Abstract
Cholangiocarcinoma is a devastating cancer of biliary origin with limited treatment options. Symptoms are usually evident after blockage of the bile duct by the tumor and, at this late stage, they are relatively resistant to chemotherapy and radiation therapy. Therefore it is imperative that alternative treatment options are explored. We present novel data indicating that the metabolism of serotonin is dysregulated in cholangiocarcinoma cell lines compared to normal cholangiocytes and in tissue and bile from cholangiocarcinoma patients. Specifically there was an increased expression of tryptophan hydroxylase 1 and a suppression of monoamine oxidase A expression (enzymes responsible for the synthesis and degradation of serotonin respectively) in cholangiocarcinoma. This resulted in an increased secretion of serotonin from cholangiocarcinoma and increased serotonin in the bile from cholangiocarcinoma patients. Increased local serotonin release may have implications on cholangiocarcinoma cell growth. Serotonin administration increased cholangiocarcinoma cell growth in vitro, whereas inhibition of serotonin synthesis decreases tumor cell growth both in vitro and in vivo. The data presented here represents the first evidence that serotonin metabolism is dysregulated in cholangiocarcinoma and that modulation of serotonin synthesis may represent an alternative target for the development of therapeutic strategies.
INTRODUCTION
Cholangiocarcinomas are devastating cancers of intrahepatic and extrahepatic origin that are increasing in both their worldwide incidence and mortality rates (1, 2). The challenges posed by these often lethal biliary tract cancers are daunting, with conventional treatment options being limited and the only hope for long-term survival being that of complete surgical resection of the tumor (1, 2). Conventional chemotherapy and radiation therapies are not effective in prolonging long-term survival (1); therefore it is important to understand the intracellular mechanisms of cholangiocarcinoma cell growth with a view to develop novel chemopreventive strategies.
Serotonin, or 5-hydroxytryptamine, is a neuromodulator, with both neuroendocrine and neurotransmitter functions, that is synthesized in serotonergic neurons in the central nervous system (3) and in enterochromaffin cells throughout the gastrointestinal tract (4). Serotonin is synthesized by the systematic hydroxylation and decarboxylation of the amino acid tryptophan by the enzymes tryptophan hydroxylase (TPH1) and amino acid decarboxylase, respectively (3). There are 16 serotonin receptor subtypes, spread over 7 receptor families through which serotonin exerts its multiple effects (5). With the exception of the serotonin 3 receptor, a ligand gated ion channel, all other serotonin receptors are G protein-coupled, seven transmembrane receptors that activate intracellular second messenger systems (5). Once serotonin has activated the receptor it is cleared from the extracellular space by specific re-uptake transporters where it undergoes catabolism (6). Degradation of serotonin is carried out primarily by the enzyme monoamine oxidase (MAO), which occurs as two molecular subtypes called MAO A and MAO B, and have some differences in their tissue and cellular distributions (7). MAO A is more selective for serotonin oxidation by being able to metabolize serotonin with a much lower Km value (and higher affinity for the substrate) than MAO B (8).
Several opposing effects of serotonin on tumor growth have been reported (9). On one hand, serotonin is known as a growth factor for several types of non-tumoral cells (10, 11), and it has been proposed to take part in the autocrine loops of growth factors contributing to cell proliferation in aggressive tumors such as small cell lung carcinoma (12), prostate cancer cells (13). human breast cancer cell lines (14), and bladder cancer (15). In contrast, several studies have also reported that serotonin can inhibit tumor growth, mainly via the specific vasoconstrictive effects of serotonin on the vessels irrigating the tumors (9, 16–18). In addition, the synthesis and secretion of serotonin has previously been shown to be dysregulated in neuroendocrine tumors with these cells possessing a higher biogenic amine content than normal cells (19–21).
Serotonin is involved in the pathogenesis of certain clinical features of cholangiopathies, pruritus, and fatigue in particular (22, 23). In animal models of chronic cholestasis, this may be due to an enhanced release of serotonin in the central nervous system and its interactions with subtype 1 serotonin receptors (23). Cholangiocytes synthesize and secrete serotonin, which is increased in proliferating rat cholangiocytes after bile duct ligation (BDL) (24). We postulate that this autocrine loop is integral in limiting the growth of the biliary tree as a result of chronic cholestasis. However, to date, nothing is known about the involvement of serotonin in the neoplastic transformation and growth of cholangiocarcinoma.
In the present study, we show a dysregulation of the cellular machinery responsible for the metabolism of serotonin in cholangiocarcinoma cell lines and human samples, which results in an increased production and secretion of serotonin from cholangiocarcinoma. Furthermore, we demonstrate that the increased secretion of serotonin has growth-promoting effects on cholangiocarcinoma cells and that inhibiting serotonin synthesis significantly blocks cholangiocarcinoma cell proliferation in vitro and in vivo.
