Abstract
The regulation of static allometry is a fundamental developmental process, yet little is understood of the mechanisms that ensure organs scale correctly across a range of body sizes. Recent studies have revealed the physiological and genetic mechanisms that control nutritional variation in the final body and organ size in holometabolous insects. The implications these mechanisms have for the regulation of static allometry is, however, unknown. Here, we formulate a mathematical description of the nutritional control of body and organ size in Drosophila melanogaster and use it to explore how the developmental regulators of size influence static allometry. The model suggests that the slope of nutritional static allometries, the ‘allometric coefficient’, is controlled by the relative sensitivity of an organ's growth rate to changes in nutrition, and the relative duration of development when nutrition affects an organ's final size. The model also predicts that, in order to maintain correct scaling, sensitivity to changes in nutrition varies among organs, and within organs through time. We present experimental data that support these predictions. By revealing how specific physiological and genetic regulators of size influence allometry, the model serves to identify developmental processes upon which evolution may act to alter scaling relationships.
Keywords: allometry, insulin, body-size regulation
1. Introduction
Biological diversity is dominated by variation in shape. This is perhaps no more apparent than in one of the most successful metazoan taxa, the insects. From a very simple body plan (head, thorax, abdomen, a pair of antenna, two pairs of wings and three pairs of legs) come myriad forms, from bees to beetles and fleas to flies. Much of this diversity is a result of variation in the relative, rather than the absolute, size of the insect body parts. The same is true for almost all other metazoan phyla. The question of how body parts scale with body size is therefore of substantial evolutionary importance and has consequently received considerable attention, through the study of allometry.
Allometric relationships, the relationship between the size of one trait and the size of another trait or the body as a whole, are traditionally modelled using the allometric equation
| (1.1) |
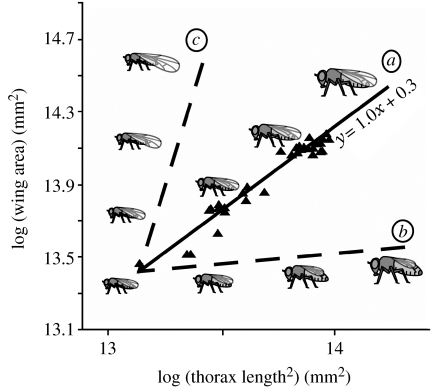
where x and y are the size of two given traits, respectively (Dubois 1897; Huxley & Tessier 1936). When x and y are the traits in conspecific individuals at the same developmental stage, the relationship is a static allometry (Schlichting & Pigliucci 1998; Shingleton et al. 2007). A log transformation of equation (1.1) produces a simple linear equation, log(y)=log(b)+α log(x) and log–log plots of the size of different traits among individuals of the same species typically reveal linear allometries with an intercept of log(b) and a slope of α, called the ‘allometric coefficient’ (Huxley & Tessier 1936). For morphological traits, as long as x and y are the same dimension, α is often close to 1, such that the relative size of the two traits is constant irrespective of absolute size. This condition is called isometry (figure 1a). However, α may also be less than or greater than 1, such that as the size of y becomes smaller or larger relative to x as both traits get absolutely larger. These conditions are called hypo- and hyperallometry, respectively (figure 1b,c). The allometric equation therefore summarizes how the relative sizes of traits scale with each other and with the overall body size, and hence capture the relationship between size and shape in complex organisms. Not all static allometries are linear, however. They may be sigmoidal or discontinuous depending on the trait, the species or the unit of measurement (Emlen & Nijhout 2000). Nevertheless, in this paper, we concentrate on those static allometries that are linear on a log–log scale.
Figure 1.
Isometry, hypoallometry and hyperallometry. The relationship between wing area and body area (thorax length2) in wild-type Drosophila melanogaster is linear and isometric (α=1.0) (a). Example of a (hypothetical) hypoallometric (b) and hyperallometric (c) relationship between wing and body size. Illustrations show example flies for each allometric relationship. See electronic supplementary material for methods.
Despite the extensive application of the allometric equation to the traits of organisms from almost every phylum, very little is understood of the developmental processes that produce linear allometries, and how these processes can be modified to generate the diversity of scaling relationships we see both within and between species (Stern & Emlen 1999; Shingleton et al. 2007). Huxley recognized that the allometric coefficient α could represent the differential growth rate of two traits growing simultaneously. That is, α=m/n, where m and n are the growth rates of the two traits (Huxley 1924; Shea 1985). In such cases, the static allometry follows the growth trajectories of the two structures and different points on the static allometry represent individuals that grow for different periods of time. However, the growth of different structures is not always synchronous. Some structures, for example, the antlers on deer, grow only after the rest of the body has stopped growing. Others, for example, the adult organs of holometabolous insects, have growth periods that only partially overlap with each other and with the growth period of the body (Williams 1980). Under such circumstances, it is unclear what developmental mechanisms regulate the slope and intercept of allometric relationships between traits (Nijhout & Wheeler 1996).
Recent elucidations of the developmental regulation of body and organ size in holometabolous insects provide the first clues as to how static allometries may be controlled (for review see Shingleton et al. 2007). These mechanisms concern the nutritional regulation of growth and so are particularly applicable to our understanding of nutritional static allometries; that is, static allometries where variation in trait size is a consequence of variation in developmental nutrition. Nutritional status is a major regulator of body and organ size in animals (Oldham et al. 2000a) and so the mechanisms that control the developmental response to nutrition probably underlie many of the static allometries observed in nature.
Here, we develop a mathematical model of nutritional static allometry regulation in holometabolous insects. The physiological and genetic mechanisms that underlie the nutritional regulation of final body and organ size in holometabolous insects have previously been proposed by Shingleton et al. (2005, 2007) and Nijhout et al. (2006). Because static allometry describes the scaling relationship between final body and organ size, the mechanisms that regulate final body and organ size necessarily regulate allometry and, if correct, should be able to explain the observed allometric relationships. However, the impact that specific aspects of these mechanisms have on allometry expression are difficult to evaluate using verbal arguments and thought experiments alone. Mathematical modelling allows us to investigate how the physiological and genetic regulators of body size interact with the physiological and genetic regulators of organ size, and whether the hypothesized interaction can generate biologically reasonable allometries. In this paper, we first summarize the features of the mechanisms regulating final body and organ size before we use them to formulate a quantitative model of allometry regulation. The timing and dynamics of organ growth are perhaps best understood in the fruit fly Drosophila, and so we will develop our model using Drosophila as an example holometabolous insect.
