SUMMARY
The transmissible agent causing canine transmissible venereal tumor (CTVT) is thought to be the tumor cell itself. To test this hypothesis, we analyzed genetic markers including major histocompatibility (MHC) genes, microsatellites, and mitochondrial DNA (mtDNA) in naturally occurring tumors and matched blood samples. In each case, the tumor is genetically distinct from its host. Moreover, tumors collected from 40 dogs in 5 continents are derived from a single neoplastic clone that has diverged into two subclades. Phylogenetic analyses indicate that CTVT most likely originated from a wolf or an East Asian breed of dog between 200 and 2500 years ago. Although CTVT is highly aneuploid, it has a remarkably stable genotype. During progressive growth, CTVT downmodulates MHC antigen expression. Our findings have implications for understanding genome instability in cancer, natural transplantation of allografts, and the capacity of a somatic cell to evolve into a transmissible parasite.
INTRODUCTION
CTVT, also known as Sticker’s sarcoma, is a histiocytic tumor that is usually transmitted among dogs through coitus but may also spread through licking, biting, and sniffing tumor-affected areas (Cohen, 1985; Das and Das, 2000). First characterized 130 years ago (Novinski, 1876), CTVT was frequently used by cancer researchers to study tumor transplantation until the development of inbred strains of rats and mice afforded syngeneic models. The notion that the tumor is naturally transmissible as an allograft came from three lines of observation. First, CTVT can only be experimentally induced by transplanting living tumor cells, and not by killed cells or cell filtrates (Cohen, 1985). Second, the tumor karyotype is aneuploid but has characteristic marker chromosomes in tumors collected in different geographic regions (Murray et al., 1969; Oshimura et al., 1973; Weber et al., 1965). Third, a long interspersed nuclear element (LINE-1) insertion near c-myc (Katzir et al., 1985) has been found in all tumors examined so far (Katzir et al., 1987) and can be used as a diagnostic marker to confirm that a tumor is CTVT (Liao et al., 2003). In two animals that had been experimentally inoculated with CTVT, the resulting tumors contained the LINE-1/c-myc insertion, whereas the normal tissues did not (Katzir et al., 1987; Liao et al., 2003). However, in natural transmission, inheritance of a LINE-1 insertion near c-myc in the germline might represent a predisposition to develop CTVT after exposure to an oncogenic agent, similar to the Mendelian LINE-1 insertion in the factor IX gene, which causes mild hemophilia B in dogs (Brooks et al., 2003).
The recent emergence of a tumor transmitted by biting in the endangered marsupial species the Tasmanian devil (Sarcophilus harrisii) (Owen and Pemberton, 2006) has attracted renewed interest in the concept of cellular transmission, for which CTVT is cited as a precedent (Pearse and Swift, 2006). However, authors of reports describing virus-like particles in CTVT (Ajello and Gimbo, 1965; Battistacci and Morriconi, 1974; Lombard and Cabanie, 1967) considered that an oncogenic virus might play a role in tumorigenesis. Although most specialists in the field accept the cellular transmission of CTVT, definitive data that this is the case have been lacking, and the concept of a contagious cancer cell has tended to be greeted with skepticism by many oncologists and immunologists.
Molecular genetic markers have not previously been used to resolve the issue of natural transmission, the breed of origin, or the age of the canine tumor. Here, we compare matched tumor and normal tissues in naturally affected dogs in three countries and analyze the genotype and diversity of further tumors collected worldwide. We provide conclusive evidence that a cancer cell has evolved into a transmissible parasite, which represents the oldest known somatic mammalian cell in continuous propagation.
RESULTS
Clonal Origin of Worldwide Specimens of CTVT
Matched tumor tissues and blood samples were collected from 16 unrelated dogs in Italy, India, and Kenya, and we also examined microdissected tumor cells derived from paraffin-embedded specimens obtained from 24 independent natural tumors from Brazil, the United States, Turkey, Spain, and Italy (Table 1). First we sought to confirm whether the LINE-1 element near c-myc previously detected in CTVT (Katzir et al., 1985) is specific to the tumor cell or whether it represents a genetic predisposition to develop CTVT after exposure to a transmissible agent. All of the naturally occurring tumors but none of the matched normal samples from 16 dogs possessed this LINE-1 insertion, as shown for 11 tumors in Figure 1A. The tumor-specific LINE-1 insertion was present in all of the archival CTVT samples (Figure 1B), as previously reported for tumors in the United States, Israel, and Taiwan (Katzir et al., 1987; Liao et al., 2003). Thus, the LINE-1 insertion appears to be a specific marker of CTVT resulting from either an insertion during the somatic evolution of the tumor or its presence in the germline of the original host. Germline insertion at this locus has not been reported; however, it has not been examined in the canine lineage from which the tumor appears to be derived (see below). Even if the LINE-1 insertion were in the germline, the chromosome pattern and the molecular genetic analysis presented below indicate that the tumor lineage itself is somatically monoclonal.
Table 1.
Sources of CTVT Samples
| Fresh Tumors with Matching Blood Sample | |
|---|---|
| Place | Number |
| Catania, Italy | 5 |
| Messina, Italy | 5 |
| Kolkata, India | 4 |
| Nairobi, Kenya | 2 |
| Paraffin-Embedded Archival Tumors | |
| Country | Number |
| Brazil | 4 |
| Italy | 5 |
| Spain | 4 |
| Turkey | 9 |
| USA | 2 |
| Total | 40 |
Details of age, sex, breed of dog, and site of tumor are in Table S1.
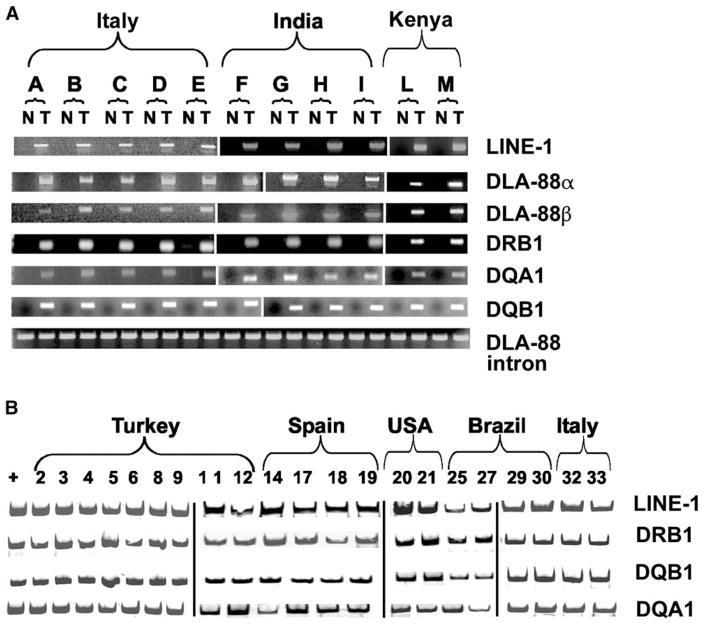
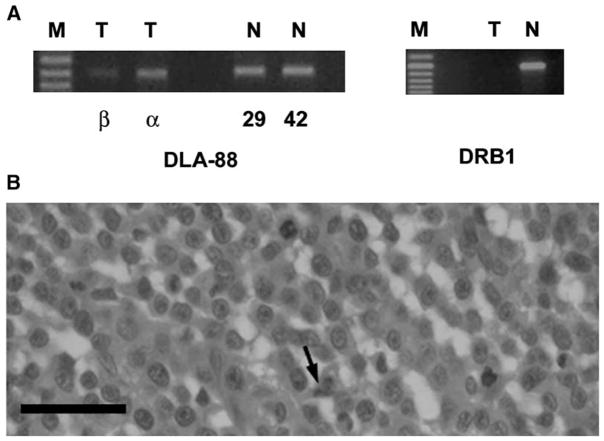
Figure 1. Specific LINE-1/c-myc and DLA Haplotype Genetic Markers for CTVT Detected by Specific PCR Amplification.