MATERIALS AND METHODS
Cell Lines
We used six human cholangiocarcinoma cell lines (Mz-ChA-1, HuH-28, HuCC-T1, CCLP1, SG231 and TFK-1) with different origins. Mz-ChA-1 cells, from human gallbladder (25) were a gift from Dr. G. Fitz (University of Texas Southwestern Medical Center, Dallas, TX). HuH-28 cells, from human intrahepatic bile duct (26), and TFK-1 cells, from extrahepatic cholangiocarcinoma (27) were acquired from Cancer Cell Repository, Tohoku University, Japan. These cells were maintained at standard conditions as described (28). In addition CCLP-1 (29), HuCC-T1 (30) and SG231 (31) also from intrahepatic bile ducts were a kind gift from Dr AJ Demetris (University of Pittsburg, PA) and were cultured as described (29–31). The human immortalized, nonmalignant cholangiocyte cell line, H69 (from Dr. G.J Gores, Mayo Clinic, Rochester, MN), was cultured as described (32).
Real time PCR
RNA was extracted from all cell lines using the RNeasy Mini Kit (Qiagen Inc, Valencia, CA) according to the instructions provided by the vendor and reverse transcribed using the Reaction Ready™ First Strand cDNA synthesis kit (SuperArray, Frederick, MD). These reactions were used as templates for the PCR assays using a SYBR Green PCR master mix (SuperArray, Frederick, MD) in the real-time thermal cycler (ABI Prism 7900HT sequence detection system) using commercially available primers designed against human TPH1, MAO A and the specific serotonin receptor subtypes (SuperArray, Frederick MD). A ΔΔCT analysis was performed using the normal cholangiocytes as the control sample. Data are expressed as relative mRNA levels ± SEM (n=3).
Immunoblotting
Following trypsinization, all cell lines (1×106 cells) were resuspended in lysis buffer (33) and sonicated. Immunoblots to detect TPH, MAO A and β-actin were performed as previously described (33) using specific antibodies against each protein (Santa Cruz Biotechnology, Santa Cruz, CA). Data are expressed as fold change (mean ± SEM) of the relative expression after normalization with β-actin.
Cholangiocarcinoma tissue array analysis
Immunoreactivity for serotonin, TPH1, MAO A, the cholangiocyte marker CK-19, and the neuroendocrine markers Chromagranin A and neuron-specific enolase (NSE) and was assessed in commercially available Accumax tissue arrays (Isu Abxis Co, LTD, Seoul, Korea) by immunohistochemistry as described (24) using specific antibodies. These tissue arrays contain 48 well-characterized cholangiocarcinoma biopsy samples from a variety of tumor differentiation grades as well as 4 control liver biopsy samples. Semi-quantitative analysis was performed by three independent observers, in a blind fashion, using the following parameters. Staining intensity was assessed on a scale from 1–4 (1=no staining, 4= intense staining) and the abundance of positively stained cells was given a score from 1 to 5 (1= no cells stained, 5 = 100% stained). The staining index was then calculated by the staining intensity multiplied by the staining abundance that gave a range from 1 to 20.
Serotonin secretion
All cell lines were trypsinized and the resulting cell pellet was resuspended in HBS buffer (1 × 107 cells/mL). Cells were then incubated for 6 hr at 37°C and the amount of serotonin released into the media assayed using a commercially available serotonin EIA kit (Invitrogen, Carlsbad, CA) according to the manufacturers instructions.
Hepatic bile was collected aseptically from T-tube drainage during post-operative day 1–3 from patients with intrahepatic stones (n=12), or gallstones in association with common bile duct stones (n=11) and from cholangiocarcinoma patients (n=14). Bile samples were immediately frozen at −80°C until analysis.
MTS cell proliferation assays
Cell lines were seeded into 96 well plates (10,000 cells/well) in a final volume of 200 μl of growth medium and allowed to adhere to the plate overnight. Cells were serum-starved for 24 hr prior to stimulation with serotonin (10−8 M to 10−6 M) or p-chlorophenylalanine (CPA; a specific TPH1 inhibitor; 0.25 mM to 1 mM; IC50 250 μM (34)) for 48 hours. In parallel experiments, cells were pretreated with commercially available specific serotonin receptor antagonists all at 10 μM (5HTR 1A antagonist, (S)-Way 100135 dihydrochloride; 5HTR 1B antagonist, SB216641 hydrochloride; 5HTR 1D antagonist, BRL 15572 hydrochloride; 5HTR 2A antagonist, TCB-2; 5HTR 2B, SB204741; 5HTR 2C, N-Desmethylclozapine; pan 5HTR 3 antagonist, Tropisetron hydrochloride; 5HTR 4 antagonist, GR125487 sulfamate; 5HTR 6 antagonist, SB258585 hydrochloride; and 5HTR 7 antagonist, SB269970 hydrochloride all purchased from Tocris Bioscience., Ellisville, MI), for 1 hr prior to the addition of serotonin (10−7 M). Cell proliferation was assessed using a colorimetric cell proliferation assay (CellTiter 96AQueous; Promega Corp., Madison, WI) and absorbance measured at 490 nm by a microplate spectrophotometer (Versamax; Molecular Devices, Sunnyvale, CA). In all cases, data were expressed as the fold change of treated cells as compared to vehicle treated controls.
