Abstract
The number of animals used in science is increasing, bringing a concomitant obligation to minimize suffering. For animals with progressive conditions, euthanasia at a ‘humane end point’ is advised if the end point is scientifically valid, predictive and accurate. Our aim was to test the hypothesis that behavioural changes would reliably precede clinical signs of disease in a progressive neurological model, using retrospective analysis. We observed 100 pair-housed female R6/1 transgenic Huntington's disease (HD) mice and 28 pair-housed female wild-type (WT) mice in standard- or resource-enriched cages. Disease progression was monitored until one member of each HD pair reached a pre-defined end point based on pathological symptoms (HD end). This mouse was then euthanized together with its cage mate (HD other) and any matched WT pairs. At euthanasia, HD mice had significantly greater absolute and relative organ weights, and significantly higher α1 acid glycoprotein concentrations than WT mice, indicating reduced welfare. HD mice initially showed significantly greater use of cage resources than WT mice but this declined progressively. Steeper declines, and earlier cessation, in the use of some climbing and exploration resources occurred in the HD end mice compared with the HD other mice. Behavioural change can be an early indicator of disease onset.
Keywords: humane end point, Huntington's disease, mouse welfare, behaviour
1. Introduction
Reliable data on animal use in scientific procedures are not widely available but it has been estimated that 50–100 million animals per annum are used in scientific procedures worldwide, with approximately 10.7 million animals used in Europe in 2002 (Nuffield Council for Bioethics 2005). The most accurate information exists for Great Britain, where the number of scientific procedures on animals declined between 1988 and 2001, but has now risen again to over 3 million per annum (Home Office 2007). Further increases seem likely, primarily due to increasing reliance on genetically modified and ‘humanized’ (i.e. where the modification incorporates human genes relevant to immune function or pharmacological response) mice and rats, as model systems for human disease and in functional genomics studies (Hudson 2007).
Humane experimentation requires researchers to consider the welfare of the animals they use. The increase in rodent use brings a concomitant obligation to minimize any suffering. For animals with progressive or chronic conditions, this can be achieved by euthanasia at an early ‘humane end point’ if it can be shown that humane end points which substitute for more severe outcomes such as severe pathologies or death, are scientifically valid, predictive and accurate (Nuffield Council for Bioethics 2005; NC3Rs 2007). However, it is a challenge to find parameters that are reliable and truly predictive of a certain stage of disease. Longitudinal studies are required whereby clinical signs are recorded throughout the animals' lives, for retrospective analysis of signs that have preceded pathology or death. This retrospective technique has been used in just a handful of published studies (Hendriksen & Morton 1999; Nemzak et al. 2004) and there is no information on the value of monitoring spontaneous or unprovoked behaviour in this context. Behaviour can be a highly sensitive indicator of pain (Roughan & Flecknell 2001) or emotional distress, and some behavioural changes are potentially generalizable across models or diseases as indicators of disease onset. Work is urgently needed to establish how behavioural changes precede pathology or death in animals with chronic or progressive conditions so that efficient automated behavioural monitoring systems can be developed (Hawkins 2002). This same approach would also be useful in identifying accurate and reliable precursors of disease for the development of new therapies targeted at early stages of disease. Thus, there are conjoint biomedical and animal welfare benefits in this approach.
Our aim was to assess whether monitoring home cage behaviour could play a role in determining humane end points for laboratory animals with progressive conditions. We tested the hypothesis that behavioural changes would reliably precede clinical signs of disease, particularly in resource-enriched (RE) cage environments. There is a growing literature on sickness behaviour, which views behavioural changes such as hyperalgesia, aversion to noxious stimulation and sleepiness as adaptive responses, mediated by cytokines, that can aid survival or recovery (Dantzer & Kelley 2007). Our hypothesis depends also on the evolutionary principle that the use of some resources is more ‘resilient’ than others. Resources that are essential for survival such as food and water are obtained at the same frequency even when conditions get harsh (McFarland 1993). The use of other resources, for example, those providing opportunities for exploration or play, is less resilient, and these are obtained with declining frequency when conditions are tough. Healthy mice reorganize their time budgets, as the cost of accessing resources increases, to defend their usage of the resources most important to them (Sherwin & Nicol 1997; Sherwin et al. 2004). The use of less important resources should also decline or cease at a relatively early stage of disease development, as animals channel their reserves to cope with the consequences of disease. The provision of an RE environment should therefore allow the most sensitive detection of disease onset, as mice retain control over the extent of physical or mental effort they invest in a range of behaviours. We wished to determine whether such subtle behavioural adjustments in the home cage could be detected in advance of obvious signs of sickness behaviour or clinical signs of disease.
We used R6/1 transgenic Huntington's disease (HD) mice that developed progressive neurological and physical deficits and compared their welfare during the study and at euthanasia with wild-type (WT) mice. Such comparisons were valid because the WT and HD mice were siblings of the same strain, differing only in the presence or absence of the transgene mutation responsible for HD. We then retrospectively compared the behaviour of HD mice that were euthanized when they reached a pre-defined clinical end point (HD end) with mice that were euthanized at the same time but had not reached a clinical end point themselves (HD other).
2. Material and methods
(a) Animals
Male and female hemizygous R6/1 transgenic mice (TgN[Hdexon1]61Gpb [R6/1]) were mated with B6CBA mice. The transgenic female mice resulting from these matings were used in our study. One hundred mice carrying the transgene (exon 1 of the gene for human huntingtin) and 28 mice without the transgene (WT) were transported from the breeding facility to the final destination and housed in same genotype, non-sibling, pairs from approximately 34 days old and monitored daily until euthanasia. Mice were individually identified by bleaching a 1 cm2 patch of fur for one mouse of each pair. Subsequent confirmatory genotyping showed that four mice had been originally misclassified. Their correct genotypes were used in subsequent statistical analysis.
(b) Housing
All mice were housed within the same room in which the lights were turned on automatically between 03.00 and 15.00 hours. Air temperature was maintained between 20 and 22°C. Cages containing either HD or WT mice were systematically allocated to racks to avoid confounding of treatment with position. Thirty-two video cameras were mounted, one between every two adjacent cages. Cages were cleaned weekly. Half of the WT and half of the HD mice were allocated to standard-enriched (SE) cages. The white polyethylene cages measured 37×21×15 cm (L×W×H) and had stainless steel wire mesh tops. A 1.5 cm layer of sawdust (Lignocell, grades 1–2, International Product Supplies), shredded paper (various sources), a semi-transparent polyethylene shelter (Amber tinted Mouse Igloo, Lillico Biotechnology) and a cardboard tube (Critter's Choice Chubes, 15 cm long, 4 cm in diameter) were provided in all cages. The remaining mice were allocated to RE cages. These contained all the features of the SE cages, but additionally contained a rope (55 cm loop of nylon rope with both ends attached to the bars of the cage lid), a wooden beam (1 cm wide, 11 cm above the cage floor) and a ladder (14×9.5 cm vertical wire mesh (0.5 cm apertures and 0.1 cm wire thickness)), attached to the cage wall to encourage physical activity. RE cages were also fitted with two 7×7 cm chambers of perforated transparent plastic (Habitrail, Rolf C Hagen (UK) Ltd), located externally to the cage at the end opposite the feed and water to encourage exploration. The left chamber was empty and anything carried into this chamber by the mice was removed daily. The right chamber contained two items selected daily and pseudo-randomly (such that the same item was not presented on 2 consecutive days) from a pool of eight (walnut, rodent chew stick, rodent fruit stick, wood chip, natural fibre rope, rawhide stick, grit paper and rawhide sheet). Both chambers were connected to the interior of the cage by 3.5 cm diameter Habitrail plastic tubes. An entry tube for the box used in the light chamber test was located between the two chambers. Water and feed were available ad libitum.