2. Nutritional regulation of body size in Drosophila
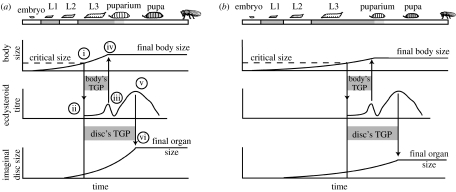
The mechanism of the nutritional regulation of body size in Drosophila is illustrated in figure 2. In fruit flies, as in all holometabolous insects, growth is restricted to the larval stages (Bakker 1959; Ashburner 1989). A developing larva feeds and grows exponentially until it reaches a critical size at the beginning of the third larval instar (figure 2a(i)) (Nijhout & Williams 1974b; Nijhout 2003; Mirth et al. 2005). By an incompletely known mechanism, attainment of critical size initiates a hormonal cascade (figure 2a(ii)) that ultimately results in the release of ecdysteroids (figure 2a(iii)) (Baehrecke 1996). The subsequent rise in ecdysteroids causes the larva to stop feeding and wander away from its food to find a place to metamorphose, called the wandering stage (Berreur et al. 1979). The cessation of feeding marks the end of body growth and, because adults possess an exoskeleton that prohibits further growth, fixes the maximum size of the adult fly (figure 2a(iv)). There is temporal separation between the attainment of critical size and the cessation of feeding and growth, called the terminal growth period (TGP) (Shingleton et al. 2007). The final size of the fly is thus determined by (i) critical size, plus the amount of growth achieved in the TGP, which is in turn determined by (ii) the duration of the TGP and (iii) the rate of growth during the TGP (Shingleton et al. 2007). Variation in developmental nutrition potentially affects final body size by influencing any or all of these three factors. However, nutrition does not appear to regulate critical size in Drosophila (De Moed et al. 1999; Shingleton et al. 2005; A. W. Shingleton, unpublished data), and has only a small effect on the duration of the body's TGP, with complete starvation causing slightly precocious pupariation (Beadle et al. 1938; Mirth et al. 2005). Rather, nutrition primarily influences final body size by regulating the body's rate of growth during its TGP (figure 2b).
Figure 2.
General model of body and imaginal disc growth in Drosophila. (a) The growth of the body and imaginal discs under optimal nutritional conditions. See main text for details. (b) A reduction in nutrition slows growth and delays attainment of critical size, extending total developmental time. Attainment of critical size initiates the same hormonal cascade that brings about the cessation of body and imaginal disc growth. The temporal dynamics of this cascade are unaffected by nutrition. Slow growth of the body and imaginal discs now reduces the amount of growth they can achieve during their TGPs, reducing final body and organ size. Hormones other than ecdsyteroids may be involved in the cessation of disc growth. L1–L3, first to third larval instar; TGP, terminal growth period.
It is the insulin/TOR-signaling (IIS) pathway that coordinates growth rate with nutritional condition in Drosophila and most metazoans (for review see Conlon & Raff 1999; Oldham et al. 2000a; Edgar 2006). In Drosophila nutrition regulates the release of insulin-like peptides (dILPs) from various tissues around the body (Brogiolo et al. 2001; Britton et al. 2002; Rulifson et al. 2002). These dILPs bind to the insulin receptor (Inr) of dividing cells and, through a well-elucidated signal transduction pathway, regulate the rate of cell growth and proliferation (Edgar 2006). Starvation down-regulates the IIS-pathway through several mechanisms. A reduction in circulating nutrients can be sensed directly by dividing cells, leading to suppression of the IIS-pathway via the target-of-rapamycin (TOR) (Oldham et al. 2000b; Neufeld 2003). Starvation also reduces the release of dILPs, down-regulating the IIS-pathway via Inr (Ikeya et al. 2002). Finally, other hormones, for example juvenile hormone (JH) (Truman et al. 2006), ecdysone (Caldwell et al. 2005; Colombani et al. 2005; Mirth et al. 2005) and imaginal disc growth factors (IDGF) (Kawamura et al. 1999), also regulate the IIS-pathway, and may themselves be nutritionally regulated. Collectively, these various humoral factors are likely dispersed uniformly throughout the developing larva, and so the IIS-pathway can coordinate growth throughout the body in response to changing nutrition (Goberdhan & Wilson 2002).
3. Nutritional regulation of organ size in Drosophila
The mechanism of the nutritional regulation of organ size, also illustrated in figure 2, is less well elucidated than for body size. In holometabolous insects like Drosophila, the adult organs develop as imaginal discs within the growing larva. Although the cells that will become the discs are specified during embryogenesis, the point in development when discs initiate growth varies among discs, with most discs starting growth in the first or second instar (Madhavan & Schneiderman 1977; Cohen 1993). Like the body, growth of the discs is approximately exponential (Martin 1982; Bryant & Levinson 1985) and ends with cell differentiation. Growth cessation appears to be regulated by the same hormonal cascade that is initiated upon attainment of critical size (Emlen & Allen 2003; Mirth 2005): Differentiation in the eye discs is regulated by the rise in ecdysteroids that causes larvae to stop feeding (Brennan et al. 1998), and differentiation in the wing and leg is also tied to changes in the levels of circulating ecdysteroids and JH ( Martin & Shearn 1980; Fristrom & Fristrom 1993; Riddiford 1993). Like the body, therefore, the imaginal discs also have TGPs that begin when a larva reaches critical size (figure 2a(i)) and end when hormone levels rise above a certain threshold (figure 2a(v),(vi)). However, the discs have different sensitivities to the hormone cascade initiated at critical size, and so their TGPs are typically longer than the TGP of the body (Garcia-Bellido & Merriam 1971; Freeman 1997).
Final organ size is therefore determined by (i) the size of the imaginal discs at critical size, (ii) the duration of the disc's TGP and (iii) the rate of growth during that TGP (Shingleton et al. 2007). Again, nutrition could affect final body size by influencing any or all of these three factors. However, the size of the discs at critical size does not appear to be affected by nutrition or changes in IIS (Shingleton et al. 2005). Further, since nutrition does not appear to substantially influence the body's TGP, it seems unlikely that it substantially affects the TGPs of the imaginal discs, since all TGPs are controlled by the same hormonal cascade. As with the body as a whole, therefore, nutrition appears to influence final organ size primarily by regulating the rate of disc growth during the discs' TGP via the IIS-pathway (figure 2b) (Shingleton et al. 2005, 2007).
4. A model of nutritional static allometry expression in Drosophila
In order to better understand the implications the mechanistic description of body and organ size regulation has for the control of static allometry (figure 2), we have formalized the mechanism into a mathematical model. The model predicts final body and organ size for a fly experiencing a constant but non-zero level of nutrition throughout development. The model assumes that this constant level of nutrition translates into a constant level of IIS. Setting different levels of IIS alters the growth rate of the body and organs during their respective TGPs and generates a variation in body and organ size. Consequently, the model can be used to explore how the body and organs scale under different nutritional conditions, and how this allometric relationship is influenced by the model's biological parameters.
Because imaginal disc growth trajectories have been best elucidated in the wing (Garcia-Bellido & Merriam 1971; Madhavan & Schneiderman 1977; Martin 1982; Bryant & Levinson 1985; Milan et al. 1996), we will use the wing as an example imaginal disc. Nevertheless, the parameters of the model can be changed to explore the allometric regulation of other discs.
(a) Exponential growth
The growth of the body (Bakker 1959) and imaginal discs (Martin 1982; Bryant & Levinson 1985) is approximately exponential in Drosophila, as is typical for insects (Nijhout et al. 2006). Changes in nutrition change the rate of growth, and in our model we will assume that this occurs exclusively through the IIS pathway and the pathways the IIS pathway interacts with. We will not assume a priori that all growth is affected by changes in nutrition. Rather, the growth rate is divided into two components: an insulin-sensitive growth rate and an insulin-insensitive, or ‘intrinsic’, growth rate. The insulin-sensitive growth rate is modified by the level of IIS, which here refers to a combination of the level of circulating dILPs, nutrients and other humoral factors that regulate the IIS pathway. In contrast, the intrinsic growth rate does not vary with the nutritional level and occurs as long as the larva has sufficient nutrients to sustain life. This does not mean that intrinsic growth is not dependent on nutrition, since a lack of nutrition precludes growth. Rather, the intrinsic growth rate does not vary with the level of nutrition.