Bromodeoxyuridine (BrdU) incorporation assays
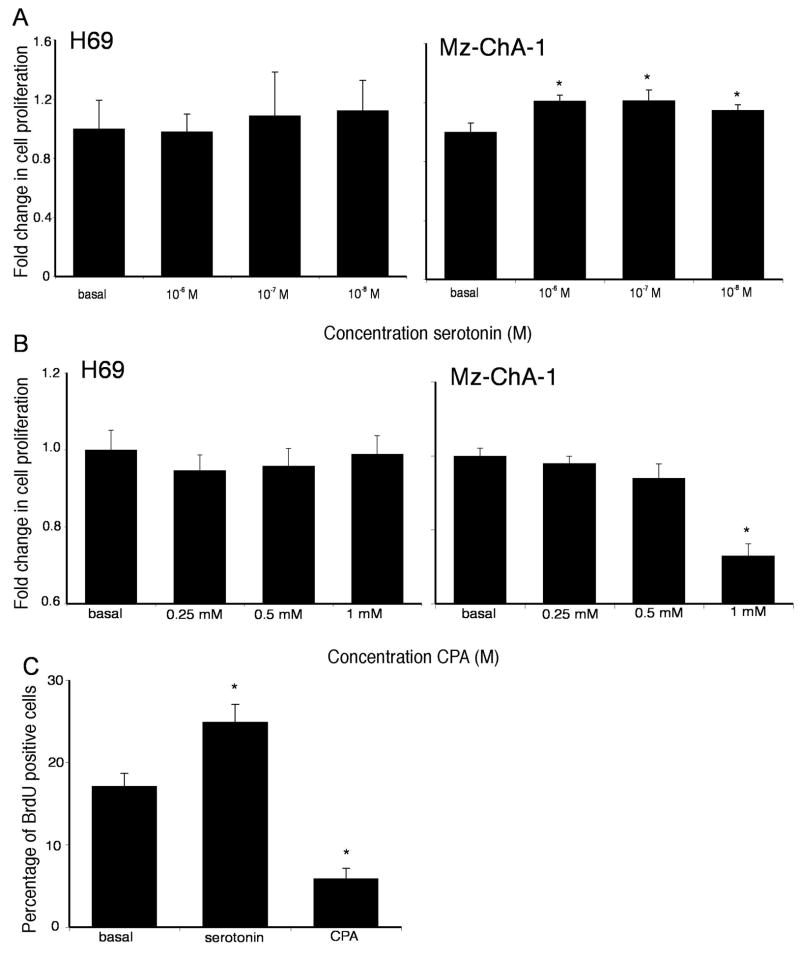
BrdU assays were performed as described previously (28) using Mz-ChA-1 cells stimulated with serotonin (1 μM) and CPA (0.25 mM) for 48 hr. The number of BrdU-positive nuclei was counted and expressed as a percentage of total cells in 5 random fields for each treatment group. Data is average ± SEM of 5 fields in 3 independent experiments.
Nude mice treatment
In vivo experiments were performed as described previously (35). Male balb/c 8-week-old nude (nu/nu) mice were kept in a temperature-controlled environment (20–22°C) with a 12-hour light–dark cycle and with free access to drinking water and to standard mouse chow. Mz-ChA-1 cells (5 × 106) were suspended in 0.25 mL of extracellular matrix gel and injected subcutaneously in the left back flank of these animals. After the establishment of the tumors, mice received CPA (150 mg/kg/day ip) injected 3 times per week. Tumor parameters were measured twice a week by an electronic calliper and volume determined as: tumor volume (mm3) = 0.5 × [length (mm) × width (mm) × height (mm)]. After approximately 2 months, mice were anaesthetized with sodium pentobarbital (50 mg/kg ip) and sacrificed according to institutional guidelines. Serum was collected and AST and ALT levels were measured using a Dimension® RxL Max Integrated Chemistry system (Dade Behring Inc., Deerfield IL) by the Scott & White Hospital, Chemistry Department. Heart, liver and kidneys were isolated, fixed in formalin, embedded in paraffin, processed for histopathology, and stained with hematoxylin-eosin (H&E) for the detection of tissue damage.