(c) Monitoring procedures
Monitoring was used to achieve three objectives. First, we assessed the progression of HD using established tests and used criteria based on these tests to determine the time of euthanasia, which was achieved by cervical dislocation. Second, we used a range of indicators taken at, or close to, euthanasia, to determine whether the welfare of HD mice had declined. Third, we took continuous behavioural records throughout the trial, and examined these retrospectively to identify associations between behavioural changes and subsequent clinical end points.
(d) Assessment of disease progression and timing of euthanasia
Disease progression was monitored every day using established tests conducted from 09.30 hours in a room adjoining the home cage rooms. Each mouse (including WT) was assessed in the home cage for normality of general activity, appearance and health using the criteria suggested by Mertens & Rulicke (1999). This was followed by an assessment using tests selected from the primary screen of the SHIRPA protocol (Rogers et al. 1999). The selected tests, spontaneous activity, tremor, limb clasp, grip strength (conducted daily), wire manoeuvre and negative geotaxis (conducted twice each week) were considered particularly informative in tracking the development of HD. Performance on each test was scored using published ordinal rankings and descriptions (http://www.mgu.har.mrc.ac.uk/facilities/mutagenesis/mutabase/shirpa_1.html), with the exception of the limb clasp test where we included an intermediate descriptor ‘attempted to clasp’ between the categories clasping absent and clasping present.
HD mice were kept until one member of each pair (HD end) reached a pre-defined humane end point based on test results and daily checks. Euthanasia decisions were taken based on any of a defined set of symptoms being shown. These included the occurrence of mild tremors or limb clasping on two consecutive tests (half the mice were only euthanized if this was accompanied by other general signs of minor ill-health) or on the first sign of any significant indicator of ill-health. It is important to stress here that the end points selected were the first sign of an obvious behavioural phenotype as the study was focused on the very early stages of disease. Whatever the precise reason for euthanasia, data for all HD end mice were pooled, following analyses that showed no effects of reason for euthanasia on any of the measures of interest in this study. Once the HD end member of each pair had reached an end point, both mice of that pair (HD end and HD other) were euthanized, and a range of post-mortem measures taken. WT mice had been pre-allocated to 14 randomly selected pairs of HD mice. These WT mice were euthanized at the same time as their allocated pair as controls.
Pearson's correlations were used to examine associations between the use of different resources in the cage, and analysis of variance was used to examine effects of genotype and cage enrichment on disease progression.
(e) Assessment of welfare at the time of euthanasia
The levels of faecal corticosterone in the weeks leading up to euthanasia were determined. Droppings had been collected 5 days per week, during SHIRPA testing. Droppings were collected using rat-tooth forceps, cleaned with approximately 70% ethanol/dH2O between mice. Droppings were immediately placed in numbered Eppendorf centrifuge tubes (Fisher Scientific, UK; 1.5 ml Safe-Lock tubes) which were frozen at −20°C within 8 hours of collection. All samples obtained from an individual mouse over the 5-day period were pooled. Once the date of euthanasia was known, samples were analysed from the week of euthanasia and from one, two, three and seven weeks prior to euthanasia. These pooled samples were dried at 25°C for 3–5 days until weight remained constant. They were then ground and duplicate 5 mg samples were taken for analysis of corticosterone using a commercially available ELISA kit (Immunodiagnostic Systems Ltd, Tyne and Wear, UK).
After euthanasia, a cardiac blood sample was taken for analysis of serum corticosterone and α1-acid glycoprotein (α1AG) concentrations. α1AG is an acute phase protein, forming part of the non-specific innate immune system that plays an important protective role in animals. α1AG concentrations rise in response not only to infection, inflammation and physical stress (reviewed by Murata et al. 2004) but also to psychological stressors such as inescapable tail shock (rats; Deak et al. 1997) and confinement (pigs, Sorrells et al. 2007). It therefore provides a useful indicator of welfare. In the present study, the concentration of the α1AG in serum was determined using immunodiffusion kits (Cardiotech Services, Inc., KY, USA). Mouse serum and standards were placed in wells containing α1AG antiserum and the diameter of the resultant precipitin rings was measured. Concentrations were determined by constructing a reference curve. Serum corticosterone was assessed using a competitive enzyme immunoassay for mouse or rat serum or plasma corticosterone (Immunodiagnostic Systems Ltd).
After blood sampling, the euthanized mice were placed on ice. The brain was removed and weighed. The body was then removed from ice and the liver and adrenal glands were removed and weighed. The effects of chronic stress on organ composition and weight are complex, but adrenal gland and liver weights generally increase (e.g. Blanchard et al. 1998; Thaxton & Puvadolpirod 2000).
Analysis of variance was used to assess the effects of genotype and cage enrichment on acute-phase protein (APP), serum corticosterone and organ weights taken at euthanasia. Repeated measures analysis of variance, using age at euthanasia as a co-variate, was used to examine changes in faecal corticosterone concentrations as the date of euthanasia approached.
(f) Retrospective assessment of behaviour and body weight
Mice were weighed twice each week to examine growth rate, the age at which maximum body weight was obtained and to monitor any falls in body weight that could be associated with disease onset.
Behavioural observations were taken during light and dark phase to establish whether early behavioural changes were reliable indicators of later disease state. Every day during the light phase, between 09.00 and 09.30 hours, the resting posture of each mouse was coded from an instantaneous behaviour sample as either ‘sitting’ or ‘laterally curled’.
During the dark phase, the behaviour of each pair of mice was recorded using sensitive cameras with built-in infrared lighting at least every 2 days, with occasional daily observations taken when additional video cameras were available. Each cage was automatically filmed for a 10-min period between 01.00 and 02.00 hours. From the video tapes, the location of each mouse within a pair was instantaneously recorded once every minute (e.g. on the cage roof, on the ladder, touching the feeder). These measures were taken as indicators of resource use. Observations within each 10-min period were highly correlated so, to ensure statistical independence, responses were coded as 1 if behaviour x was performed at any minute in the 10-min period and as 0 otherwise. This resulted in 5925 periods within the 128 mice, for retrospective analysis.