Growth of the body is modelled as
| (4.1) |
and growth of the disc is modelled as
| (4.2) |
where Bt and Dt are the size of the body and disc, respectively, at time t; c is the intrinsic growth rate; s is the insulin-sensitive growth rate; and i is an index of the level of IIS, which varies between 0 and 1. Growth rate (c+s·i) therefore varies between c, when IIS is minimal and i=0, and c+s, when IIS is maximal and i=1. The parameter s thus captures the extent to which growth rate changes with the changes in IIS, and can be thought of as a measure of ‘insulin sensitivity’. Both c and s can be the growth rate in any dimension (length, area and volume) and in any unit (cell number, mm, μg, etc.).
(b) Growth rate and final body size
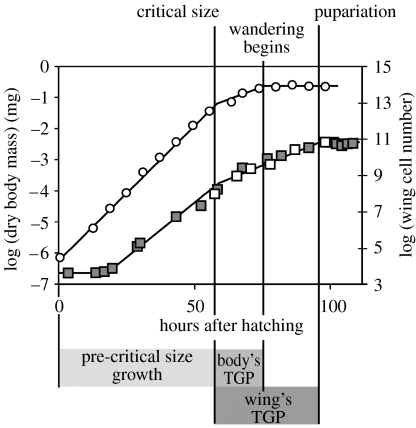
In Drosophila, the body's growth rate is not constant throughout development. Growth of the body (dry body mass) is exponential both before and after critical size, but the exponent of growth is lower once critical size is attained (Bakker 1959). Growth rate also appears to show a decline immediately before growth stops at the beginning of the wandering larval stage (Bakker 1959). Because this decline is relatively slight, however, we will approximate body growth as two exponential growth periods, the first from hatching to critical size and the second from critical size to the beginning of the wandering stage (figure 3). We will model growth rate of the body before critical size as in equation (4.1) and growth rate after critical size as
| (4.3) |
where c′ and s′ are the intrinsic and insulin-sensitive growth rates after critical size.
Figure 3.
The fit between the model and observed growth trajectories for the body and wing. Growth of the body and wing imaginal discs can be modelled as two exponential periods of growth, one before the attainment of critical size and one after the attainment of critical size. Growth of the body begins at hatching and ends at the beginning of larvae wandering. Growth of the wing imaginal discs begins towards the end of the first larval instar and pauses at pupariation. Points show published data: open circles, body (Bakker 1959); open squares, wing (Martin 1982); filled squares, wing (Bryant & Levinson 1985). Lines show modelled growth trajectory using parameter values from table 1.
Final body size is the critical size plus the amount of growth achieved during the body's TGP (figure 2). Neither critical size nor TGP are substantially affected by changes in nutrition. Final body size can therefore be modelled by solving equation (4.3) for t=T0, where T0 is the duration of the body's TGP, i.e. the time between the attainment of critical size and the beginning of the wandering stage. Thus,
| (4.4) |
where BF is final body size under level of IIS i and L is the critical size.
(c) Growth rate and final disc size
Growth of the wing imaginal discs is also not constant throughout development. The wing discs begin growth midway through the first larval instar but the growth slows after the attainment of critical size and stops temporarily at pupariation (Bryant & Levinson 1985). Cell division resumes after the wing discs have everted (Garcia-Bellido & Merriam 1971; Milan et al. 1996) before permanently stopping when the wing discs differentiate, 24 hours after pupariation at 25°C (Milan et al. 1996). This later stage of growth has not, however, been well characterized. Further, the size of the wing disc at pupariation is thought to be the major determinant of final wing size (Day & Lawrence 2000). We will therefore only model wing disc growth up to the point of pupariation.
Like the body, wing disc growth can be considered as two exponential growth periods. The first is from midway through the first larval instar to critical size, the duration of which depends on the rate of the body's growth to critical size, and the second is from after critical size to pupariation (figure 3). We will describe growth rate of the disc before critical size using equation (4.2) and growth rate after critical size as
| (4.5) |
where and are the intrinsic and insulin-sensitive growth rates after critical size, respectively. We will model the final wing disc size by analysing each of these growth periods separately.
Discs begin their growth after the body has started growing (Cohen 1993). It is not clear what initiates disc growth but a reasonable hypothesis is that it begins when the body reaches a certain size. In our model, disc growth begins when the body reaches proportion a of critical size L, at time T2 after the hatching of embryo to larva. The value of T2 therefore depends on the body's growth rate up to aL. We can model this by solving equation (4.2) for t=T2
| (4.6) |
where B0 is the size of the body at hatching.
It follows that
| (4.7) |
By the same argument, critical size (L) is reached at time T1 after the larva hatches, such that
| (4.8) |
Again, T1 depends on the body's growth rate up to L.
We can model the size of the disc at critical size at a given level of insulin signalling by solving equation (4.2) for t=T1−T2, that is the amount of time between the beginning of disc growth and the attainment of critical size, such that
| (4.9) |
where DL is the size of the disc at critical size and D0 is the size of the disc at the beginning of growth. Inserting the values for T1 and T2 (equations (4.7) and (4.8), respectively) into equation (4.9), the size of the disc at critical size is
| (4.10) |
Once critical size is reached the disc continues to grow through its TGP. Because the disc's TGP is probably regulated by the same hormone fluctuations that regulate the body's TGP (T0), we can hypothesize that the former is some multiple of the latter. Final disc size can be modelled by solving equation (4.5) for t=T0
| (4.11) |
where DF is the final size of the disc and K is the factor relating the disc's TGP to the body's TGP. Inserting the values of DL gives the final disc size as
| (4.12) |
(d) Fitting parameter values: growth under optimal conditions
The parameters of the model are real biological parameters and their values can be determined empirically. Several authors have published data for the growth of the body and wing imaginal discs of wild-type Drosophila reared on 100% food at 25°C (Bakker 1959; Madhavan & Schneiderman 1977; Martin 1982; Bryant & Levinson 1985). We have used these data to fit approximate parameter values to our model (table 1). Because the data were collected from flies reared under optimal conditions, we will assume that for these data the level of insulin signalling is maximal and that i=1.
Table 1.