Tumor tissues were also excised from the flank of these mice and fixed in formalin, and embedded in paraffin. Histological tests included H&E staining for histopathology, or Masson’s trichrome staining for collagen visualization. Tumor protein selectively expressed by cholangiocytes was evaluated after CK-7 immunohistochemical staining (36). Furthermore, the expression of specific neuroendocrine markers chromagranin A and NSE was assessed using immunohistochemistry (36). In each case, sections were counterstained with haematoxylin prior to analysis.
Light microscopy and immunohistochemistry observation were taken by BX-51 light microscopy (Olympus, Tokyo, Japan) with a videocam (Spot Insight; Diagnostic Instrument, Inc., Sterling Heights, MI) and processed with an Image Analysis System (IAS; Delta Sistemi, Rome, Italy). Three pathologists independently performed analysis in a blind manner. The degree of inflammation and fibrosis was evaluated in 5 randomly non overlapping fields (magnification ×20) for each slide using light microscopy of Masson’s-stained sections as previously described (37); the necrotic mass was evaluated by quantitative morphometry on LM images as previously described (38, 39) and expressed as area of necrosis/total area of tumor × 100. For each sample, more than 5 non-overlapping fields (magnification ×20) were studied.
RESULTS
Expression of metabolic enzymes for serotonin is dysregulated in cholangiocarcinoma
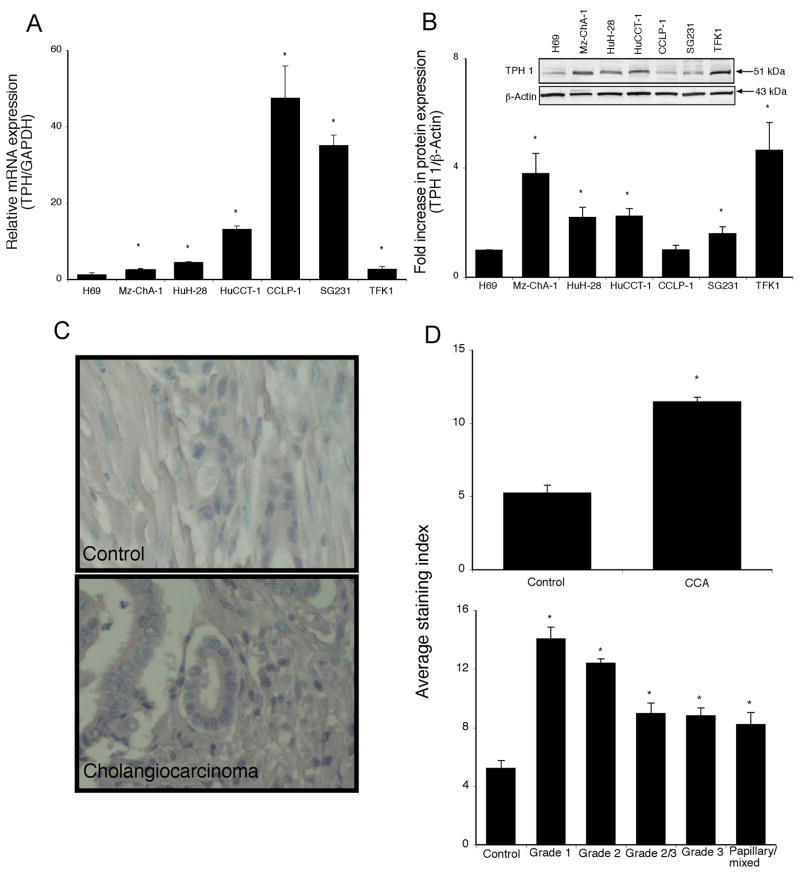
The de novo synthesis of serotonin is predominantly performed by TPH1 in the gastrointestinal tract (3, 34). The expression of TPH1 mRNA was significantly upregulated (from 2.5 to 50 fold) in 5 out of 6 cholangiocarcinoma cell lines when compared to the non-malignant H69 cells (Figure 1A). This trend was confirmed by TPH1 protein expression as demonstrated by immunoblotting (Figure 1B). In addition, immunohistochemical analysis of human liver biopsy samples indicated that there is also increased TPH1 immunoreactivity in cholangiocarcinoma samples compared to control as assessed by three independent observers (Figure 1C and 1D and data not shown). Analysis of the TPH1 immunoreactivity as a function of the differentiation grade of the tumor showed a correlation between staining intensity and the degree of differentiation (Figure 1D). More specifically, while there is an increased expression of TPH1 in all cholangiocarcinoma samples compared to normal liver samples, the increase is more evident in tumors with a differentiation grade of 1 (well differentiated) compared to the less differentiated grade 3 (Figure 1D).