Changes in emotional and cognitive response were examined using a light chamber test given twice per week, which required no handling. The mice were presented with a trade-off where highly palatable food was placed within a well-lit (mildly aversive) area. Light chambers (8×8×15 cm high with a wire mesh top) were constructed from proprietary rodent houses (Safari rooms, Habitrail) that had been sprayed with matt black paint. One light chamber was attached between the two fixed chambers of each cage, using a 3.5 cm plastic tube. A 6 V lamp was inserted through a hole 8 cm high in the back of the box. When switched on, the light level was approximately 180 lux at mouse height. Testing took place for 15 min at 16.00 hours, when approximately 20 g of hamster mix was placed on the floor of the light chamber and the light switched on. The latency of each mouse to enter the chamber, and the number and duration of visits made were recorded from video.
Statistical analysis of the retrospective data was achieved using an innovative approach. The extent to which the behaviour of individual mice housed in pairs was statistically independent was accounted for by using a multilevel approach, which partitioned variation arising at mouse, cage or higher (e.g. treatment, strain) levels. Multilevel curve fitting for analysis of body weight data was conducted by fitting separate quadratic curves for HD end, HD other and WT mice, and including a random effect for each mouse to account for individual mouse differences using the MLwiN software (Rasbash et al. 2000) developed by one of the authors (W.B.). The differential patterns of resource use shown by WT, HD end and HD other mice in the RE cages were analysed similarly. For each behaviour, for each daily period of observation, a 1 was recorded if the particular behaviour was observed and a 0 otherwise. Logistic regression models for each behaviour included random effects to account for differences due to individual mice and cages. Mouse-type covariates and linear and quadratic age terms plus interactions were included to produce separate probability curves for WT, HD end and HD other mice.
The above analyses examined differences between groups of WT, HD end and HD other mice, but decisions about when to euthanize a mouse would need to be made at an individual mouse level. For traits where clear significant differences were detected between WT, HD end and HD other mice, we assessed the diagnostic value of that trait by calculating sensitivity (the proportion of HD end individuals that would have been correctly identified in advance of the clinical end points that we used) and specificity (the proportion of WT or HD other individuals that would have been correctly identified in advance of the clinical end points that we used).
3. Results
(a) Disease progression using established tests
The average age of the mice at euthanasia was 144 s.d. 31 days. The mice used the additional resources provided in the RE cages. During the dark phase, the chambers were used approximately 7.5% of observed time, and the beam, rope and ladder approximately 1.5% of observed time, primarily at the expense of the time spent climbing the cage roof or using the cardboard tubes in the SE cages. There were positive correlations between the average use of resources by individual mice. Thus, mice that used the left chamber a lot also used the right chamber a lot (r=0.51; p<0.001). Weaker correlations were also found for the climbing equipment: beam and rope (0.21; p<0.10), beam and ladder (0.22; p<0.10) and rope and the ladder (0.29; p<0.05).
For validation, we checked that the SHIRPA tests used to monitor disease progression and inform euthanasia decisions, revealed differences between HD and WT mice. One test, negative geotaxis, did not discriminate between these genotypes, with no significant differences in average scores at any time, little evidence of progression (i.e. first occurrence of low score followed by later occurrence of a high score) and little consistency within individuals. There was a trend towards a difference between genotypes in spontaneous activity (F1,126=4.06; p=0.06), and strongly significant differences between genotypes in limb clasping (F1,126=19.7; p<0.001), grip strength (F1,126=22.2; p<0.001) and wire manoeuvre (F1,126=14.1; p<0.001) test scores in the expected direction. These tests were therefore used to examine possible housing effects on disease progression. The most specific test, with the fewest false positive scores from WT mice, was the wire manoeuvre test.
Restricting analyses to RE and SE HD mice only, there was no significant effect of housing on age at euthanasia, but type of housing influenced the time of onset of some SHIRPA signs. The age at first occurrence of a score indicating impaired activity was noted for each mouse for each test. If a mouse never exhibited a particular score then its age at euthanasia was entered as its individual default value. A significant delay was found for the age at first occurrence of ‘difficulty grasping wire’ (RE 125.3 s.e.m. 5.4 days; SE 108.8 s.e.m. 5.4 days; F=4.69; p=0.03) in the wire manoeuvre test. There was, however, an opposite effect of enrichment on age at first occurrence of ‘no grip’ in the grip strength test (RE 119.8 s.e.m. 5.0 days, SE 143.6 s.e.m. 5.0 days; F=11.3; p=0.001) and of ‘moderate’ (but not slow) movement in the spontaneous activity test (RE 49.0 s.e.m. 3.5 days, SE 61.0 s.e.m. 3.6 days; F=5.75; p=0.02).
(b) Comparison of the welfare of HD and WT mice
Our aim was to assess whether welfare had declined in the HD mice at the time of euthanasia. Our end points had been selected based on current best practice, so we predicted that there would be no signs of significantly impaired welfare.
However, at the time of euthanasia, we found indications that the welfare of the HD mice had diminished, including significantly greater APP (α1 acid glycoprotein) concentrations (μg ml−1; HD 365.1 s.e.m. 19.62 versus WT 261.5 s.e.m. 38; F1,74=5.85; p<0.02). These concentrations were numerically but not significantly greater in HD (end) than in HD (other) mice (369.7 s.e.m. 26.2 versus 358.9 s.e.m. 30.0). In addition, both absolute and relative liver weights were greater in HD than WT mice (absolute weight (g) HD1.12 s.e.m. 0.02; WT 1.00 s.e.m. 0.03; p<0.005). The relative weight of the left adrenal gland was also significantly greater in HD mice (p<0.01) while absolute and relative brain weights (as expected) were significantly lower (absolute weight (g) HD 0.407 s.e.m. 0.003; WT 0.459 s.e.m. 0.006; p<0.001).
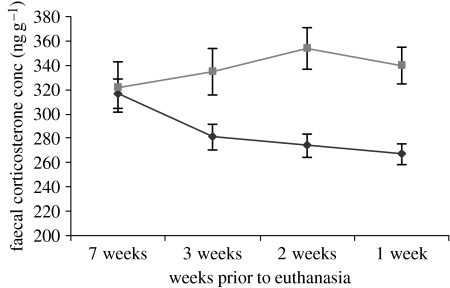
Contrary to previous reports, serum corticosterone levels were significantly lower in HD than WT mice (HD 120.3 s.e.m. 10.3, WT 160.6 s.e.m. 19.3 ng ml−1; p=0.02). There was also a significant interaction between whether a mouse was HD end or HD other, and whether she had been housed in an SE or RE cage. HD end mice, housed in RE cages, had the highest serum corticosterone concentrations (interaction p=0.01). For mice housed in the RE cages, there was a significant positive correlation between mean use of the climbing resources throughout the study and the serum corticosterone levels at the end of the study (p=0.01). Analysis of the faecal corticosterone concentrations in the weeks leading up to euthanasia confirmed the finding that HD mice had lower levels. Faecal corticosterone concentrations (ng g−1) declined significantly as the date of euthanasia approached (effect of time: F1,100=7.06; p=0.009) but this occurred in the HD mice only (interaction between time and genotype: F1,100=3.89; p=0.05; figure 1). There was no significant difference between HD end and HD other mice.