(A) For each of 11 dogs (A–M), fresh normal and tumor samples are indicated as N and T, respectively. The panel is assembled from three separate gels visualized by ethidium bromide. The invariant DLA-88 intron sequence serves as a positive control for each of the 22 specimens.
(B) PCR amplification of DNA using Cy5-labeled forward primers from 21 microdissected tumor cells from paraffin-embedded specimens. The panel is assembled from four separate gels.
Next we analyzed the sequence of the most polymorphic genes (Kennedy et al., 2002b) of the canine MHC (also known as dog leukocyte antigen [DLA]): the class I gene (DLA-88 exons 2 and 3) and three class II genes (exon 2 of DRB1, DQB1, and DQA1). Using generic intron PCR primers followed by sequencing of the amplified DLA alleles, we found that, in each case, the CTVT DLA haplotype was different from those of the hosts but was identical among tumors. We therefore designed PCR amplification primers for the DLA alleles that are tumor specific and confirmed that all of the tumors shared the same alleles in all four DLA genes, which were not present in matched normal tissue (Figure 1A). The tumor-specific alleles were also detected in the paraffin-embedded specimens collected worldwide (Figure 1B).
The DLA genotyping indicated that the class II genes in CTVT are either homozygous or hemizygous, except for the DRB1 gene, which possesses two alleles that differ by one nonsynonymous substitution distant from the peptide binding groove. Quantitative PCR was therefore performed to determine DLA gene dosage. While the DLA-88, DRA, and DRB1 genes were diploid in all samples (data not shown), the DQB1 locus was haploid (hemizygous) in 5 of 11 fresh tumors, and the DQA1 locus was haploid in 12 of 29 tumors analyzed, indicating a frequent loss of class II DQ alleles (see Figure S1 in the Supplemental Data available with this article online).
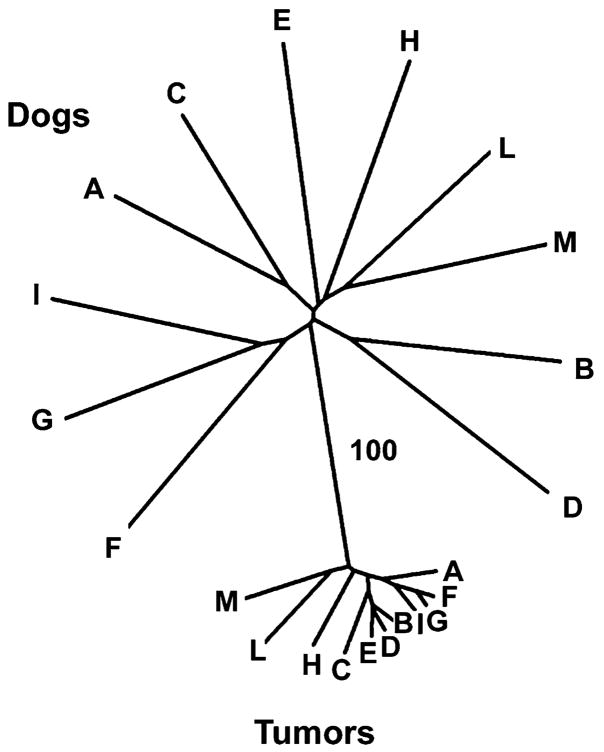
Microsatellite genotyping was conducted by PCR amplification of 21 canine microsatellite markers that are widely dispersed across different chromosomes in the normal karyotype (Parker et al., 2004). These were chosen to compare tumor and normal DNA in 11 of the dogs from which both types of tissue were available. A neighbor-joining tree was constructed using chord distance (Figure 2), which showed that the tumors and the hosts were genetically separate, with all tumors clustered together. A neighbor-joining tree based on the proportion of alleles shared between pairs of samples gave a similar result (Figure S2). None of the host dogs showed close relatedness to any of the others, consistent with the fact that they came from three locations in Europe, Asia, and Africa and were mongrels.
Figure 2. Microsatellite DNA Analysis of 11 Fresh Tumors and Matched Host Samples.
Unrooted neighbor-joining tree based on chord distance compiled from 21 microsatellite loci. A similar neighbor-joining tree based on allele sharing is provided in Figure S2.
A further polymorphic marker analyzed was the mitochondrial DNA (mtDNA) control region. A 580 bp sequence was amplified from 11 fresh specimens and compared to the mtDNA of their hosts. In order to place our data in the context of dog and wolf mtDNA sequences, we analyzed our data in conjunction with sequences collected in previous studies of canids (Vila et al., 1997; Savolainen et al., 2002). All of the tumor sequences grouped together into two distinct clusters (Figure S3), both of which lie within clade A of the canine mtDNA tree; this clade includes ~70% of all dog mtDNA sequences (Savolainen et al., 2002). In contrast, the sequences from the mtDNA from the blood of host dogs bearing the tumor were scattered across clades A, B, and C. Although sequence variation in the mtDNA control region can arise by somatic mutation in human tumors (Vega et al., 2004), the lack of genetic relatedness between matched normal and CTVT mtDNA haplotypes, and the genetic clustering of the tumors, confirmed that the tumors in the dogs from Italy, India, and Kenya were distinct from their hosts.