Figure 1.
Tryptophan hydroxylase 1 expression is increased in cholangiocarcinoma. TPH1 levels were assessed in six cholangiocarcinoma cell lines as well as a non-malignant cholangiocyte cell line H69, by real time PCR (A) and immunoblotting (B). In each case, data are expressed as average ± SEM (n=3). Asterix denotes significance (p<0.05) compared with TPH1 expression in H69 cells. TPH1 levels were also assessed in biopsy samples from 48 cholangiocarcinoma patients and healthy controls by immunohistochemistry. Representative photomicrographs of the TPH1 immunoreactivity are shown (C; magnification X40). Staining intensity was assessed as described in the methods and expressed as an average ± SEM of all cholangiocarcinoma patients compared to control samples (D), as well as a function of tumor grade (degree of differentiation (D). Asterix denotes significance (p<0.05) compared with TPH1 immunoreactivity in control biopsy samples.
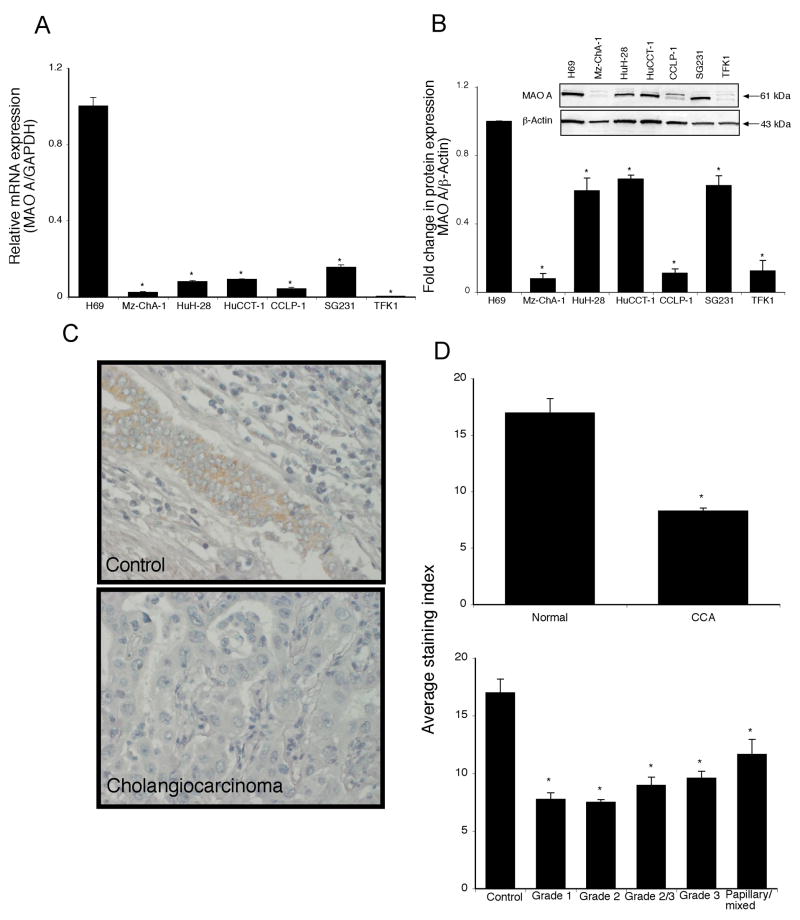
In contrast to TPH1 expression, the MAO A mRNA levels were significantly decreased in cholangiocarcinoma cell lines compared to H69 cells (Figure 2A), which was paralleled by the protein expression as demonstrated by immunoblotting (Figure 2B). Similarly, immunohistochemical analysis showed a suppression of MAO A expression in human biopsy samples, as assessed by 3 independent observers (Figure 2C and 2D and data not shown). However, when MAO A immunoreactivity was expressed as a function of tumor differentiation grade, there was no correlation between the degree of differentiation and the expression of MAO A (Figure 2D).
Figure 2.
Monoamine oxidase A expression is decreased in cholangiocarcinoma. MAO A levels were assessed in six cholangiocarcinoma cell lines as well as a non-malignant cholangiocyte cell line H69, by real time PCR (A) and immunoblotting (B). In each case, data are expressed as average ± SEM (n=3). Asterix denotes significance (p<0.05) compared with MAO A expression in H69 cells. MAO A levels were also assessed in biopsy samples from 48 cholangiocarcinoma patients and healthy controls by immunohistochemistry. Representative photomicrographs of the MAO A immunoreactivity is shown (C; magnification X40). Staining intensity was assessed as described in the methods and expressed as an average ± SEM of all cholangiocarcinoma patients compared to control samples (D), as well as a function of tumor grade (degree of differentiation (D). Asterix denotes significance (p<0.05) compared with MAO A immunoreactivity in control biopsy samples.