Figure 1.
Faecal corticosterone concentrations (ng g−1) decline in HD mice as date of euthanasia approaches. Diamonds, HD mice; squares, WT mice.
(c) Retrospective assessment of behavioural and body weight changes preceding clinical disease
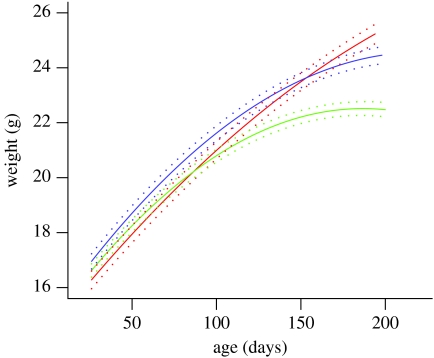
There were no significant differences between the weight of HD and WT mice on arrival, their average body weight throughout the experiment, or maximum body weight attained. Initial curve fitting, using the quadratic curve function in SPSS, predicted an earlier age at peak body weight (when growth curve reaches plateau) for HD mice (228 s.e.m. 10.9 day) compared with WT mice (264 s.e.m. 19.4 days). Multilevel modelling showed no significant difference between the growth curves of the WT and HD other mice at any age, although the analysis confirmed that WT mice were likely to reach their peak body weight later than HD mice. From approximately 100 days of age, HD end mice had significantly slowed growth (p<0.05) compared with HD other mice (figure 2).
Figure 2.
Multilevel modelling fitted growth curves for each category of mouse (±1 s.e.). From approximately 100 days of age, HD end mice had significantly slowed growth compared with the HD other mice (red, WT; blue, HD other; green, HD end).
The light-phase resting posture of the HD and WT mice differed. The resting posture of pairs of mice was highly correlated so, to avoid false replication, one mouse from each pair was randomly selected and a score of 0 allocated on days when the mouse was observed sitting and a score of 1 allocated on days when the mouse was observed laterally curled. These scores were plotted by day for each mouse from arrival until euthanasia. Overall, lateral curling was observed more often in RE than SE cages, (F1,60=16.54; p=0.001) but there was no difference between HD and WT mice in the average amount of lateral curling observed over the full experimental period. Despite this, plots for individual WT mice showed a clear shift in resting posture with time. Change in resting posture was examined by fitting a linear regression line over the experimental period for each mouse, and then entering the resultant slope values for each mouse into an analysis of variance. During the experiment, the WT mice showed a progressive increase in lateral-curling with age, indicated by significantly higher slope values than HD mice (F1,62=11.57; p=0.0012). By contrast, lateral curling remained constant with age for the HD mice. Thus, the HD levels of lateral curling were greater than those of the WT mice when young, but less than those of the WT mice when older.
Multilevel modelling showed that the only behaviour that discriminated between HD and WT mice in both RE and SE cages was drinking behaviour, which declined with age in WT mice, but increased in HD mice. Between approximately 100 and 160 days of age, this difference was significant (p<0.05) but, as the number of mice alive declined, this difference ceased to be significant. There was a marked cage effect, with significantly more drinking occurring in the SE cages than in the RE cages (p<0.05). Drinking behaviour did not differ between HD end and HD other mice.
Further analysis of the mice housed in the RE cages only, showed that patterns of resource use discriminated between WT and HD mice and between HD end and HD other mice. For each mouse in the RE cages, we calculated the number of days between the last observed use of an enrichment or cage resource and the date of euthanasia (difference between last observed use and euthanasia, DLOUE). Occasionally, an individual mouse was never observed to use a particular resource. If so, a default value was entered being the number of days that mouse was in the study. We predicted that the DLOUE should be longer for mice that were developing disease than for mice that were not developing disease, or were developing disease at a slower rate. Mice that are developing disease should ‘give up’ using resources earlier. We therefore compared the DLOUE values for mice that were euthanized because they showed clinical symptoms (HD end), with those of mice that were euthanized so that they would not be left singly housed (HD other). For all resources, HD end mice had an earlier last observed date of use compared with HD other mice, although not all of these individually reached statistical significance. The strongest effects were noted for the climbing resources in the RE cages. For example, the DLOUE period for use of the rope was 75.25 s.e.m. 9.30 days for HD end mice and 51.81 s.e.m. 6.41 days for HD other mice (p=0.04). These results give average last-observed times not actual last-used times.
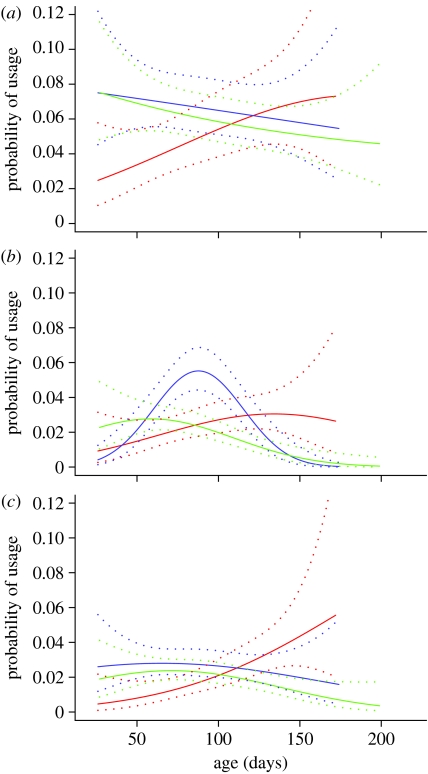
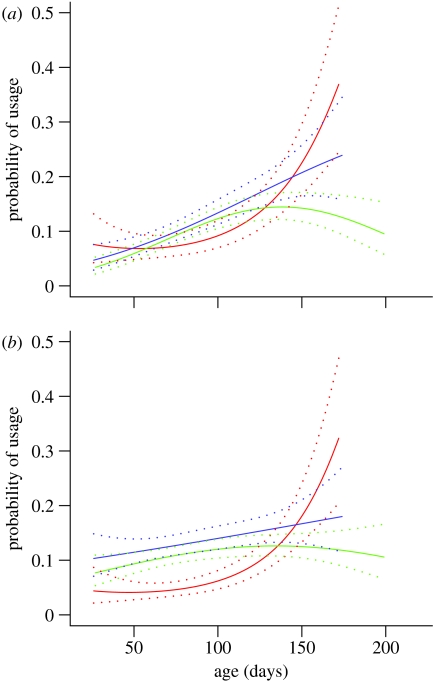
The probability of observing a WT mouse using climbing resources (figure 3) or chambers (figure 4) increased significantly (p<0.05) with age. The HD mice were initially more active, but their use of all resources declined. For all resources, the HD end mice showed steeper drops in resource use from a relatively early age than the mice euthanized with their cage mates (HD other) There were significant differences between WT and HD other mice in the combined use of the exploratory chambers between days 70–100 (p<0.05). Owing to low sample sizes, some of the lines are not statistically significantly different from each other, or indicated trends (p<0.1), but significant differences occurred for the use of the rope between ages 80 and 110 days between HD end and HD other (p<0.05; figure 3) and for the right chamber between WT and HD other between ages 50 and 110 days (p<0.05; figure 4). However the similarities in pattern across all the different resources provided in the RE cages are striking.