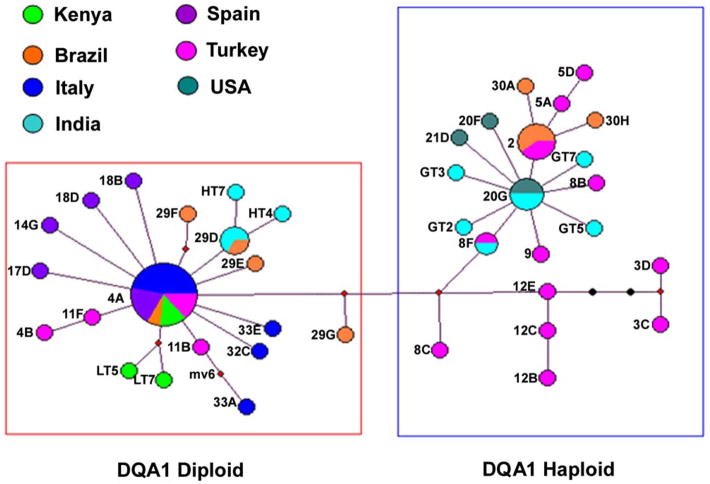
A shorter 257 bp amplified fragment of mtDNA was analyzed in 21 microdissected tumor cells from paraffin-embedded specimens in addition to the fresh tumor. Several amplicons from each tumor specimen were sequenced because there was some variation within tumors. Figure 3 shows that the majority of tumor mtDNA haplotypes grouped into two clusters. Interestingly, all tumors in mtDNA cluster 1 were homozygous diploid for DLA DQA1, while all of those in cluster 2 except for dog 9 were haploid. This observation indicates that the ancestral tumor clone split into two distinguishable subclades, each of which has become broadly distributed in many countries.
Figure 3. Analysis of mtDNA in CTVT.
Tumor haplotypes from fresh and paraffin-embedded tissues showing two main clusters of mtDNA. Diameter of each circle is proportional to the number of tumor samples. Each branch represents one base pair change, with black dots representing intermediates not found in the tumor samples analyzed. The outlined boxes indicate that tumor samples with homozygous (diploid) and hemizygous (haploid) DQA1 genes coincide with mtDNA clusters, except for tumor 9, which is diploid.
We also used the program PAUP* (Swofford, 2003) to estimate a maximum-likelihood tree for a subset of 21 tumor mtDNA sequences and the previously obtained dog and wolf sequences (Figure S4). Although there is considerable uncertainty in the tree, in part due to substantial rate heterogeneity across sites leading to recurrent mutations (Savolainen et al., 2002), the tumor sequences again fall into two main clusters within canid clade A. However, two amplicons from two of the fixed tumor samples contained mtDNA unrelated to the two clusters. The two outliers appear to be incompatible with a monophyletic origin of the amplified mtDNA. In these two tumors, the microdissection may not have removed all of the host cells because other mtDNA sequences from the same tumors were within the clusters. We estimate from histopathology (see below) that approximately 10% of cells in tumors represent host hematopoietic or stromal cells.
Origin of CTVT
We sought to determine the genetic ancestry of CTVT by phylogenetic alignment with previously published data based on DLA typing (Kennedy et al., 2002b; Seddon and Ellegren, 2002) and microsatellite analysis (Parker et al., 2004). The DLA class II DQB1 and DQA1 alleles that we detected in CTVT have been previously described: DQB1 03501 was reported in North American wolves and dogs, and DQA1 04202 was reported in huskies. The tumors contained previously undescribed alleles of DLA-88 and DRB1; the two CTVT DRB1 alleles differ from each other by only one nonsynonymous substitution and are related to alleles 04101 of North American wolves and 04701 of Alaskan and Siberian huskies (Figure S5). The DLA data are consistent with a tumor origin in wolves or “old” breeds of dog in or related to the Spitz group.
To further investigate the origin of CTVT, we genotyped 73 microsatellite loci in three tumor samples (one each from India, Italy, and Kenya) and a subset of 18 of those microsatellites in an additional 24 CTVT samples of diverse geographic origins. These microsatellites are a subset of the loci genotyped by Parker et al. (2004) in a sample of 8 wolves and 414 dogs representing 85 breeds. Analysis of the dog genome (Lindblad-Toh et al., 2005) confirms that the domestic dog (Canis familiaris) and the gray wolf (Canis lupus) are one species. Contaminating normal alleles were excluded from the analysis (see Experimental Procedures).
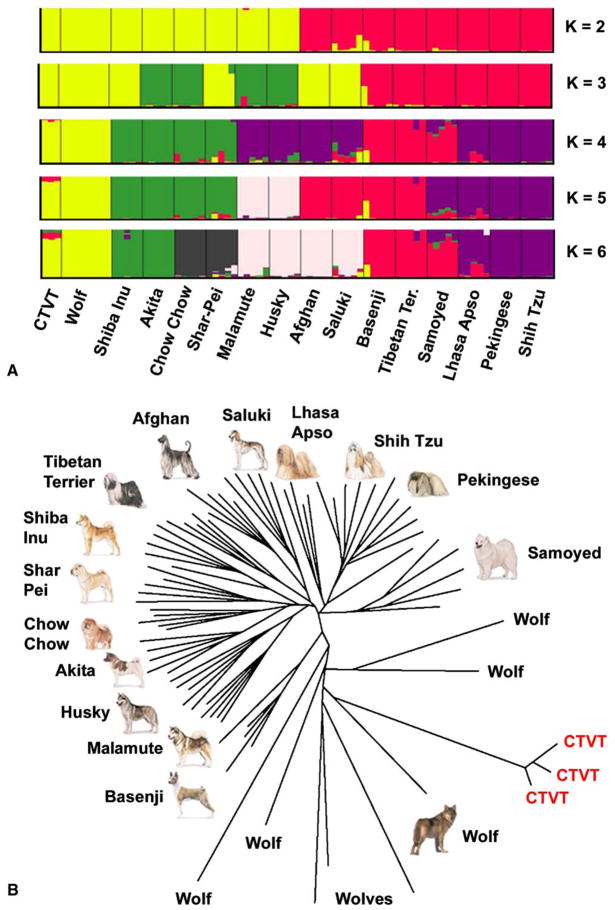
A model-based clustering algorithm, Structure, was used to investigate the relationship between the CTVT samples and the canid data (Falush et al., 2003; Pritchard et al., 2000). This method groups individuals in a sample into a prespecified number of clusters (K). Our initial analyses applied Structure without giving it any information about the origin of the samples (i.e., dog breed, wolf, or CTVT; Figure S6). At K = 2, the tumor samples grouped with the wolves and a set of dog breeds that was previously identified by Parker et al. (2004) as being genetically most similar to wolves. At higher values of K, all of the tumor samples clustered into a unique group that is distinct from the dogs and wolves, again indicating that the samples have a single shared origin.
To determine the specific origin of CTVT, we performed additional Structure analyses (Figure 4A), focusing on wolves and the subset of breeds that showed some similarity to CTVT in the initial analysis. The model used allowed the CTVT samples to have mixed ancestry, so that if the progenitor animal was a mongrel dog, then the CTVT ancestry should be spread across two or more breeds. In this analysis, the tumor samples clustered most strongly with wolves. A second method of analysis applied a nonparametric clustering technique (a neighbor-joining tree based on pairwise allele sharing among genotypes) and again indicated similarity between CTVT and the wolf samples (Figure 4B). Thus, both the model-based method and the neighbor-joining method for microsatellites are consistent with our DLA analysis and indicate that CTVT may have originated in wolves. However, some caution is required due to the small sample sizes of each breed and the fact that the available dog data were limited to pedigree breeds (Parker et al., 2004), so an origin in domestic dogs is not excluded.
Figure 4. Relationship of CTVT to Wolves and Dog Breeds.