Serotonin secretion is increased in cholangiocarcinoma
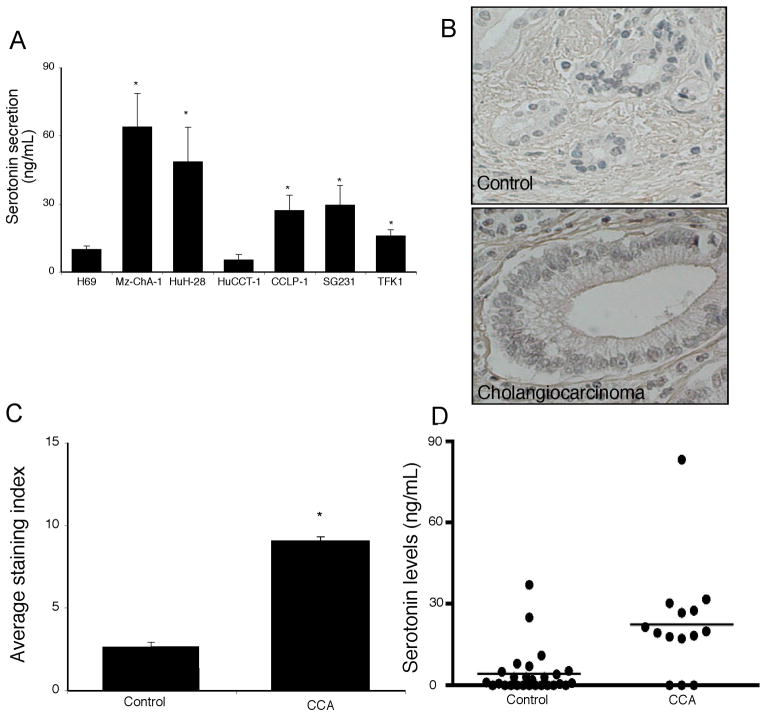
Taking the changes in expression of the serotonin synthesis and degradation enzymes together, it would be reasonable to expect an overall increase in serotonin production and secretion from cholangiocarcinoma cells. Indeed, the secretion of serotonin was increased in 5 out of 6 cholangiocarcinoma cell lines (Figure 3A). Serotonin immunoreactivity was also increased in the human biopsy samples contained on the cholangiocarcinoma tissue array, as assessed by 3 independent observers (Figure 3B and 3C and data not shown). Analysis of serum samples from cholangiocarcinoma patients versus age-matched controls revealed no significant difference in serotonin levels (data not shown) which is not surprising given that the normal tissue surrounding the tumor presumably has normal degradation machinery (i.e., MAO A expression) and would possibly take up the excess serotonin and metabolize it. We then performed a pilot study with a limited number of bile samples taken from cholangiocarcinoma patients. As controls, we used bile samples collected from patients affected by intrahepatic cholelithiasis (e.g. non malignant disease). Analysis of these treatment groups revealed an increase in serotonin levels in cholangiocarcinoma patients compared to “controls” (Figure 3D).
Figure 3.
Serotonin secretion in cholangiocarcinoma. Serotonin levels in the supernatant of cell suspensions of cholangiocarcinoma cell lines and the non-malignant cholangiocyte cell line H69 were determined by EIA after 6 hr (A). Data are expressed as average serotonin concentration (ng/mL) ± SEM (n=3). Asterix denotes significance (p<0.05) compared with serotonin levels secreted from H69 cells. Serotonin immunoreactivity was assessed in cholangiocarcinoma biopsy samples by immunohistochemistry. Representative photomicrographs of the serotonin immunoreactivity are shown (B; magnification X40). Staining intensity was assessed as described in the methods and expressed as an average ± SEM of all cholangiocarcinoma patients compared to control samples (C). Asterix denotes significance (p<0.05) compared with serotonin immunoreactivity in control biopsy samples. Serotonin levels in bile samples from cholangiocarcinoma patients and patients with intrahepatic cholelithiasis by EIA (D). Data are expressed as average serotonin concentration (ng/mL) ± SEM.