Figure 3.
Probability of using the climbing resources in the RE cages modelled with mouse age, using MLwiN (±1 s.e.). (a) beam, (b) rope and (c) ladder. The curves have greatest validity within the range 40–170 days. The tails of the curves at ages up to 40 days and from 170 days onwards must be interpreted with caution as there were fewer data readings for mice this young (due to differing age at trial entry) or old (due to variable euthanasia age; red, WT; blue, HD other; green, HD end).
Figure 4.
(a,b) Probability of using the chambers in the RE cages modelled with mouse age using MLwiN (±1 s.e.). The curves have the greatest validity within the range 40–170 days (red, WT; blue, HD other; green, HD end).
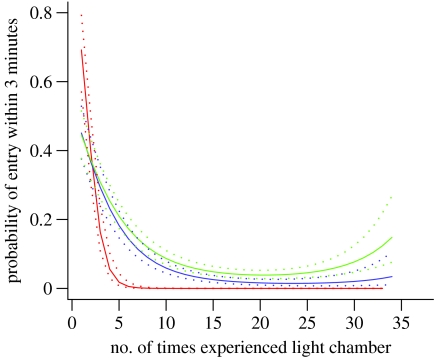
Next, we examined the responses of the mice in the light chamber test. Data for latency to enter the chamber were bi-modal so a threshold of 3 min was taken to distinguish between mice that entered the chamber quickly and those that entered slowly or not at all. We found that the HD mice were initially quicker to enter than the WT mice (figure 5) but they took longer to enter the chamber as disease progressed. When confidence envelopes were calculated, these differences were significant (p<0.05) from the third test onwards. There were no significant differences in the latencies for HD end and HD other mice.
Figure 5.
Latency to enter the light chamber. The x-axis measures the number of experiences of the light chamber (including the one where measurement taken) as tests were conducted once every 4 days (±1 s.e.). The y-axis records whether the chamber was entered within 3 min (score 0) or with a latency exceeding 3 min (score 1; red, WT; blue, HD other; green, HD end).
Finally, we assessed the diagnostic value of a decrease in body weight or a change in light chamber behaviour as potential criteria for making early euthanasia decisions about individual mice. A decrease in weight of more than 5% was selected as one criterion, after examination of growth curves, with 56.6% of HD mice showing a decrease in this magnitude either once or twice during the experiment. However, HD end and HD other mice were not well distinguished using this criterion (sensitivity 61.1% and specificity 51.1%). We then categorized mice according to whether they refused to enter the chamber on at least one occasion after previously exhibiting entering behaviour, with 28.6% of HD mice showing this tendency. Again however, mice were not well distinguished (sensitivity 32.0%; specificity for HD other 62.5%; specificity for HD other plus WT 80.5%).
4. Discussion
(a) Disease progression in SE and RE cages
The primary aim of this study was to investigate whether behavioural changes in the home cage reliably predict the onset of clinical disease, as detected by existing humane end points, and whether such predictions can be made earlier in a RE cage that allows the expression of a wider variety of behaviour patterns. A complicating factor was that provision of additional cage resources might alter the timing of onset of clinical disease, as such delays have been found previously for R6 HD mice housed in enriched cages (cardboard, paper and plastic objects replaced every 2 days) compared with their counterparts housed in non-enriched cages (Van Dellen et al. 2000; Spires et al. 2004; Lazic et al. 2006). However, the effect of level of enrichment was examined by Hockly et al. (2002) who compared R6/2 mice housed in non-enriched, minimally enriched or maximally enriched cages. Both minimal and maximal levels of enrichment delayed disease progression to a similar extent relative to non-enriched controls. In our study, we also found no consistent effects of the level of enrichment on disease progression. RE R6/1 HD mice showed a delay in the onset of difficulty grasping wire relative to SE mice, but there was an opposite effect on the onset of no grip and ‘moderate movement’ indicators of disease. A lack of a clear effect of resource provision in our study may therefore be because we did not provide the non-enriched conditions that appear to accelerate disease progression (Hockly et al. 2002).
(b) Early predictors of disease onset
Retrospective analysis of growth data and home cage behaviour revealed some differences between WT and HD mice prior to reaching the humane end point. HD mice from both cage types appeared to be on course to reach the point at which their growth curve flattened out earlier than WT mice. They also showed an increase in drinking with age whereas WT mice showed a decrease, and their levels of sitting and lateral curling remained constant with age, in contrast to the WT mice whose resting posture changed with age.
There were also interesting differences in behaviour, observed in the RE cages only, between WT mice, those HD mice who reached a humane end point (HD end), and their cage mates who were euthanized at the same time without having reached an end point (HD other). WT mice showed a progressive increase with age, from low starting levels, in the use of resources such as the rope, beam, ladder and chambers in the RE cages. HD mice, on the other hand, showed a relatively high level of use during early life (especially of the beam, rope and ladder), which then peaked and subsequently decreased to levels well below those of WT mice of the same age. Furthermore, this decrease in the observed use of resources was steeper for HD end mice than for HD other mice and the last observation of HD end mice using specific resources was consistently earlier than for HD other mice, and significantly so for the use of the rope. In addition, HD mice initially showed significantly shorter latencies to enter the light chamber when it was made available, relative to WT mice.