(A) Results of a Structure analysis of the canids that appeared most closely related to CTVT (yellow at K = 2 in Figure S6). The clustering was based on the nontumor samples only, and the three tumor samples with nearly complete data were then assigned to the appropriate clusters.
(B) Neighbor-joining tree based on pairwise differences among the same set of individuals as in (A). The relationship between wolves and CTVT is similar when the tree is constructed using all dogs (Figure S6).
Estimating the Age of CTVT
We investigated whether CTVT represents an epidemic of a recently emerged tumor or whether it has a more ancient origin and is in effect a stable parasite of dog populations. The Novinski (1876) report would argue that CTVT is at least 130 years old, assuming that the tumors he observed were clonal with modern tumors. The tumors available to us were collected over a period of 28 years (1976–2003) and thus enable us to test whether CTVT has a modern origin. Moreover, given the wide geographic distribution of the tumor and existing knowledge of the time of divergence of different dog breeds (Vila et al., 1997; Sundqvist et al., 2006), it is possible to estimate minimum and maximum time limits on the emergence of the tumor clone that is currently circulating as a cellular parasite among dogs.
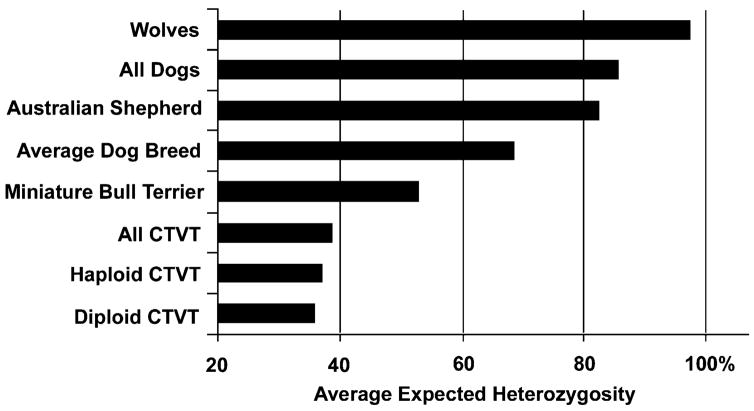
We examined the level of microsatellite variation across tumors (Figure 5). The CTVT isolates were far less variable than wolves and dogs as a whole, and even less variable than the most inbred (miniature bull terrier) of the 85 dog breeds studied by Parker et al. (2004). This observation argues strongly against an ancient date of origin for the common ancestor of CTVT. In 17 of the 18 microsatellites genotyped in the tumors, there is a single genotype that is present in at least half the tumors. We assume that this modal genotype represents that of the ancestral CTVT. There is no trend of distance from the modal genotype as a function of the age of our tumor samples, implying that the common ancestor’s date of origin must be substantially older than the 28 year range of our fixed and fresh samples.
Figure 5. Average Pairwise Divergence of Microsatellite DNA in Wolves, Dogs, and CTVT.
The expected heterozygosity at microsatellite loci in different subsets of canids and CTVT was plotted. Non-CTVT data were derived from Parker et al. (2004). The Australian shepherd represents the most diverse defined breed and the miniature bull terrier the least diverse breed for microsatellite DNA.
Ignoring back mutation (which is a small effect when most genotypes are identical), the probability that a genotype matches the ancestral genotype is exp(−μt), where μ is the mutation rate per genotype per year and t is the time in years since the common ancestor. Unfortunately, the microsatellite mutation rate in CTVT is unclear. Microsatellite mutation rates per allele in humans are in the range of 10−3 to 10−4 per generation for typical loci (Huang et al., 2002). If one assumes that genotype mutation rates per year for CTVT are in this range, then the age estimates range from 250 to 2500 years. If mutation rates are higher in the tumor, as observed in some human tumors (Raptis and Bapat, 2006), CTVT would be at the younger end of this range.
We also computed the average expected heterozygosity of the microsatellites separately for the two CTVT subclades defined by diploid and haploid DQA1 alleles and by mtDNA clusters. The subclades were only slightly less diverse than all CTVT samples (Figure 5). This finding indicates that the two major subclades split relatively soon after the emergence of CTVT, consistent with the wide geographic range of both subclades.
Another potential source of information about the age of CTVT is the levels of mtDNA sequence variability within CTVT samples and relative to dog mtDNA in general. Savolainen et al. (2002) have previously estimated that the most recent common ancestor of dog clade A lived about 41,000 years ago, assuming a star-shaped genealogy. We found that pairwise variability of mtDNA within CTVT is larger than the variability within dog clade A (1.38% versus 0.73% pairwise divergence), but these samples included the two dogs with mtDNA apparently unrelated to the two defined clusters (Figure S4). Most likely these samples contained mtDNA from host stromal cells because, unlike the microsatellite analysis, contaminating normal mtDNA was not omitted. However, a possibility remains of mitochondrial heteroplasmy within the tumor cells of some tumors if CTVT became parasitized by host mitochondria during serial passage. While the analysis of mtDNA would suggest a much older origin, such a conclusion seems implausible given the strikingly low microsatellite variation observed. A high mtDNA mutation rate, together with admixture of host mtDNA or heteroplasmy in the samples, may explain the discrepancy.
In summary, the data on microsatellite variation, including the lack of significant accumulation of new genotypes over the 28 years of tumor collection, and the historical observations on transplantable CTVT since 1876 indicate that CTVT has been transmitted among dogs for two centuries or more. The microsatellite variability of CTVT is only 56% of that for the least variable dog breed (Figure 5, miniature bull terrier), which is probably less than 200 years old (Parker et al., 2004; Sundqvist et al., 2006). However, since miniature bull terriers were presumably founded by several individuals, the most recent common ancestor of their alleles is probably considerably older than 200 years. These data indicate that the tumor cannot have existed in wolves or dogs since ancient times. Thus, the current clone of CTVT would not appear to have been a parasite for more than 2500 years, and probably is considerably younger.
Downregulation of MHC Expression in CTVT
The foregoing analysis shows that CTVT has been transmitted as an allograft across many DLA types through innumerable hosts. Although dogs that have recovered from CTVT are immune to tumor development upon reinoculation, naive dogs of many breeds are susceptible to tumor growth (Cohen, 1985). A recent study indicated that secretion of tumor growth factor β(TGF-β1) may play a role in local immune suppression during progressive growth but that interleukin 6 secretion by tumor-infiltrating lymphocytes aids eventual immune destruction during tumor regression (Hsiao et al., 2004). However, the expression of MHC antigens in CTVT has not been analyzed in detail. One study based on immunostaining indicated that β2-microglobulin could not be detected on CTVT cells (Cohen et al., 1984), but MHC mRNA expression has previously not been examined.