Expression of neuroendocrine markers in cholangiocarcinoma samples
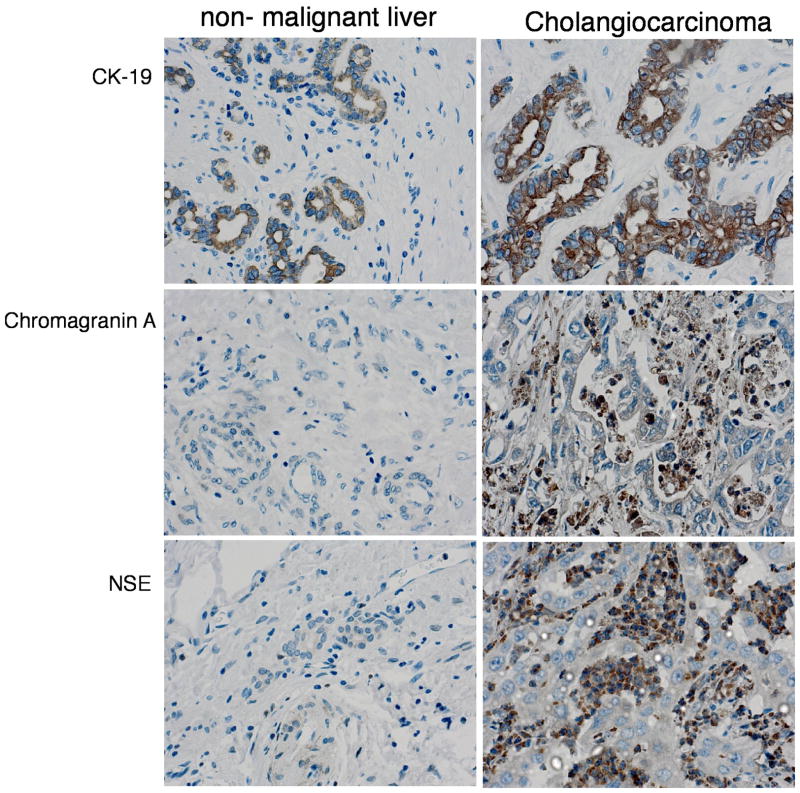
Because serotonin production is dramatically increased in a number of neuroendocrine tumors, we assessed the expression of neuroendocrine markers and cholangiocyte markers in the tumor biopsy samples. CK-19 immunoreactivity was observed to a similar intensity in cholangiocarcinoma tissue and non-malignant liver tissue (Figure 4). There was no observable expression of the neuroendocrine markers chromagranin A and NSE in non-malignant livers, but was expressed in a number of the cholangiocarcinoma biopsy samples studied (44% and 63% of cholangiocarcinoma samples studied for chromagranin A and NSe respectively), suggesting the emergence of a neuroendocrine, or “carcinoid-like” phenotype
Figure 4.
Cholangiocarcinoma display features of a neuroendocrine tumor. Immunohistochemical analysis of biopsy samples from non-malignant liver and cholangiocarcinoma tumors were performed using antibodies against CK-19, (cholangiocyte marker), chromagranin A- and NSE- (neuroendocrine markers). Representative photomicrographs of the immunoreactivity are shown. (magnification X40).
Increased local serotonin secretion has implications on cholangiocarcinoma cell growth in vitro
The potential implications of the increased local serotonin secretion were explored in vitro using the cholangiocarcinoma cell lines. Treatment of the SV40-transformed human cholangiocyte cell line, H69, with various concentrations of serotonin had no significant impact on cell proliferation (Figure 5A), whereas treatment of all of the cholangiocarcinoma cell lines with serotonin (10−7 to 10−6 M) caused a significant increase in cell proliferation after 48 hr as demonstrated by MTS cell proliferation assays (Figure 5A and Supplemental Figure 1). Cholangiocarcinoma cell lines expressed all serotonin receptor subtypes, with the vast majority being upregulated compared to the H69 cholangiocyte cell line except 5HTR 1B, 5HTR 1F, 5HTR 2B, 5HTR 3C and 5HTR 7 (which are all downregulated; Supplemental table 1). Furthermore, specific inhibition of the serotonin receptors 5HTR 1A, 5HTR 2A, 5HTR 2B, 5HTR 4 and 5HTR 6 effectively prevented the growth promoting effects of serotonin (Supplemental Figure 2).
Figure 5.