Parameters used in the model and their values, if known, from published data.
| parameter value | |||
|---|---|---|---|
| parameter | meaning | body | disc |
| B0/D0 | initial body/disc size (mg and cell number, respectively) | 0.0022 | 38 |
| c | intrinsic (insulin-insensitive) growth rate | — | |
| s | insulin-sensitive growth rate | — | |
| i | level of insulin signalling (ranges from 0 to 1) | — | |
| c+s | growth rate under optimal conditions (i=1), before critical size | 0.088 | 0.11 |
| c′+s′ | growth rate under optimal conditions (i=1), after critical size | 0.041 | 0.075 |
| L | critical size (mg) | 0.21 | — |
| T0 | body's TGP | 21 | — |
| T1 | time from hatch to attainment of critical size | — | — |
| T2 | time from hatch to initiation of disc growth | — | — |
| a | body size (as proportion of L) at which disc starts growing | — | 0.035 |
| K | disc's TGP (as multiple of T0) | — | 1.9 |
Newly hatched larvae weigh 0.0022 mg (dry weight) and grow exponentially up to the critical size (Bakker 1959). The exponent of growth before critical size (cb+sb)=0.088 (Bakker 1959). Critical size (L) is 0.21 mg and is attained 53 hours after hatching (Bakker 1959). After critical size, the growth remains exponential but the rate is approximately halved, such that the exponent (Bakker 1959). Growth continues until larvae stop feeding, 74 hours after hatching (Bakker 1959). The TGP of the body (T0) is therefore 21 hours.
The wing imaginal discs initially constitute approximately 38 cells at hatching, and start growing 15 hours later (Madhavan & Schneiderman 1977). At this point, the body size is approximately 0.008 mg (Bakker 1959), hence a=0.035. Like the body, the growth of the wing discs appears to slow down slightly after critical size. The exponent of growth before critical size (cd+sd) is 0.11, and the exponent after critical size is 0.075 (Bryant & Levinson 1985). Cell proliferation pauses at pupariation (93 hours, K=1.9) when the wing discs constitute approximately 45–50 000 cells (Bryant & Levinson 1985).
Using these parameter values in equations (4.4) and (4.12) predicts a maximum body size (dry mass) of 0.50 mg, with 50 000 cells in the wings at pupariation, a close match to measurements made in wild-type flies (Bakker 1959; Bryant & Levinson 1985). The predicted growth trajectories of the body and the wings also closely match those observed in wild-type flies (figure 3).
(e) Fitting variables: growth under suboptimal nutritional conditions and static allometry
We are interested in how the developmental regulators of body and organ size affect static allometry. In our model, nutrition influences final body and organ size through its influence on growth rate via the level of IIS (i). By plotting the predicted body and organ size for different values of i, we can use our model to generate static allometries; that is we make i a variable. While the maximal growth rates of both the body and the wing imaginal discs (c+s) are known (Bakker 1959; Bryant & Levinson 1985), how growth rate varies with changes in IIS has not been measured directly. Consequently, the individual values of the body and organs' insulin-sensitive (s) and intrinsic growth rates (c) are unknown. Nevertheless, our model, as well as additional empirical evidence, allows us to predict what these parameter values are likely to be and explore how these values affect allometry.
Under maximal nutritional conditions (i=1), the body's growth rate in wild-type larvae is 0.088 before critical size and 0.041 after critical size (Bakker 1959). In contrast, larvae carrying a temperature-sensitive mutation of Inr (Shingleton et al. 2007) stop growing when transferred to a restrictive temperature, eliminating Inr activity (electronic supplementary material, figure S1). This suggests that the body has no intrinsic growth rate and that cb and . It follows that for the body sb=0.088 and .
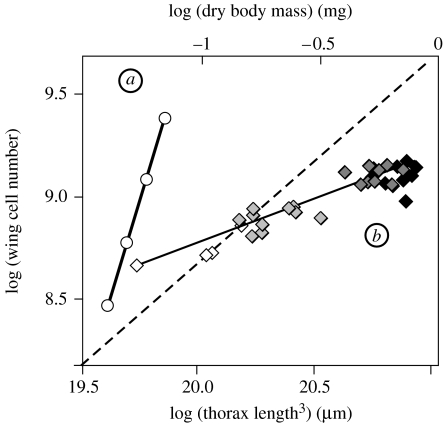
We can extend these assumptions to the wing. Under maximal nutritional conditions (i=1), the wing's growth rate is 0.11 before critical size (cd+sd) and 0.075 after critical size . If the wing also has no intrinsic growth rate, that is cd and , it follows that sd=0.11 and (Bryant & Levinson 1985). Using these parameter values, the model predicts a highly hyperallometric relationship between wing-cell number and body size as i varies, with an allometric coefficient (α) of 3.5 (figure 4a). However, the observed nutritional static allometry between adult wing cell number and body size has an allometric coefficient of 0.43 (figure 4b). The incompatibility of our model with the observed allometry suggests that our assumption that growth of the wings is entirely insulin-sensitive is incorrect. Consequently, the wings may have an intrinsic growth rate, either before or after the attainment of critical size.
Figure 4.
Modelled and observed allometric relationship between wing size and body size in Drosophila. (a) When both the wing and the body have no intrinsic growth rate (cb, , cd and ) the model predicts a highly hyperallometric relationship between wing cell number and body size (values as in table 1). (b) In fact, the nutritional static allometry between wing cell number and body volume (thorax length3) is linear but hypoallometric, such that α=0.43. Flies reared on diets of increasing nutritional quality (2–100% of standard diet). White diamonds, 2% diet; grey diamonds, 5% diet; dark grey diamonds, 10% diet; black diamonds, 100% diet. Dashed line is isometry. See electronic supplementary material for methods.
The model suggests that the wings do not have intrinsic growth before attainment of critical size. When cd>0 the body:wing allometry is nonlinear (figure 5c). This is because at very low levels of IIS, the time to critical size is long (electronic supplementary material, figure S2). If wing discs grow even when IIS is low, this developmental delay will lead to an increase in the disc size at critical size. This compensates for slow growth of the discs during their TGP and results in larger wings at low compared with intermediate levels of IIS (figure 5c). However, the allometric relationship between wing and body size created by rearing flies under different nutritional conditions is positive and linear (figure 4b): flies reared on low-quality diets, and those experiencing low levels of IIS, do not have larger wings than those reared on intermediate and high-quality diets. This suggests that the wings do not have intrinsic growth before the attainment of critical size.
Figure 5.
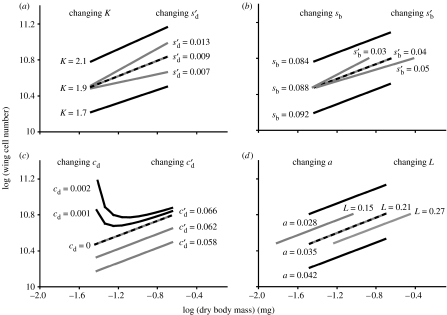
(a–d) The effect of changing parameter values on the modelled allometry between wing cell number and body size in Drosophila. Dashed line is the predicted wild-type allometry, based on the observed data. Values at the end of each line show the parameter values used; parameter values in black correspond to the black lines and parameter values in grey correspond to the grey lines. All other parameter values are from table 1.
By contrast, the model does generate a linear static allometry when the wings have an intrinsic growth rate after critical size; that is . It can be shown (electronic supplementary material) that when cb and cd=0 the allometric coefficient (α) between body and organ size can be modelled as
| (4.13) |
For an allometric coefficient of 0.43, if K=1.9 (table 1) then . Thus to produce the observed hypoallometry between wing cell number and body size, the insulin-sensitive growth rate of the discs must be considerably less than that of the body: if , then . However, published data indicate that under optimal nutritional conditions (i=1), the exponent of wing growth (Bryant & Levinson 1985). This suggests that and that the wing does have an intrinsic growth rate after the attainment of critical size.