We therefore performed RT-PCR with tumor-specific and host-specific primers within the tumor tissue of Sicilian dog C in order to investigate differential expression of tumor and host DLA genes. Figure 6A shows that class I expression was lower in tumor cells than in stromal cells (which serve as a loading control) and that class II expression was absent. This result indicates significant downmodulation of DLA expression in the tumor cells because they were the majority population (~90%) in the microdissected tumor tissue (Figure 6B). If class I genes were wholly unexpressed, NK cells might eliminate the tumor; hence, our finding of low expression appears more plausible than the suggestion of defective β2-microglobulin (Cohen et al., 1984). A systematic and quantitative analysis of several tumors during different phases of growth and regression would be required to elucidate this phenomenon more thoroughly, but that is beyond the scope of this study. Nonetheless, our finding of DLA downregulation at the transcriptional level is consistent with previous suggestions (Cohen, 1985; Hsiao et al., 2004) that, during progressive growth, CTVT has adapted to evade host immune responses.
Figure 6. MHC Expression in CTVT.
(A) Expression of DLA class I (DLA-88) and class II (DRB1) in the penile tumor of dog C by RT-PCR using tumor-cell-specific primers (T) and primers specific to the alleles of this animal for host stromal cells and infiltrating normal cells (N). M = marker lanes.
(B) Histopathology of a hematoxylin-and-eosin-stained 4 μm section of the same tumor. Scale bar = 30 μm. Cells with large round nuclei are tumor cells, and mitoses are apparent. A stromal cell is indicated with an arrow.
DISCUSSION
Our results, based on several independent genetic markers in tumor-bearing dogs living on five continents, show that CTVT arose from a common ancestral neoplastic cell. Early in its evolution, the clone diverged into two subclades, each of which now has a broad geographic distribution. Many breeds of dog tend to be homozygous for DLA class II genes (Kennedy et al., 2002a), and CTVT is also homozygous for these genes when they are diploid as in subclade 1. Our microsatellite and DLA typing indicate that CTVT first arose in a wolf or in a dog related to the “old” East Asian breeds.
The precise date when CTVT first occurred is difficult to determine. From its indistinguishable histopathology and its ability to grow as an allograft, it is likely that Novinski (1876) studied the same clone, and CTVT could have become established centuries before this date. Our analysis of divergence of microsatellites indicates that the tumor arose between 200 and 2500 years ago. Whether this time period represents the time the tumor first arose or whether it represents a later bottleneck in the tumor’s dispersion as a parasite cannot be resolved. While this estimated date indicates a relatively recent evolutionary origin, CTVT represents the oldest known mammalian somatic cell in continuous propagation, having undergone countless mitoses and host-to-host transfers.
Although the tumor is highly aneuploid, the karyotype is remarkably constant in tumors from the United States, Kenya, and Japan (Murray et al., 1969; Oshimura et al., 1973; Weber et al., 1965). Therefore, its genome diversity at the chromosomal level appears to have stabilized early in its emergence as a transmissible parasite, and our studies revealed only moderate diversification of microsatellite DNA sequences. CTVT has active telomerase (Chu et al., 2001), and we surmise that if telomerase activation occurred after the generation of aneuploidy, the subsequent maintenance of the remaining telomeres may have stabilized the abnormal karyotype. Long-established human tumor cell lines, such as HeLa cells, may be similar in this regard. Other than expression of c-myc (Katzir et al., 1987), activation of oncogenes and deletion of tumor-suppressor genes have not yet been studied in CTVT.
Based on our analysis of 73 widely dispersed microsatellites, there is no evidence of significant genome loss or progressive genome instability in this longest lived of all known tumor clones. CTVT does not appear to exhibit a mutator phenotype (Raptis and Bapat, 2006) in terms of microsatellite instability, and neither does it exhibit progressive chromosome instability (Brumer et al., 2006) following the gross rearrangements early in its emergence.
Both naturally and experimentally transplanted CTVT exhibit an initial stage of rapid and progressive growth, which is typically followed by spontaneous regression 3 to 9 months later, unless the dog is elderly, is in poor condition, or is immunosuppressed (Cohen, 1985). After tumor regression, the host is immune to rechallenge, and passive transfer of serum from a recovered dog also confers immunity. Experimentally, CTVT can be transplanted into immunocompetent animals of other canine species, such as foxes, coyotes, and jackals (Cohen, 1985), as well as into immunodeficient mice (Harmelin et al., 2001; Holmes, 1981). CTVT is a histiocytic tumor (Marchal et al., 1997), and histiocytic tumors with markers of the myeloid dendritic cell lineage that express DLA class II antigens are relatively frequent in dogs of several breeds (Affolter and Moore, 2002). What has led a single clone to become sexually transmissible as an allograft remains obscure. A recent study (Hsiao et al., 2004) shows that, during progressive growth, secretion of TGF-β1 by CTVT acts as a potent local inhibitor of host immune responses, as does the downmodulation of DLA class I and II expression observed by us and others (Cohen et al., 1984). Thus, the evasion of host immune responses has enabled the tumor to survive and grow until it can be further transmitted.
Allorecognition of nonself from self predates the evolution of the highly polymorphic MHC system and is seen in yeast mating types, sponges, and cellular slime molds. However, natural chimeras (Buss, 1982) do occur in metazoans including colonial urochordates (Rinkevich, 2004), and CTVT can be regarded a special case of somatic cell chimerism. The driving selection for the evolution of the MHC system and cell-mediated adaptive immunity in early jawed vertebrates may have been as much to protect against malignancy as to protect against infectious disease because invasive and metastatic tumors develop only in vertebrates, whereas infections are universal. Although recent discussion of cancer immunosurveillance has focused on recognition within the host (Dunn et al., 2002), the rejection of malignant allografts may have been a factor in MHC evolution. Nonetheless, CTVT has evolved into a cellular parasite that has gained independence from and long outlived its original host. Since CTVT is an asexually reproducing cell that cannot “cleanse” its genome of accumulated deleterious mutations through recombination, it may be expected that, over evolutionary time, its genome may suffer from slow degradation through the process of Muller’s ratchet (Muller, 1964). However, there is no evidence that Muller’s ratchet has yet exerted an effect.
In humans, occult tumors in donor organs have emerged on rare occasions in immunosuppressed transplant recipients (Barozzi et al., 2003; Kauffman et al., 2002; MacKie et al., 2003), and choriocarcinoma represents a malignant version of the hemiallogeneic fetal trophoblast. We are not aware of any reports on the sexual transmission of tumor cells (for example, prostate or cervical carcinoma) between humans, but the possibility merits investigation in transplant recipients and immunodeficient individuals with AIDS. Cohen (1985) suggested that the emergence of CTVT may have been favored because of the copulatory and postcoital tie in canid species that provides a tight contact between injured vaginal and penile mucosae for a sufficient time to allow the implantation of tumor cells. It is not evident from our data whether the “infective dosage” is a single cell or a bolus of tumor tissue; the latter seems more likely from a report (Holmes, 1981) that only ~13% of experimentally injected tumor cells survive to develop into a tumor.