Overall, these findings indicate that HD mice are more active and exploratory than WT mice during early life, and may also be less anxious than WT mice, as reflected by a shorter latency to enter the well-lit area of the light chamber and a greater use of the curled posture when resting during the light phase. However, WT mice gradually increase their activity, ‘boldness’, and the use of resources with age, whereas HD mice show the opposite trend (the increase in latency to enter the light chamber with age may reflect diminished boldness, activity or motivation for the food reward provided). This pattern of change in HD mice relative to WT mice, along with the observation of increased drinking behaviour with age in HD mice, may indicate the onset of disease. A similar pattern has been detected in other studies. Menalled et al. (2002) found evidence of a biphasic motor phenotype in a 94 CAG knock-in model with increased rearing at two months and decreased locomotor activity at four months. At one month, transgenic HD rats showed improved motor performance, but by six months motor impairments began. On a beam-walking test, there were no differences at one to six months, but HD rats showed difficulties from eight months (Nguyen et al. 2006). In the current study, differences between HD end and same-strain HD other mice provide more convincing evidence. The finding that HD end mice tend to show a steeper decline in activity and exploration behaviours than do HD other mice further supports the hypotheses that these patterns of behavioural change may indeed be predictors of disease onset in this strain. That these are most easily detected in RE cages also supports our hypothesis that the provision of additional home cage resources, allowing expression of a wider range of behaviours, facilitates detection of subtle behavioural changes that may predict impending disease.
There is considerable interest in the detection of preclinical markers of Huntington's (and other degenerative) disease, so that novel drug therapies can be targeted at early stages of disease development. HD is composed of a motor, cognitive and psychiatric symptoms but its diagnosis has historically relied on the emergence of motor dysfunction. In N-terminal transgenic mouse models of HD, early deficits have been identified in tests of motor function in the widely used rapid onset R6/2 model compared with WT mice, before clinical symptoms become apparent (Stack et al. 2005; Hickey et al. 2007), although not in R6/1 mice using rotarod tests at 10 or 25 weeks (Lazic et al. 2006) or footprint analysis of gait abnormality between 7 and 36 weeks (Naver et al. 2003). There is also variation between studies in the reported age of onset and proportion of individuals showing limb clasping (Clifford et al. 2002; Naver et al. 2003). In another HD model with rapid onset, N171-82Q, mice fail to gain weight by 12 weeks, and show gait abnormalities, hypokinesis and tremor from 12 weeks, leading to death at 20–24 weeks. However, the earliest sign of disease onset in this model may be reduced rearing behaviour at eight weeks (Klivenyi et al. 2006). Full-length models like knock-in or YAC transgenic mice show a less severe and a more progressive disease course than the N-terminal models, making them better models of the human disease. But the slow rate of disease progression makes the search for early predictive markers particularly important in developing new therapies.
Recent studies suggest that early cognitive deficits are detectable prior to motor symptoms in people (e.g. Wahlin et al. 2007) and there has been some interest in detecting cognitive or emotional preclinical changes in rodent models. Naver et al. (2003) found that R6/1 mice were less anxious than WT in the elevated plus maze between 6 and 12 weeks, but File et al. (1998) found that exploratory and non-exploratory behaviours were reduced in a hole-board test from 8 weeks. No differences in anxiety were reported for N171-82Q mice (Klivenyi et al. 2006). By contrast, at all ages, transgenic HD rats showed reduced anxiety in social and elevated plus maze tests.
Our results indicate that home cage behaviour, especially in RE cages, may provide another potentially powerful way of detecting preclinical signs of HD. The importance of this for the use of novel drug therapies is clear, but there are also strong animal welfare arguments for reliably detecting preclinical signs of disease. Criteria for end points are rarely reported in papers, but when they are they can be severe rather than ‘humane’. For example, ‘the criterion for euthanasia was the point in time when mice could no longer right themselves after 30 s when placed on their side. This was confirmed by two separate observers and was considered the time of death’ (Stack et al. 2005). In order to further improve early/preclinical detection of disease onset, truly longitudinal studies of the sort carried out here are required to accurately identify links between specific behaviour changes and subsequent clinical signs. Such studies are rare, but vitally important if progress is to be made in this area.
The current study was designed to identify differences between groups of WT, HD end and HD other mice. Future studies will need to take this approach forward by investigating the precise temporal relationship between onset of preclinical signs and development of disease in individual animals. Automated recording of home cage behaviour would both avoid stressful handling and enable the collection of vastly more data for individual mice than we could collect using video. Such an approach would enable the development of diagnostic tests with much greater sensitivity and specificity than obtained in this study. Automated recording would also permit analysis of circadian rhythms that are progressively disrupted in HD. Systems that may be suitable for this are increasingly available, e.g. Clever Systems, Inc. (Reston, VA), IntelliCage (Lipp et al. 2005).
Our findings also emphasize that the behaviour of WT mice changes with time and so single constant values cannot be used as benchmarks against which to compare HD mouse models. Rather, it should be appreciated that the behaviour of WT mice provides an age-specific ‘moving baseline’ for comparison.
(c) Welfare at euthanasia
Another important aim of our study was to investigate the link between humane end points and independent measures of the welfare of the mice at the time of euthanasia. The end points used in this study were probably earlier than in other studies (see above), but suggestions that the animals may have had reduced welfare were nevertheless found. Specifically, HD mice showed elevated levels of APPs when compared with WT mice, and these were higher, though not significantly so, when HD end compared with HD other mice. APPs of the sort measured in this study (α1-acid glycoprotein) usually rise in response to infection, inflammation or stressful situations such as restraint or inescapable tail shock in rodents (Murata et al. 2004). HD may be associated with inflammatory changes in the brain (Bonifati & Kishore 2007), as well as physiological stress responses that may be intrinsic to the disease, or caused by perception of symptoms (e.g. Leblhuber et al. 1995; Petersen & Bjorkqvist 2006). Either or both may have played a role in the elevated levels of APP observed, indicating reduced welfare in the HD mice. However, this conclusion would be strengthened by baseline, early life measures of APP showing no differences between HD and WT mice.
We detected significant absolute and relative increases in most organ weights, as previously reported for full-length huntingtin YAC models (Van Raamsdonk et al. 2006), even at the early stage of disease when mice were euthanized. It is not known whether the changes in body composition might be associated with pain, but this would be worth further investigation.
Faecal and serum corticosterone concentrations were unexpectedly lower in HD mice, relative to WT mice at the time of euthanasia. Contrary to this general trend, HD end mice in RE cages showed particularly high serum levels, possibly as a result of frustration at no longer being able to use the climbing resources to which they had grown accustomed. A complicating factor is that HPA activity may be altered in HD mice due to the disease itself. Although relative adrenal gland weight was increased in the HD mice in our study, the brain regions that control CRH production may have been affected by the disease, although this does not explain why our results contrast with previous findings. Bjorkqvist et al. (2006) found increased ACTH and corticosterone levels in R6/2 HD mice, and increased cortisol levels have also been observed in humans with HD (e.g. Leblhuber et al. 1995; Petersen & Bjorkqvist 2006), though not always (Markianos et al. 2007). Given this background, the finding of lower levels of corticosterone in HD mice in this study is difficult to explain. The low corticosterone concentrations may have been a consequence of declining activity in the HD mice although previous work has found no effect of either short periods of intense exercise or long periods of moderate exercise on glucocorticoid levels that are only affected once fat and carbohydrate stores have been depleted under prolonged extreme exercise (reviewed by Lane 2006). We consider it perhaps more likely that a chronic stress state in HD mice might lead to eventual fatigue and hypocortisolaemia close to the time of death, as there is some evidence for a link between fatigue and low corticosteroid levels in human patients (e.g. Chaudhuri & Behan 2004). Clearly, corticosterone cannot be used as a simple ‘welfare indicator’ in a chronic disease model unless these underlying influences are better understood.