Equation (4.13) can be generalized to apply to any two organs, neither of which is the body, again assuming that neither the organs nor the body have an intrinsic growth rate prior to critical size. The more general equation is
| (4.14) |
where αpq is the allometric coefficient for traits p and q. For example, the relationship between the size of the maxillary palp and the male genitals of Drosophila is highly hypoallometric (figure 1), such that α=0.35–0.5, depending on the genotype. This suggests that either the genitals have a growth rate that is relatively insensitive to changes in IIS compared with other organs, or that the genitals' TGP is relatively short compared with other organs.
Equation (4.13) suggests that the parameters that primarily influence an organ's allometric coefficient with body size are its post-critical size insulin-sensitive growth rate relative to that of the body and the relative duration of its TGP (K). Using different values of , and K in the model generates a range of allometries with different slopes (figure 5a). Changes in K, while slightly affecting the slope of the allometry, also affect the minimal disc size and so have a more dramatic effect on the allometry's intercept. By contrast, changes in and have a substantial effect on the allometry's slope, but do not affect the minimal body or disc size and so have little effect on the allometry's intercept. Changes in when the imaginal discs start growing prior to critical size (a), the insulin-sensitive growth rate of the disc and the body before critical size (sd and sb, respectively), critical size (L), the disc's intrinsic growth rate after critical size all influence the intercept but not the slope of the allometry (figure 5b–d).
5. Discussion
Simple allometry, modelled as y=bxα, is widely applicable to scaling relationships in myriad animals. Nevertheless, the biological meaning of the equation's parameters, in particular the allometric coefficient α, is unclear for any animal except those whose body parts grow synchronously (Nijhout & Wheeler 1996). Many organisms do not grow synchronously, including holometabolous insects. Rather, different structures grow at different points in development and for different periods. In such situations, it is not immediately obvious what the biological regulators of α are.
Our simple model of nutritional static allometry in holometabolous insects suggest that α is regulated by the relative sensitivity of an organ's growth rate to changes in IIS and the duration of development when IIS affects the final organ size (equation (4.13)). This has an intuitive appeal. The slope of a static allometry captures the extent to which factors that affect body size also affect organ size. For example, an organ will be hyperallometric to body size if the conditions that alter body size have a greater affect on organ size. For both the organs and the body, their insulin-sensitive growth rates and their TGP will affect how much their final size is affected by the changes in IIS. Thus, the greater an organ's insulin-sensitive growth rate is compared with the body's , or the longer its relative TGP (K), the more the organ's final size will be affected by changes in IIS relative to the body, and the more hyperallometric will be its relationship to body size.
(a) Allometry and insulin sensitivity
The possibility that different discs differ in their growth response to IIS has been hypothesized before (Nijhout 2003; Emlen et al. 2007; Shingleton et al. 2007). Reduced nutrition and IIS produce smaller individuals, yet with ostensibly normal proportions (except for certain organs such as the male genitals (Shingleton et al. 2005)). Proportionality is maintained if each organ grows at a particular rate relative to each other (Nijhout 2003). However, all organs are probably exposed to the same level of circulating growth factors. Consequently, for different organs to maintain the correct relative growth rate, they must grow at different rates in response to a particular level of circulating insulin (Nijhout 2003). Our model suggests an additional nuance to this observation. Two organs may have very different growth rates under the same level of IIS, but will only maintain proportionality as IIS varies if they share the same level of insulin-sensitive growth and the same TGP. Thus, there are two aspects to a disc's response to IIS: the rate at which a disc grows at a particular level of circulating insulin and how this rate changes with changes in IIS. This latter aspect is captured by the insulin-sensitive growth rate s in our model, and we restrict the term insulin sensitivity to the magnitude of s.
The model suggests that if two discs differ in either their insulin sensitivity or in their TGP then, all other things being equal, their scaling relationship will not be isometric. Conversely, if two organs are isometric, but differ in their TGP, then they should also differ in their insulin sensitivity. The point in development when a disc stops cell proliferation and differentiates into its final adult form varies among imaginal discs in Drosophila. The eye and the wing are both approximately isometric to body size, yet differ in their TGPs (Basler & Hafen 1989; Milan et al. 1996). These arguments suggest that we should see frequent, but possibly subtle, differences among discs in the way changes in IIS affects their growth rate.
(b) Allometry and intrinsic growth
The model predicts that the effect of IIS on disc growth varies through development, for wing discs at least: before attainment of critical size, and when changes in nutrition can delay development, the model suggests that the growth rates of the body and the wing discs are entirely insulin-sensitive. After attainment of critical size, when the final duration of growth is fixed, the model suggests that there is a decline in the wing discs' insulin-sensitive growth rate, with the discs taking on an intrinsic growth rate in order to sustain their overall rate of growth. Changing a disc's post-critical size intrinsic growth rate alters the intercept but not the slope of its allometry (figure 5c), while changing the disc's insulin sensitivity alters the slope but not the intercept (figure 5a). Consequently, separating intrinsic from insulin-sensitive growth may allow discs to regulate the slope of their allometry independent of the intercept of their allometry. We might therefore expect intrinsic growth to be a common feature of imaginal discs in holometabolous insects.
There is good experimental evidence that discs in holometabolous insects have an intrinsic growth rate. It is possible to manipulate the IIS pathway in developing larvae using a temperature-sensitive mutation of the Inr (Shingleton et al. 2005). Switching off the IIS pathway in all tissues after attainment of critical size almost eliminates body growth (electronic supplementary material, figure S1). By contrast, the wing discs continue to grow (electronic supplementary material figure S1, figure 6), suggesting that they have intrinsic growth even when their cells have severely reduced IIS.
Figure 6.
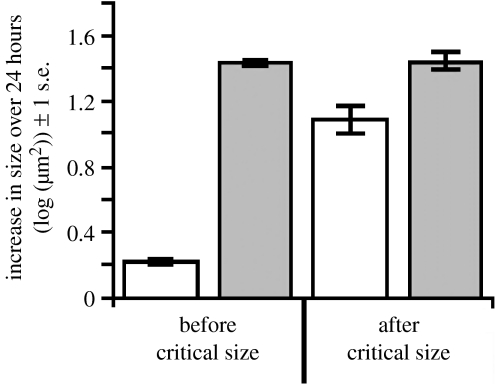
The wings of pre- and post-critical size larvae differ in their response to starvation. Third-instar larvae were starved (open bars) or fed (filled bars) for 24 hours, either before or after attainment of critical size (approx. 8 hours after the moult from second to third instar), and the increase in their wing area (μm2) over that period was measured. Each bar represents 10–18 discs. See electronic supplementary material for methods.
Further evidence for intrinsic growth comes from work on the tobacco hornworm Manduca sexta. In this insect, disc growth responds only to changes in IIS in the presence of JH (Truman et al. 2006). In animals that have had their corpora allata excised (the gland that produces JH), starvation reduces but does not prevent disc growth. This remaining growth, which the authors term ‘morphogenetic growth’, is presumably an intrinsic growth that does not require nutritional input. The authors argue that morphogenetic growth sets the lower limit of disc size. In our model, intrinsic growth also sets the lower limit of disc size. Hypothetical larva in which IIS is eliminated immediately after attainment of critical size exhibit no body growth but their discs continue to grow until final disc size is appropriate for the much reduced body size.