Given that MHC expression is downregulated in many tumors (Khong and Restifo, 2002), it is not clear why parasitic tumors have not emerged more frequently. However, the natural transmissibility of CTVT does not appear to be unique. Based on karyotype, a transmissible tumor was reported in a colony of Syrian hamsters (Cooper et al., 1964) and can even be transmitted via mosquitoes (Banfield et al., 1965); like CTVT, this tumor is histiocytic. The recent emergence of a contagious tumor spread by biting in the Tasmanian devil (Pearse and Swift, 2006) also appears to represent an example of cellular transmission according to karyotype, although a definitive analysis based on DNA markers such as we used for CTVT is awaited.
As a sexually transmitted cell, CTVT would not have been able to colonize dogs worldwide if it killed them too quickly; the host must survive in a fit state long enough to transmit the tumor, which in the case of females probably entails an estrous cycle. Thus, it will be interesting to model the restraints preventing the emergence of more aggressive subclones within the host and whether epigenetic factors affect the progressive and regressive phases of tumor growth. CTVT cells with their stabilized genomes may reflect kinship selection and reduced virulence, thus aiding host survival and onward tumor transmission (Frank, 1996), whereas the evolutionary dynamics of a “selfish,” dead-end tumor typically progresses toward greater autonomy and malignancy (Greaves, 2002; Michor et al., 2004).
In contrast to CTVT, the Tasmanian devil facial tumor is highly virulent, killing most of the affected animals by obstructing their ability to feed (Pearse and Swift, 2006). If the devil facial tumor does not eradicate its entire host population, it will be interesting to investigate whether the newly emerged tumor cell lineage eventually evolves toward a less aggressive phenotype.
In the hamster and Tasmanian devil examples, the tumors spread among animals that have little genetic diversity (Cooper et al., 1964; Jones et al., 2004; Owen and Pemberton, 2006). The fact that CTVT is nearly homozygous in each of the DLA class II loci and also has closely related class I alleles may similarly have facilitated the origin and spread of CTVT within a partially inbred population, but today its chief reservoir is among mixed-breed dogs, particularly strays. Thus, CTVT is not a temporary, localized outbreak within a high-kinship group of animals; rather, it represents the evolution of a cancer cell into a successful parasite of worldwide distribution.
EXPERIMENTAL PROCEDURES
Tissue and DNA Sources
CTVT tissue and normal blood were obtained from dogs in different countries; age and sex are as listed in Table S1. Canine tissue and DNA was brought to the UK with Department for Environment, Food and Rural Affairs permission. For histopathology to confirm diagnosis and for microdissection, 4 μm sections were cut from paraffin-embedded tumor blocks. Histological examination was performed using standard hematoxylin and eosin staining (Figure 6B). Tumor tissue was separated from host tissue using manual microdissection under stereomicroscopic observation. DNA was extracted from the microdissected samples using the QIAamp DNA Micro Kit (QIAGEN). PCR primers for the genetic loci examined are provided in Table S2.
LINE-1 insertion
The LINE-1 insertion site upstream of the c-myc gene (Katzir et al., 1987) was probed using a forward PCR primer in LINE-1 and a reverse primer in the 3′-flanking sequence (Table S2).
DLA Sequence, Gene Dosage, and Expression
To clone the DLA genes, PCR products were extracted and purified via gel extraction kit (QIAGEN). The purified products were ligated into the pGEM-T vector (Promega) and cloned using the TOP10 cell strain (Invitrogen). For normal and tumor samples, 7 and 10 colonies were randomly chosen, respectively, and the positive plasmid DNA was extracted, purified, and sequenced (Miniprep Kit, QIAGEN). In order to determine the gene dosage of the DLA genes, real-time PCR based on SYBR Green I fluorescence with a light cycler was used on DNA extracted directly from tumor tissue.
For RT-PCR investigation of DLA gene expression, total RNA was isolated from tumor and blood samples using Trizol Reagent (GIBCO), and mRNA was isolated from total RNA using the Oligotex kit (QIAGEN). Single-strand cDNA was synthesized from RNA by using the SuperScript II RNase H-Reverse Transcriptase kit (Invitrogen). DLA-88 and DLA-DRB1 allele-specific primers and PCR conditions for CTVT and host C are described in Table S2.
Microsatellite Genotyping
Twenty-one microsatellites were chosen for their high polymorphism in order to test genetic differences between matched tumor and host tissues (Figure 2). These markers are widely distributed across the canine genome (Lindblad-Toh et al., 2005). The comparison between tumor genotypes and host genotypes was standardized with the same positive control sample as used by Parker et al. (2004) to align canine genotypes for breed analysis. To investigate the breed and date of origin of CTVT, 73 microsatellites from the loci genotyped by Parker et al. (2004) were genotyped in three CTVT samples, one each from India, Italy, and Kenya; a subset of 15–18 of these microsatellites were genotyped in an additional 24 CTVT samples from five continents. The primer sequences are from the Dog Genome Project website (http://research.nhgri.nih.gov/dog_genome/). The alleles were analyzed using GeneScan software (PE Applied Biosystems). To determine the specific tumor genotype, any normal contaminating alleles were excluded. In fresh specimens, normal alleles were apparent from analysis of matched normal blood samples; in paraffin-embedded specimens, the host alleles presented only minor peaks in GeneScan because stromal cells represented no more than 10% of the tumor sample.
Population Structure Analysis
Model-based clustering of the microsatellite data was performed using the Structure algorithm (Falush et al., 2003; Pritchard et al., 2000), which clusters individuals into groups. We performed separate analyses both with and without using prior information about breed of origin of DNA samples. For all analyses, the admixture model was used with correlated allele frequencies; each run consisted of 10,000 repetitions after 20,000 burn-in steps. Each plotted result was the run with highest posterior probability out of five independent runs with the same parameters. For the analyses in Figure S6, all 27 tumor samples for which microsatellite data was available were used; for Figure 4A, only the three tumor samples with nearly complete data were used. For Figure 4A, the Structure option PFROMPOPFLAGONLY was used, which allowed us to exclude the tumor samples when updating the allele frequencies for each cluster. This forced the tumor samples to cluster with one or more dog/wolf clusters rather than allowing the tumors to create their own cluster. The wolf sample was larger than the breed samples (eight individuals versus five). To check whether this biased the tumor samples to group with wolves, sets of three wolves were dropped, and the analysis was rerun. The posterior assignment of tumors to the wolf cluster dropped slightly (e.g., from ~0.74 to ~0.66 at K = 4). The figures were prepared using Distruct (Rosenberg, 2004).
Nonparametric Clustering of Genotypes
The neighbor-joining tree in Figure 2 was based on chord distance and in Figure S2 on the proportion of shared alleles. The neighbor-joining tree in Figure 4B was based on a pairwise distance matrix among all individuals. The distance between individuals was computed as −log(p), where p is the proportion of alleles that match between two individuals, averaged across all microsatellite loci. The plots should be interpreted as showing nonparametric clustering of individuals based on similarity rather than being an actual evolutionary tree, since the loci do not come from a single linked region of the genome (Felsenstein, 1989).
