. 1987;24:312–319. doi: 10.1111/j.1469-8986.1987.tb00301.x. [DOI] [PubMed] [Google Scholar]
- Stanke M, Geissen M, Götz R, Ernsberger U, Rohrer H. The early expression of VAChT and VIP in mouse sympathetic ganglia is not induced by cytokines acting through LIFRß or CNTFRα. Mech. Devel. 2000;91:91–96. doi: 10.1016/s0925-4773(99)00275-0. [DOI] [PubMed] [Google Scholar]
- Uno H. Sympathetic innervation of the sweat glands and piloarrector muscles of macaques and human beings. J Invest Dermatol. 1977;69:112–120. doi: 10.1111/1523-1747.ep12497915. [DOI] [PubMed] [Google Scholar]
- Uno H, Montagna W. Catecholamine-containing nerve terminals of eccrine sweat glands of macaques. Cell Tiss. Res. 1975;158:1–13. doi: 10.1007/BF00219948. [DOI] [PubMed] [Google Scholar]
- Weihe E, Eiden LE. Chemical neuroanatomy of the vesicular amine transporters. FASEB J. 2000;14:2435–2449. doi: 10.1096/fj.00-0202rev. [DOI] [PubMed] [Google Scholar]
- Weihe E, Schäfer MK-H, Erickson JD, Eiden LE. Localization of vesicular monoamine transporter isoforms (VMAT1 and VMAT2) to endocrine cells and neurons in rat. J. Mol. Neurosci. 1994;5:149–164. doi: 10.1007/BF02736730. [DOI] [PubMed] [Google Scholar]
- Weihe E, Tao-Cheng J-H, Schäfer MK-H, Erickson JD, Eiden LE. Visualization of the vesicular acetylcholine transporter in cholinergic nerve terminals and its targeting to a specific population of small synaptic vesicles. Proc. Natl. Acad. Sci. USA. 1996;93:3547–3552. doi: 10.1073/pnas.93.8.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf JE, Maibach HI. Palmar eccrine sweating--the role of adrenergic and cholinergic mediators. Br. J. Dermatol. 1974;91:439–446. doi: 10.1111/j.1365-2133.1974.tb13084.x. [DOI] [PubMed] [Google Scholar]
- Yang B, Slonimsky JD, Birren SJ. A rapid switch in sympathetic neurotransmitter release properties mediated by the p75 receptor. Nat Neurosci. 2002;5:539–545. doi: 10.1038/nn0602-853. [DOI] [PubMed] [Google Scholar]