An important aspect of intrinsic growth is that it is not present throughout development. In particular, our model suggests that, in order to express a linear static allometry, discs should not have intrinsic growth prior to attainment of critical size, when the time to the cessation of growth is not yet fixed. This appears to be the case in the buckeye butterfly Precis coenia, where starvation prevents growth of both the body and the wing discs in larvae that are still feeding (Miner et al. 2000). Further, the wing size at critical size is not influenced by changes in IIS in Drosophila (Shingleton et al. 2007), providing indirect evidence that the wing discs do not have intrinsic growth early in development. However, to test this directly, we starved pre- and post-critical size larvae and measured the extent of wing-disc growth over the subsequent 24 hours. Starvation prevents body growth and severely inhibits insulin signalling in a developing larva. The wing discs showed almost no additional growth in larvae starved before critical size, but grew considerably in larvae starved after critical size (figure 6). The fact that the wings grew slightly in larvae starved before critical size probably reflects a lag in the cessation of disc growth after a larva is removed from food rather than an intrinsic growth rate, although a direct manipulation of the insulin-signalling pathway in developing wing discs would confirm this. Nevertheless, our model and our data suggest that there is reprogramming of the imaginal discs' response to nutrition at or around the attainment of critical size.
The finding that JH is responsible for linking disc growth with nutrition in M. sexta makes it an excellent candidate for reprogramming the imaginal discs. In M. sexta, and possibly all holometabolous insects, the attainment of critical size is followed by a decline in JH levels (Nijhout & Williams 1974a). In Coleoptera and Lepidoptera, this decline is essential in ensuring that the succeeding moult is larval–pupal rather than larval–larval (Nijhout & Williams 1974a; Safranek et al. 1980; Gilbert et al. 1996). In Drosophila, there is also a decline in JH after critical size (Buhrlen et al. 1984; Bownes & Rembold 1987). The application of JH throughout larval life does not prevent pupariation, but does prevent differentiation of the abdomen during the pupal–adult moult (Postlethwait 1974). Our model suggests a second role for the decline in JH in holometabolous insects, that of regulating the switch from wholly insulin-dependent growth to partially insulin-independent growth, essential in the regulation of allometry.
(c) Expanding the model
Although the model has been developed to explore the primary developmental factors that influence allometry in Drosophila, it can easily be applied to other holometabolous insects. Like Drosophila, all holometabolous insects have organs that develop as imaginal discs and are thought to have a critical size for metamorphosis. Unlike Drosophila, however, this critical size may be influenced by nutrition, for example in M. sexta. The effect of nutrition on critical size can be built into our model by making L, critical size, a function of i, the level of IIS. Similarly, while the duration of the body's TGP in Drosophila appears to be only slightly affected by nutrition (Beadle et al. 1938; Mirth et al. 2005), this effect may be more substantial in other insects. This can be built into our model by making T0, the body's TGP, a function of i. Finally, in many holometabolous insects, the imaginal discs do not begin growth until after the attainment of critical size. This is thought to be the ancestral state (Truman et al. 2006). The TGP for such discs is effectively their entire growth period. This can easily be accommodated in our model by replacing DL, disc size at critical size, with D0, disc size at the beginning of growth, in equation (4.11).
6. Conclusions
The model suggests that two factors, insulin sensitivity and TGP, are fundamental regulators of the slope of allometries in holometabolous insects. Data concerning the insulin sensitivity and TGP of different imaginal discs in Drosophila, or any holometabolous insect, are lacking. Consequently, it is not yet possible to test whether the model can accurately predict the nutritional static allometries of different Drosophila organs. Nevertheless, the model makes a number of testable hypotheses concerning the mechanisms that regulate allometry in animals with asynchronous growth. First, even among organs that are isometric to each other and to body size, there should be differences in insulin sensitivity if these organs also differ in the period in which IIS influence their final size. In holometabolous insects, this is the TGP. The organs of many animals, including humans, begin and stop growth at different points in development, and so will have different periods during which their growth can be influenced by IIS. We might expect that these organs also differ in their insulin sensitivity. Second, the model suggests that there is reprogramming of imaginal discs after the attainment of critical size so that they take on an intrinsic growth rate. Our experimental data confirm this reprogramming in Drosophila, while data from M. sexta suggest that the reprogramming may be regulated by changes in the level of JH. Thus, the model provides a developmental reason for the finding that JH interacts with IIS to influence disc growth in M. sexta.
The model clarifies how altering specific developmental parameters may alter the expressed allometric relationship between organ size and body size. Changing key parameters, for example, insulin-sensitivity (s), duration of TGP (K) and critical size (L), all influence aspects of the an organ's static allometry (figure 5). Variation in static allometry underlies variation in animal shape, and so the evolution of allometric relationships is a major factor in the generation of phenotypic diversity. Hitherto, there has been very little understanding of the developmental processes that regulate static allometries, essential if we are to identify candidate developmental mechanisms, pathways and genes upon which selection may act. Our model provides such candidates. The next stage is to explore whether the variation in these candidates correlates with that in expressed allometries in holometabolous insects, both intra- and interspecifically.
More than 100 years after the problem of allometry was first recognized and formulated by those studying the brain:body ratios in humans (Lapicque 1898), we are finally beginning to identify the developmental mechanisms that are involved in the regulation of nutritional static allometry. The relevance of these mechanisms to other forms of environmental static allometry created by other regulators of size, for example, temperature and oxygen levels, and to genetic static allometry and evolutionary allometry has yet to be determined. Nevertheless, this model and models similar to it (Nijhout et al. 2006) provide a firm developmental context for continued research.
Acknowledgments
We thank Bradley Stieper for his help generating the body and imaginal disc growth data. We thank Tony Frankino, Ian Dworkin, Kyle Miller and two anonymous referees for their comments on early versions of this paper. PWB acknowledges support from NSF DMS-0401708 and DARPA FunBio HR 0011-05-1-005.