Mitochondrial DNA Sequence and Phylogenetic Analysis
The 722 bp mtDNA control region was amplified from fresh normal and tumor tissues as previously described (Table S2), and a 580 bp sequence within it was used for phylogenetic analysis (Savolainen et al., 2002). Tumor and normal mtDNA sequences were superimposed on a network of published mt haplotypes (Figure S3). For paraffin-embedded tumor tissues, newly designed primers for a 290 bp fragment were used (Table S2), with 257 bp used for phylogenetic analysis of both fresh and archival tumors (Figure 3 and Figure S4). Between seven and ten clones from each tumor were used for DNA sequence analysis.
Supplemental Data
Supplemental Data include Supplemental Experimental Procedures, Supplemental References, two tables, and six figures and can be found with this article online at http://www.cell.com/cgi/content/full/126/3/477/DC1/.
Acknowledgments
We thank the following veterinarians for kindly providing tissues: V. Fazzino, Italy; A. Zanghi, Italy; A.K. Das, India; E. Sanna, Italy; M. de la Heras, Spain; N. Magre, Kenya; G. Tolga, Turkey; M. Varaschin, Brazil; C. Wanchick, USA. We thank A.R. Fooks (Veterinary Laboratory Agency, Weybridge, UK) for testing for rabies virus sequences in the fresh Italian specimens; E. Ostrander for providing data on breed-specific microsatellite combinations; S. Hué and O. Pybus for advice on phylogenetic analyses; and C. Boshoff, C. Gale, P. Kellam, A. Mitchison, and G. Towers for reading the manuscript. C.M. was a Wellcome Trust Clinical Veterinary Research Training Fellow, and A.F. is a Wellcome Trust University Research Fellow. R.A.W. was supported by grants from the Special Trustees of the Middlesex Hospital and the Medical Research Council.
Footnotes
Accession Numbers
Novel DLA sequences detected in the tumor samples have been deposited in GenBank with the accession numbers DQ056267–DQ056284.
References
- Affolter VK, Moore PF. Localized and disseminated histiocytic sarcoma of dendritic cell origin in dogs. Vet Pathol. 2002;39:74–83. doi: 10.1354/vp.39-1-74. [DOI] [PubMed] [Google Scholar]
- Ajello P, Gimbo A. Presenza di particelle virali nelle cellule del tumore di Sticker. Atti Soc Ital Sci Nat. 1965;19:736–739. [Google Scholar]
- Banfield WG, Woke PA, Mackay CM, Cooper HL. Mosquito transmission of a reticulum cell sarcoma of hamsters. Science. 1965;148:1239–1240. doi: 10.1126/science.148.3674.1239. [DOI] [PubMed] [Google Scholar]
- Barozzi P, Luppi M, Facchetti F, Mecucci C, Alu M, Sarid R, Rasini V, Ravazzini L, Rossi E, Festa S, et al. Post-transplant Kaposi sarcoma originates from the seeding of donor-derived progenitors. Nat Med. 2003;9:554–561. doi: 10.1038/nm862. [DOI] [PubMed] [Google Scholar]
- Battistacci M, Morriconi F. Ricerche ultastrutturali sul sarcoma di Sticker. Nuova Vet. 1974;50:226–236. [Google Scholar]
- Brooks MB, Gu W, Barnas JL, Ray J, Ray K. A LINE-1 insertion in the Factor IX gene segregates with mild hemophilia B in dogs. Mamm Genome. 2003;14:788–795. doi: 10.1007/s00335-003-2290-z. [DOI] [PubMed] [Google Scholar]
- Brumer Y, Michor F, Shakhnovich EI. Genetic instability and the quasispecies model. J Theor Biol. 2006;241:216–222. doi: 10.1016/j.jtbi.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Buss LW. Somatic cell parasitism and the evolution of somatic tissue compatibility. Proc Natl Acad Sci USA. 1982;79:5337–5341. doi: 10.1073/pnas.79.17.5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu RM, Lin CY, Liu CC, Yang SY, Hsiao YW, Hung SW, Pao HN, Liao KW. Proliferation characteristics of canine transmissible venereal tumor. Anticancer Res. 2001;21:4017–4024. [PubMed] [Google Scholar]
- Cohen D. The canine transmissible venereal tumor: a unique result of tumor progression. Adv Cancer Res. 1985;43:75–112. doi: 10.1016/s0065-230x(08)60943-4. [DOI] [PubMed] [Google Scholar]
- Cohen D, Shalev A, Krup M. Lack of beta 2-microglobulin on the surface of canine transmissible venereal tumor cells. J Natl Cancer Inst. 1984;72:395–401. [PubMed] [Google Scholar]
- Cooper HL, Mackay CM, Banfield WG. Chromosome studies of a contagious reticulum cell sarcoma of the Syrian hamster. J Natl Cancer Inst. 1964;33:691–706. [PubMed] [Google Scholar]
- Das U, Das AK. Review of canine transmissible venereal sarcoma. Vet Res Commun. 2000;24:545–556. doi: 10.1023/a:1006491918910. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. PHYLIP–Phylogeny Inference Package (Version 3.2) Cladistics. 1989;5:164–166. [Google Scholar]
- Frank SA. Models of parasite virulence. Q Rev Biol. 1996;71:37–78. doi: 10.1086/419267. [DOI] [PubMed] [Google Scholar]
- Greaves M. Cancer causation: the Darwinian downside of past success? Lancet Oncol. 2002;3:244–251. doi: 10.1016/s1470-2045(02)00716-7. [DOI] [PubMed] [Google Scholar]
- Harmelin A, Pinthus JH, Katzir N, Kapon A, Volcani Y, Amariglio EN, Rehavi G. Use of a murine xenograft model for canine transmissible venereal tumor. Am J Vet Res. 2001;62:907–911. doi: 10.2460/ajvr.2001.62.907. [DOI] [PubMed] [Google Scholar]
- Holmes JM. Measurement of the rate of death of canine transmissible venereal tumour cells transplanted into dogs and nude mice. Res Vet Sci. 1981;30:248–250. [PubMed] [Google Scholar]
- Hsiao YW, Liao KW, Hung SW, Chu RM. Tumor-infiltrating lymphocyte secretion of IL-6 antagonizes tumor-derived TGF-beta 1 and restores the lymphokine-activated killing activity. J Immunol. 2004;172:1508–1514. doi: 10.4049/jimmunol.172.3.1508. [DOI] [PubMed] [Google Scholar]
- Huang QY, Xu FH, Shen H, Deng HY, Liu YJ, Li YZ, Li JL, Recker RR, Deng HW. Mutation patterns at dinucleotide microsatellite loci in humans. Am J Hum Genet. 2002;70:625–634. doi: 10.1086/338997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ME, Paetkau D, Geffen E, Moritz C. Genetic diversity and population structure of Tasmanian devils, the largest marsupial carnivore. Mol Ecol. 2004;13:2197–2209. doi: 10.1111/j.1365-294X.2004.02239.x. [DOI] [PubMed] [Google Scholar]