5. Conclusions
Changes in behaviour across time that predicted disease onset in HD mice were detectable in home cages, especially those with additional resource enrichment, even when dark phase behaviour was monitored for just 10 min every few days. The extent to which such changes may be used to detect preclinical signs of disease and humane end points in individual mice now requires further research. Home cage monitoring may offer a new and sensitive way of predicting future disease problems.
Acknowledgments
All procedures were conducted under UK Home Office Licence and with approval from the appropriate institutional and university animal ethics committees.
The work was funded by the National Centre for the 3Rs. We would like to thank Dr Ian Inglis for his comments on the corticosterone results.
References
- Bjorkqvist M, et al. Progressive alterations in the hypothalamic–pituitary–adrenal axis in the R6/2 transgenic model of Huntington's disease. Hum. Mol. Genet. 2006;15:1713–1721. doi: 10.1093/hmg/ddl094. doi:10.1093/hmg/ddl094 [DOI] [PubMed] [Google Scholar]
- Blanchard R.J, Nikulina J.N, Sakai R.R, McKittrick C, McEwen B, Blanchard D.C. Behavioral and endocrine change following chronic predatory stress. Physiol. Behav. 1998;63:561–569. doi: 10.1016/s0031-9384(97)00508-8. doi:10.1016/S0031-9384(97)00508-8 [DOI] [PubMed] [Google Scholar]
- Bonifati D.M, Kishore U. Role of complement in neurodegeneration and neuroinflammation. Mol. Immunol. 2007;44:999–1010. doi: 10.1016/j.molimm.2006.03.007. doi:10.1016/j.molimm.2006.03.007 [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Behan P.O. Fatigue in neurological disorders. Lancet. 2004;363:978–988. doi: 10.1016/S0140-6736(04)15794-2. doi:10.1016/S0140-6736(04)15794-2 [DOI] [PubMed] [Google Scholar]
- Clifford J.J, Drago J, Natoli A.L, Wong J.Y.F, Kinsella A, Waddington J.L, Vaddadi K.S. Essential fatty acids given from conception prevent topographies of motor deficit in a transgenic model of Huntington's disease. Neuroscience. 2002;109:81–88. doi: 10.1016/s0306-4522(01)00409-2. doi:10.1016/S0306-4522(01)00409-2 [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley K.W. Twenty years of research on cytokine-induced sickness behavior. Brain Behav. Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. doi:10.1016/j.bbi.2006.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak T, Meriwether J.L, Fleshner M, Spencer R.L, Abouhamze A, Moldawer L.L, Grahn R.E, Watkins L.R, Maier S.F. Evidence that brief stress may induce the acute phase response in rats. Am. J. Physiol. 1997;273:R1998–R2004. doi: 10.1152/ajpregu.1997.273.6.R1998. [DOI] [PubMed] [Google Scholar]
- File S.E, Mahal A, Mangiarini L, Bates G.P. Striking changes in anxiety in Huntington's disease transgenic mice. Brain Res. 1998;805:234–240. doi: 10.1016/s0006-8993(98)00736-7. doi:10.1016/S0006-8993(98)00736-7 [DOI] [PubMed] [Google Scholar]
- Hawkins P. Recognizing and assessing pain, suffering and distress in laboratory animals: a survey of current practice in the UK with recommendations. Lab. Anim. 2002;36:378–395. doi: 10.1258/002367702320389044. doi:10.1258/002367702320389044 [DOI] [PubMed] [Google Scholar]
- Hendriksen, C. F. M. & Morton, D. B. 1999 Humane endpoints in animal experiments for biomedical research. In Proc. Int. Conf., Zeist, The Netherlands London, UK: Royal Society of Medicine.
- Hickey M.A, Gallant K, Gross G.G, Levine M.S, Chesselet M.F. Early behavioral deficits in R6/2 mice suitable for use in preclinical drug testing. Neurobiol. Dis. 2007;20:1–11. doi: 10.1016/j.nbd.2005.01.024. doi:10.1016/j.nbd.2005.01.024 [DOI] [PubMed] [Google Scholar]
- Hockly E, Cordery P.M, Woodman B, Mahal A, van Dellen A, Blakemore C, Lewis C.M, Hannan A.J, Bates G.P. Environmental enrichment slows disease progression in R61/2 Huntington's disease mice. Ann. Neurol. 2002;51:235–242. doi: 10.1002/ana.10094. doi:10.1002/ana.10094 [DOI] [PubMed] [Google Scholar]
- Home Office 2007 Statistics of scientific procedures on living animals: Great Britain 2006 Cm 7153. London, UK: The Stationery Office.