Supplementary Material
References
- Ashburner M. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. Drosophila: a laboratory handbook. [Google Scholar]
- Baehrecke E.H. Ecdysone signaling cascade and regulation of Drosophila metamorphosis. Arch. Insect Biochem. Physiol. 1996;33:231–244. doi: 10.1002/(SICI)1520-6327(1996)33:3/4<231::AID-ARCH5>3.0.CO;2-V. doi:10.1002/(SICI)1520-6327(1996)33:3/4<231::AID-ARCH5>3.0.CO;2-V [DOI] [PubMed] [Google Scholar]
- Bakker K. Feeding period, growth, and pupation in larvae of Drosophila melanogaster. Entomol. Exp. Appl. 1959;2:171–186. doi:10.1007/BF00302537 [Google Scholar]
- Basler K, Hafen E. Dynamics of Drosophila eye development and temporal requirements of sevenless expression. Development. 1989;107:723–731. doi: 10.1242/dev.107.4.723. [DOI] [PubMed] [Google Scholar]
- Beadle G, Tatum E, Clancy C. Food level in relation to rate of development and eye pigmentation in Drosophila melanogaster. Biol. Bull. 1938;75:447–462. doi:10.2307/1537573 [Google Scholar]
- Berreur P, Porcheron P, Berreur-Bonnenfant J, Simpson P. Ecdysteroid levels and pupariation in Drosophila melanogaster. J. Exp. Zool. 1979;210:347–352. doi:10.1002/jez.1402100218 [Google Scholar]
- Bownes M, Rembold H. The titre of juvenile hormone during the pupal and adult stages of the life cycle of Drosophila melanogaster. Eur. J. Entomol. 1987;164:709–712. doi: 10.1111/j.1432-1033.1987.tb11184.x. [DOI] [PubMed] [Google Scholar]
- Brennan C.A, Ashburner M, Moses K. Ecdysone pathway is required for furrow progression in the developing Drosophila eye. Development. 1998;125:2653–2664. doi: 10.1242/dev.125.14.2653. [DOI] [PubMed] [Google Scholar]
- Britton J.S, Lockwood W.K, Li L, Cohen S.M, Edgar B.A. Drosophila's insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev. Cell. 2002;2:239–249. doi: 10.1016/s1534-5807(02)00117-x. doi:10.1016/S1534-5807(02)00117-X [DOI] [PubMed] [Google Scholar]
- Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, Hafen E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. 2001;11:213–221. doi: 10.1016/s0960-9822(01)00068-9. doi:10.1016/S0960-9822(01)00068-9 [DOI] [PubMed] [Google Scholar]
- Bryant P.J, Levinson P. Intrinsic growth control in the imaginal primordia of Drosophila, and the autonomous action of a lethal mutation causing overgrowth. Dev. Biol. 1985;107:355–363. doi: 10.1016/0012-1606(85)90317-3. doi:10.1016/0012-1606(85)90317-3 [DOI] [PubMed] [Google Scholar]
- Buhrlen U, Emmerich H, Rembold H. Titer of juvenile hormone-Iii in Drosophila hydei during metamorphosis determined by Gc-Ms-Mis. Z. Naturforsch. C Biosci. 1984;39:1150–1154. [Google Scholar]
- Caldwell P.E, Walkiewicz M, Stern M. Ras activity in the Drosophila prothoracic gland regulates body size and developmental rate via ecdysone release. Curr. Biol. 2005;15:1785–1795. doi: 10.1016/j.cub.2005.09.011. doi:10.1016/j.cub.2005.09.011 [DOI] [PubMed] [Google Scholar]
- Cohen S.M. Imaginal disc development. In: Bate M, Martinez Arias A, editors. The development of Drosophila. Cold Spring Harbour Press; New York, NY: 1993. pp. 747–841. [Google Scholar]
- Colombani J, Bianchini L, Layalle S, Pondeville E, Dauphin-Villemant C, Antoniewski C, Carré C, Noselli S, Léopold P. Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science. 2005;310:667–670. doi: 10.1126/science.1119432. doi:10.1126/science.1119432 [DOI] [PubMed] [Google Scholar]
- Conlon I, Raff M. Size control in animal development. Cell. 1999;96:235–244. doi: 10.1016/s0092-8674(00)80563-2. doi:10.1016/S0092-8674(00)80563-2 [DOI] [PubMed] [Google Scholar]
- Day S.J, Lawrence P.A. Measuring dimensions: the regulation of size and shape. Development. 2000;127:2977–2987. doi: 10.1242/dev.127.14.2977. [DOI] [PubMed] [Google Scholar]
- De Moed G.H, Kruitwagen C, De Jong G, Scharloo W. Critical weight for the induction of pupariation in Drosophila melanogaster: genetic and environmental variation. J. Evol. Biol. 1999;12:852–858. doi:10.1046/j.1420-9101.1999.00103.x [Google Scholar]
- Dubois E. Sur le rapport de l'encéphale avec la grandeur du corps chez les Mammiferes. Bull. Soc. Anthropol. (Paris) 1897;8:337–374. [Google Scholar]
- Edgar B.A. How flies get their size: genetics meets physiology. Nat. Rev. Genet. 2006;7:907–916. doi: 10.1038/nrg1989. doi:10.1038/nrg1989 [DOI] [PubMed] [Google Scholar]
- Emlen D.J, Allen C.E. Genotype to phenotype: physiological control of trait size and scaling in insects. Integr. Comp. Biol. 2003;43:617–634. doi: 10.1093/icb/43.5.617. doi:10.1093/icb/43.5.617 [DOI] [PubMed] [Google Scholar]
- Emlen D.J, Nijhout H.F. The development and evolution of exaggerated morphologies in insects. Annu. Rev. Entomol. 2000;45:661–708. doi: 10.1146/annurev.ento.45.1.661. doi:10.1146/annurev.ento.45.1.661 [DOI] [PubMed] [Google Scholar]
- Emlen D.J, Corley Lavine L, Ewen-Campen B. On the origin and evolutionary diversification of beetle horns. Proc. Natl Acad. Sci. USA. 2007;104(Suppl. 1):8661–8668. doi: 10.1073/pnas.0701209104. doi:10.1073/pnas.0701209104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M. Cell determination strategies in the Drosophila eye. Development. 1997;124:261–270. doi: 10.1242/dev.124.2.261. [DOI] [PubMed] [Google Scholar]
- Fristrom, D. & Fristrom, J. W. 1993 The metamorphic development of the adult epidermis. In The development of Drosophila melanogaster, vol. 2 (eds M. Bate & A. Martinez Arias), pp. 843–897. Cold Spring Harbor, NY: Cold Spring Harbor Press.
- Garcia-Bellido A, Merriam J.R. Parameters of the wing imaginal disc development of Drosophila melanogaster. Dev. Biol. 1971;24:61–87. doi: 10.1016/0012-1606(71)90047-9. doi:10.1016/0012-1606(71)90047-9 [DOI] [PubMed] [Google Scholar]
- Gilbert L.I, Rybczynski R, Tobe S. Endocrine cascade in insect metamorphosis. In: Gilbert L.I, Tata J.R, Atkinson B.G, editors. Metamorphosis: ostembryonic reprogramming of gene expression in amphibian and insect cells. Academic Press; San Diego, CA: 1996. pp. 59–107. [Google Scholar]
- Goberdhan D.C, Wilson C. Insulin receptor-mediated organ overgrowth in Drosophila is not restricted by body size. Dev. Genes Evol. 2002;212:196–202. doi: 10.1007/s00427-002-0226-3. doi:10.1007/s00427-002-0226-3 [DOI] [PubMed] [Google Scholar]
- Huxley J.S. Constant differential growth-ratios and their significance. Nature. 1924;114:895–896. doi:10.1038/114895a0 [Google Scholar]
- Huxley J.S, Tessier G. Terminology of relative growth. Nature. 1936;137:780–781. doi:10.1038/137780b0 [Google Scholar]
- Ikeya T, Galic M, Belawat P, Nairz K, Hafen E. Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr. Biol. 2002;12:1293–1300. doi: 10.1016/s0960-9822(02)01043-6. doi:10.1016/S0960-9822(02)01043-6 [DOI] [PubMed] [Google Scholar]
- Kawamura K, Shibata T, Saget O, Peel D, Bryant P.J. A new family of growth factors produced by the fat body and active on Drosophila imaginal disc cells. Development. 1999;126:211–219. doi: 10.1242/dev.126.2.211. [DOI] [PubMed] [Google Scholar]
- Lapicque, L. 1898 Sur la relation du poids de l'encéphale aux poids du corps. Comptes Rendus Séances Soc. Biol. Fil., (Sér 10)5, 62–63.