- Katzir N, Rechavi G, Cohen JB, Unger T, Simoni F, Segal S, Cohen D, Givol D. Retroposon” insertion into the cellular oncogene c-myc in canine transmissible venereal tumor. Proc Natl Acad Sci USA. 1985;82:1054–1058. doi: 10.1073/pnas.82.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzir N, Arman E, Cohen D, Givol D, Rechavi G. Common origin of transmissible venereal tumors (TVT) in dogs. Oncogene. 1987;1:445–448. [PubMed] [Google Scholar]
- Kauffman M, McBride MA, Cherikh WS, Pamela C, Marks WH, Roza AM. Transplant tumor registry: donor related malignancies. Transplantation. 2002;74:358–362. doi: 10.1097/00007890-200208150-00011. [DOI] [PubMed] [Google Scholar]
- Kennedy LJ, Barnes A, Happ GM, Quinnell RJ, Bennett D, Angles JM, Day MJ, Carmichael N, Innes JF, Isherwood D, et al. Extensive interbreed, but minimal intrabreed, variation of DLA class II alleles and haplotypes in dogs. Tissue Antigens. 2002a;59:194–204. doi: 10.1034/j.1399-0039.2002.590303.x. [DOI] [PubMed] [Google Scholar]
- Kennedy LJ, Barnes A, Happ GM, Quinnell RJ, Courtenay O, Carter SD, Ollier WE, Thomson W. Evidence for extensive DLA polymorphism in different dog populations. Tissue Antigens. 2002b;60:43–52. doi: 10.1034/j.1399-0039.2002.600106.x. [DOI] [PubMed] [Google Scholar]
- Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao KW, Lin ZY, Pao HN, Kam SY, Wang FI, Chu RM. Identification of canine transmissible venereal tumor cells using in situ polymerase chain reaction and the stable sequence of the long interspersed nuclear element. J Vet Diagn Invest. 2003;15:399–406. doi: 10.1177/104063870301500501. [DOI] [PubMed] [Google Scholar]
- Lindblad-Toh K, Wade CM, Mikkelsen TS, Karlsson EK, Jaffe DB, Kamal M, Clamp M, Chang JL, Kulbokas EJ, 3rd, Zody MC, et al. Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature. 2005;438:803–819. doi: 10.1038/nature04338. [DOI] [PubMed] [Google Scholar]
- Lombard C, Cabanie P. [Considerations on the nature and studies of the ultrastructure of the Sticker sarcoma of the dog] Bull Cancer. 1967;54:357–365. [PubMed] [Google Scholar]
- MacKie RM, Reid R, Junor B. Fatal melanoma transferred in a donated kidney 16 years after melanoma surgery. N Engl J Med. 2003;348:567–568. doi: 10.1056/NEJM200302063480620. [DOI] [PubMed] [Google Scholar]
- Marchal T, Chabanne L, Kaplanski C, Rigal D, Magnol JP. Immunophenotype of the canine transmissible venereal tumour. Vet Immunol Immunopathol. 1997;57:1–11. doi: 10.1016/s0165-2427(96)05757-1. [DOI] [PubMed] [Google Scholar]
- Michor F, Iwasa Y, Nowak MA. Dynamics of cancer progression. Nat Rev Cancer. 2004;4:197–205. doi: 10.1038/nrc1295. [DOI] [PubMed] [Google Scholar]
- Muller HJ. The relation of recombination to mutational advance. Mutat Res. 1964;106:2–9. doi: 10.1016/0027-5107(64)90047-8. [DOI] [PubMed] [Google Scholar]
- Murray M, James ZH, Martin WB. A study of the cytology and karyotype of the canine transmissible venereal tumour. Res Vet Sci. 1969;10:565–568. [PubMed] [Google Scholar]
- Novinski MA. Zur Frage uber die Impfung der Krebsigen Geschwulste. Zentralbl Med Wissensch. 1876;14:790–791. [Google Scholar]
- Oshimura M, Sasaki M, Makino S. Chromosomal banding patterns in primary and transplanted venereal tumors of the dog. J Natl Cancer Inst. 1973;51:1197–1203. doi: 10.1093/jnci/51.4.1197. [DOI] [PubMed] [Google Scholar]
- Owen D, Pemberton D. Tasmanian Devil: A Unique and Threatened Animal. London: Allen & Unwin; 2006. [Google Scholar]
- Parker HG, Kim LV, Sutter NB, Carlson S, Lorentzen TD, Malek TB, Johnson GS, DeFrance HB, Ostrander EA, Kruglyak L. Genetic structure of the purebred domestic dog. Science. 2004;304:1160–1164. doi: 10.1126/science.1097406. [DOI] [PubMed] [Google Scholar]
- Pearse AM, Swift K. Allograft theory: transmission of devil facial-tumour disease. Nature. 2006;439:549. doi: 10.1038/439549a. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raptis S, Bapat B. Genetic instability in human tumors. EXS. 2006;96:303–320. doi: 10.1007/3-7643-7378-4_13. [DOI] [PubMed] [Google Scholar]
- Rinkevich B. Primitive immune systems: are your ways my ways? Immunol Rev. 2004;198:25–35. doi: 10.1111/j.0105-2896.2004.0114.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg N. DISTRUCT: a program for the graphical display of population structure. Mol Ecol. 2004;4:127–138. [Google Scholar]
- Savolainen P, Zhang YP, Luo J, Lundeberg J, Leitner T. Genetic evidence for an East Asian origin of domestic dogs. Science. 2002;298:1610–1613. doi: 10.1126/science.1073906. [DOI] [PubMed] [Google Scholar]
- Seddon JM, Ellegren H. MHC class II genes in European wolves: a comparison with dogs. Immunogenetics. 2002;54:490–500. doi: 10.1007/s00251-002-0489-x. [DOI] [PubMed] [Google Scholar]
- Sundqvist AK, Bjornerfeldt S, Leonard J, Hailer F, Hedhammar A, Ellegren H, Vila C. Unequal contribution of sexes in the origin of dog breeds. Genetics. 2006;172:1121–1128. doi: 10.1534/genetics.105.042358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford DL. PAUP* 4.0: Phylogenetic Analysis Using Parsimony (and Other Methods) Version 4. Sunderland, MA, USA: Sinauer Associates, Inc; 2003. [Google Scholar]
- Vega A, Salas A, Gamborino E, Sobrido MJ, Macaulay V, Carracedo A. mtDNA mutations in tumors of the central nervous system reflect the neutral evolution of mtDNA in populations. Oncogene. 2004;23:1314–1320. doi: 10.1038/sj.onc.1207214. [DOI] [PubMed] [Google Scholar]
- Vila C, Savolainen P, Maldonado JE, Amorim IR, Rice JE, Honeycutt RL, Crandall KA, Lundeberg J, Wayne RK. Multiple and ancient origins of the domestic dog. Science. 1997;276:1687–1689. doi: 10.1126/science.276.5319.1687. [DOI] [PubMed] [Google Scholar]
- Weber WT, Nowell PC, Hare WC. Chromosome studies of a transplanted and a primary canine venereal sarcoma. J Natl Cancer Inst. 1965;35:537–547. doi: 10.1093/jnci/35.3.537. [DOI] [PubMed] [Google Scholar]