- Hudson M. Why do the numbers of laboratory animal procedures conducted continue to rise? An analysis of the Home Office statistics of scientific procedures on living animals: Great Britain 2005. Altern. Lab. Anim. 2007;35:177–187. doi: 10.1177/026119290703500110. [DOI] [PubMed] [Google Scholar]
- Klivenyi P, Bende Z, Hartai Z, Penke Z, Nemeth H, Toldi J, Vecsei L. Behaviour changes in a transgenic model of Huntington's disease. Behav. Brain Res. 2006;169:137–141. doi: 10.1016/j.bbr.2006.01.003. doi:10.1016/j.bbr.2006.01.003 [DOI] [PubMed] [Google Scholar]
- Lane J. Can non-invasive glucocorticoid measures be used as reliable indicators of stress in animals? Anim. Welf. 2006;15:331–342. [Google Scholar]
- Lazic S.E, Grote H.E, Blakemore C, Hannan A.J, van Dellen A, Phillips W, Barker R.A. Neurogenesis in the R6/1 transgenic mouse model of Huntington's disease: effects of environmental enrichment. Eur. J. Neurosci. 2006;23:1829–1838. doi: 10.1111/j.1460-9568.2006.04715.x. doi:10.1111/j.1460-9568.2006.04715.x [DOI] [PubMed] [Google Scholar]
- Leblhuber F, Peichl M, Neubauer C, Reisecker F, Steinparz F.X, Windhager E, Maschek W. Serum dehydroepiandrosterone and cortisol measurements in Huntington's Chorea. J. Neurol. Sci. 1995;132:76–79. doi: 10.1016/0022-510x(95)00114-h. doi:10.1016/0022-510X(95)00114-H [DOI] [PubMed] [Google Scholar]
- Lipp H.-P, et al. IntelliCage: inter-laboratory comparisons and validation with exploratory behavior and spatial learning. In: Noldus L.P.J.J, Grieco F, Loijens L.W.S, Zimmerman P.H, editors. Proceedings of measuring behaviour. Noldus Information Technology; Wageningen, The Netherlands: 2005. pp. 66–69. [Google Scholar]
- Markianos M, Panos M, Kalfakis N, Vassilopoulos D. Plasma testosterone, dehydroepiandrosterone sulfate and cortisol in female patients with Huntington's disease. Neuroendocrinol. Lett. 2007;28:199–203. [PubMed] [Google Scholar]
- McFarland D. Longman Scientific; London, UK: 1993. Animal behaviour. [Google Scholar]
- Menalled L.B, Sison J.D, Wu Y, Olivieri M, Li X.J, Li H, Zeitlin S, Chesselet M. Early motor dysfunction and striosomal distribution of huntingtin microaggregates in Huntington's disease knock-in mice. J. Neurosci. 2002;22:8266–8276. doi: 10.1523/JNEUROSCI.22-18-08266.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens C, Rulicke T. Score sheets for the monitoring of transgenic mice. Anim. Welf. 1999;8:433–438. [Google Scholar]
- Murata H, Shimada N, Yoshioka M. Current research on acute phase proteins in veterinary diagnosis: an overview. Vet. J. 2004;168:28–40. doi: 10.1016/S1090-0233(03)00119-9. doi:10.1016/S1090-0233(03)00119-9 [DOI] [PubMed] [Google Scholar]
- Naver B, Stub C, Moller M, Fenger K, Hansen A.K, Hasholt L, Sorensen S.A. Molecular and behavioral analysis of the R6/1 Huntington's disease transgenic mouse. Neuroscience. 2003;122:1049–1057. doi: 10.1016/j.neuroscience.2003.08.053. doi:10.1016/j.neuroscience.2003.08.053 [DOI] [PubMed] [Google Scholar]
- NC3Rs 2007 Humane endpoints. See www.nc3Rs.org/category.asp?catID=21
- Nemzak J.A, Xiao H.-Y, Minard A.E, Bolgos G.L, Remick D.G. Humane endpoints in shock research. Shock. 2004;21:17–25. doi: 10.1097/01.shk.0000101667.49265.fd. doi:10.1097/01.shk.0000101667.49265.fd [DOI] [PubMed] [Google Scholar]
- Nguyen H.P, et al. Behavioral anomalies precede neuro-pathological markers in rats transgenic for Huntington's disease. Hum. Molec. Genet. 2006;15:3177–3194. doi: 10.1093/hmg/ddl394. [DOI] [PubMed] [Google Scholar]
- Nuffield Council for Bioethics 2005 The ethics of research involving animals. See http://www.nuffieldbioethics.org/go/ourwork/animalresearch/introduction
- Petersen A, Bjorkqvist M. Hypothalamic–endocrine aspects in Huntington's disease. Eur. J. Neurosci. 2006;24:961–967. doi: 10.1111/j.1460-9568.2006.04985.x. doi:10.1111/j.1460-9568.2006.04985.x [DOI] [PubMed] [Google Scholar]
- Rasbash J, et al. 2nd edn. Institute of Education; London, UK: 2000. A user's guide to MLwiN. [Google Scholar]
- Rogers D.C, Jones D.N.C, Nelson P.R, Jones C.M, Quilter C.A, Robinson T.L, Hagan J.J. Use of SHIRPA and discriminant analysis to characterize marked differences in the phenotype of six inbred mouse strains. Behav. Brain Res. 1999;105:207–217. doi: 10.1016/s0166-4328(99)00072-8. doi:10.1016/S0166-4328(99)00072-8 [DOI] [PubMed] [Google Scholar]
- Roughan J.V, Flecknell P.A. Behavioural effects of laparotomy and analgesic effects of ketoprofen and carprofen in rats. Pain. 2001;90:65–74. doi: 10.1016/s0304-3959(00)00387-0. doi:10.1016/S0304-3959(00)00387-0 [DOI] [PubMed] [Google Scholar]
- Sherwin C.M, Nicol C.J. Behavioural demand functions of caged laboratory mice for additional space. Anim. Behav. 1997;53:67–74. doi:10.1006/anbe.1996.0278 [Google Scholar]
- Sherwin C.M, Haug E, Terkelsen N, Vadgama M. Studies on the motivation for burrowing by laboratory mice. Appl. Anim. Behav. Sci. 2004;88:343–358. doi:10.1016/j.applanim.2004.03.009 [Google Scholar]
- Sorrells A.D, Eicher S.D, Harris M.J, Pajor E.A, Richert B.T. Periparturient cortisol, acute phase cytokine and acute phase protein profiles of gilts housed in groups or stalls during gestation. J. Anim. Sci. 2007;85:1750–1757. doi: 10.2527/jas.2007-0025. doi:10.2527/jas.2007-0025 [DOI] [PubMed] [Google Scholar]
- Spires T.L, Grote H.E, Varshney N.K, Cordery P.M, van Dellen A, Blakemore C, Hannan A.J. Environmental enrichment rescues protein deficits in a mouse model of Huntington's disease, indicating a possible disease mechanism. J. Neurosci. 2004;24:2270–2276. doi: 10.1523/JNEUROSCI.1658-03.2004. doi:10.1523/JNEUROSCI.1658-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack E.C, Kubilus J.K, Smith K, Cormier K, Del Signore S.J, Guelin E, Ryu H, Hersch S.M, Ferrante R.J. Chronology of behavioural symptoms and neuropathological sequel in R6/2 Huntington's disease transgenic mice. J. Comp. Neurol. 2005;490:354–370. doi: 10.1002/cne.20680. doi:10.1002/cne.20680 [DOI] [PubMed] [Google Scholar]
- Thaxton J.P, Puvadolpirod S. Model of physiological stress in chickens: quantitative evaluation. Poult. Sci. 2000;79:391–395. doi: 10.1093/ps/79.3.391. [DOI] [PubMed] [Google Scholar]
- Van Dellen A, Blakemore C, Deacon R, York D, Hannan A.J. Delaying the onset of Huntington's in mice. Nature. 2000;404:721–722. doi: 10.1038/35008142. doi:10.1038/35008142 [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk J.M, Gibson W.T, Pearson J, Murphy Z, Lu G, Leavitt B.R, Hayden M.R. Body weight is modulated by levels of full-length Huntingtin. Hum. Mol. Genet. 2006;15:1513–1523. doi: 10.1093/hmg/ddl072. doi:10.1093/hmg/ddl072 [DOI] [PubMed] [Google Scholar]
- Wahlin T.B.R, Lundin A, Dear K. Early cognitive deficits in Swedish gene carriers of Huntington's disease. Neurospsychology. 2007;21:31–44. doi: 10.1037/0894-4105.21.1.31. doi:10.1037/0894-4105.21.1.31 [DOI] [PubMed] [Google Scholar]