- Madhavan M.M, Schneiderman H.A. Histological analysis of dynamics of growth of imaginal disks and histoblast nests during larval development of Drosophila melanogaster. Wilhelm Roux's Arch. Dev. Biol. 1977;183:269–305. doi: 10.1007/BF00848459. doi:10.1007/BF00848459 [DOI] [PubMed] [Google Scholar]
- Martin P.F. Direct determination of the growth rate of Drosophila imaginal discs. J. Exp. Zool. 1982;222:97–102. doi:10.1002/jez.1402220113 [Google Scholar]
- Martin P, Shearn A. Development of Drosophila imaginal discs in vitro: effects of ecdysone concentration and insulin. J. Exp. Zool. 1980;211:291–301. doi:10.1002/jez.1402110306 [Google Scholar]
- Milan M, Campuzano S, Garcia-Bellido A. Cell cycling and patterned cell proliferation in the Drosophila wing during metamorphosis. Proc. Natl Acad. Sci. USA. 1996;93:11 687–11 692. doi: 10.1073/pnas.93.21.11687. doi:10.1073/pnas.93.21.11687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner A.L, Rosenberg A.J, Nijhout H.F. Control of growth and differentiation of the wing imaginal disk of Precis coenia (Lepidoptera: Nymphalidae) J. Insect Physiol. 2000;46:251–258. doi: 10.1016/s0022-1910(99)00177-8. doi:10.1016/S0022-1910(99)00177-8 [DOI] [PubMed] [Google Scholar]
- Mirth C. Ecdysteroid control of metamorphosis in the differentiating adult leg structures of Drosophila melanogaster. Dev. Biol. 2005;278:163–174. doi: 10.1016/j.ydbio.2004.10.026. doi:10.1016/j.ydbio.2004.10.026 [DOI] [PubMed] [Google Scholar]
- Mirth C, Truman J.W, Riddiford L.M. The role of the prothoracic gland in determining critical weight for metamorphosis in Drosophila melanogaster. Curr. Biol. 2005;15:1796–1807. doi: 10.1016/j.cub.2005.09.017. doi:10.1016/j.cub.2005.09.017 [DOI] [PubMed] [Google Scholar]
- Neufeld T.P. Body building: regulation of shape and size by PI3K/TOR signaling during development. Mech. Dev. 2003;120:1283–1296. doi: 10.1016/j.mod.2003.07.003. doi:10.1016/j.mod.2003.07.003 [DOI] [PubMed] [Google Scholar]
- Nijhout H.F. The control of body size in insects. Dev. Biol. 2003;261:1–9. doi: 10.1016/s0012-1606(03)00276-8. doi:10.1016/S0012-1606(03)00276-8 [DOI] [PubMed] [Google Scholar]
- Nijhout H.F, Wheeler D.E. Growth models of complex allometries in holometabolous insects. Am. Nat. 1996;148:40–56. doi:10.1086/285910 [Google Scholar]
- Nijhout H.F, Williams C.M. Control of moulting and metamorphosis in the tobacco hornworm, Manduca sexta (L.): cessation of juvenile hormone secretion as a trigger for pupation. J. Exp. Biol. 1974a;61:493–501. doi: 10.1242/jeb.61.2.493. [DOI] [PubMed] [Google Scholar]
- Nijhout H.F, Williams C.M. Control of moulting and metamorphosis in the tobacco hornworm, Manduca sexta (L.): growth of the last-instar larva and the decision to pupate. J. Exp. Biol. 1974b;61:481–491. doi: 10.1242/jeb.61.2.481. [DOI] [PubMed] [Google Scholar]
- Nijhout H.F, Davidowitz G, Roff D.A. A quantitative analysis of the mechanism that controls body size in Manduca sexta. J. Biol. 2006;5:16.1–16.15. doi: 10.1186/jbiol43. doi:10.1186/jbiol43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham S, Bo¨hni R, Stocker H, Brogiolo W, Hafen E. Genetic control of size in Drosophila. Phil. Trans. R. Soc. B. 2000a;355:945–952. doi: 10.1098/rstb.2000.0630. doi:10.1098/rstb.2000.0630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham S, Montagne J, Radimerski T, Thomas G, Hafen E. Genetic and biochemical characterization of dTOR, the Drosophila homolog of the target of rapamycin. Genes Dev. 2000b;14:2689–2694. doi: 10.1101/gad.845700. doi:10.1101/gad.845700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwait J.H. Juvenile hormone and the adult development of Drosophila. Biol. Bull. 1974;147:119–135. doi: 10.2307/1540573. doi:10.2307/1540573 [DOI] [PubMed] [Google Scholar]
- Riddiford L.M. Hormones and Drosophila development. In: Bate M, Martinez Arias A, editors. The development of Drosophila melangogaster. Cold Spring Harbour Laboratory Press; New York, NY: 1993. pp. 899–939. [Google Scholar]
- Rulifson E.J, Kim S.K, Nusse R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science. 2002;296:1118–1120. doi: 10.1126/science.1070058. doi:10.1126/science.1070058 [DOI] [PubMed] [Google Scholar]
- Safranek L, Cymborowski B, Williams C.B. Effects of juvenile hormone on ecdysone-dependent development in the tobacco hornworm, Manduca sexta. Biol. Bull. 1980;158:248–256. doi:10.2307/1540934 [Google Scholar]
- Schlichting C.D, Pigliucci M. Sinaeur; Sunderland, MA: 1998. Phentypic evolution—a reaction norm perspective. [Google Scholar]
- Shea B.T. Bivariate and multivariate growth allometry—statistical and biological considerations. J. Zool. 1985;206:367–390. [Google Scholar]
- Shingleton A.W, Das J, Vinicius L, Stern D.L. The temporal requirements for insulin signaling during development in Drosophila. PLoS Biol. 2005;3:e289. doi: 10.1371/journal.pbio.0030289. doi:10.1371/journal.pbio.0030289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingleton A.W, Frankino W.A, Flatt T, Nijhout H.F, Emlen D.J. Size and shape: the developmental regulation of static allometry in insects. BioEssays. 2007;29:536–548. doi: 10.1002/bies.20584. doi:10.1002/bies.20584 [DOI] [PubMed] [Google Scholar]
- Stern D.L, Emlen D.J. The developmental basis for allometry in insects. Development. 1999;126:1091–1101. doi: 10.1242/dev.126.6.1091. [DOI] [PubMed] [Google Scholar]
- Truman J.W, Hiruma K, Allee J.P, Macwhinnie S.G, Champlin D.T, Riddiford L.M. Juvenile hormone is required to couple imaginal disc formation with nutrition in insects. Science. 2006;312:1385–1388. doi: 10.1126/science.1123652. doi:10.1126/science.1123652 [DOI] [PubMed] [Google Scholar]
- Williams C.M. Growth in insects. In: Locke M, Smith D.S, editors. Insect biology in the future. Academic Press; New York, NY: 1980. pp. 369–383. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.