(A) Serotonin increases cholangiocarcinoma cell proliferation in vitro. Mz-ChA-1 cells and the non-malignant cholangiocyte cell line, H69 were treated with various concentrations of serotonin (10−8 M to 10−6 M) for 48 hr. Cell proliferation was assessed using an MTS cell proliferation assay. Data are expressed as fold change in proliferation (average ± SEM, n=7) and the asterix denotes p<0.05 compared to basal treatment within each cell line. (B) Inhibition of serotonin synthesis decreases cholangiocarcinoma cell proliferation in vitro. Mz-ChA-1 cells and the non-malignant cholangiocyte cell line, H69 were treated with various concentrations of CPA (0.25 mM to 1 mM) for 48 hr. Cell proliferation was assessed using an MTS cell proliferation assay. Data are expressed as fold change in proliferation (average ± SEM, n=7) and the asterix denotes p<0.05 compared to basal treatment within each cell line. (C) BrdU labelling of cholangiocarcinoma cells indicates changes in cell cycle progression after serotonin and CPA. Mz-ChA-1 cells were treated with serotonin (10−6M) and CPA (0.25 mM) for 48 hr and BrdU uptake was determined. The number of BrdU-positive cells was expressed as a percentage of total cells. Data was expressed as the average ± SEM from 5 random fields from 3 independent experiments. * denotes significance (p<0.05) when compared to basal treatment.
Conversely, blocking serotonin synthesis CPA, a specific TPH1 inhibitor, markedly inhibited cholangiocarcinoma cell proliferation, particularly at 1 mM (Figure 5B and Supplemental Figure 3). Once again, no effect was observed in H69 cells. Taken together this indicates that the increased serotonin secretion from cholangiocarcinoma cells may have a growth promoting effect on tumor progression.
The effects of serotonin and CPA on cell cycle progression were assessed by BrdU uptake assays in Mz-ChA-1 cells. Consistent with the MTS assays described above, there was an increase in the number of BrdU-positive nuclei per field after serotonin treatment (Figure 5C), whereas treatment with CPA decreased the number of BrdU-positive cells (Figure 5C). This suggests that increased serotonin secretion from cholangiocarcinoma cells may exert its effects by regulating cell cycle progression.
Inhibition of serotonin synthesis inhibits cholangiocarcinoma tumor growth in vivo
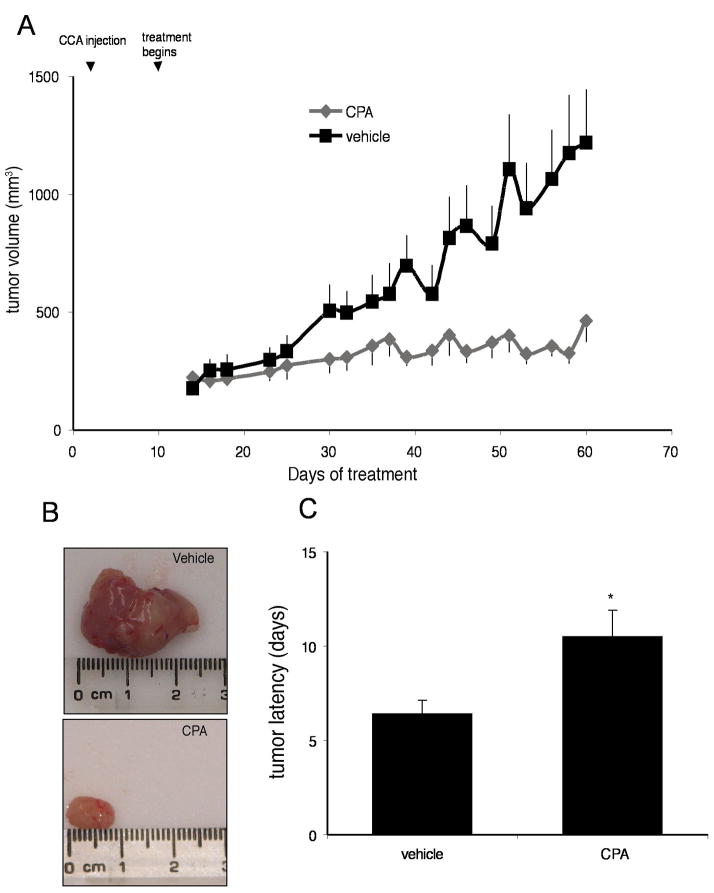
By treating an in vivo xenograft model of cholangiocarcinoma tumors with the TPH inhibitor, we significantly suppressed tumor growth (Figure 6A and 6B). In addition, the latency of tumor growth (i.e., time taken for tumor volume to increase to 150% of the original size) was increased after CPA treatment compared to vehicle treatment (Figure 6C). Analysis of liver enzymes in the serum revealed that there was no significant difference in AST (Vehicle, 96.0 ± 11.7 vs CPA, 75.6 ± 13.5) and ALT levels (Vehicle, 42.3 ± 13.1 vs CPA, 33.0 ± 4.4) between CPA-treated and vehicle treated animals, both of which fell within normal range suggesting that the CPA treatment was well tolerated and did not cause any liver damage. Histological analysis of liver, heart, and kidney also indicated no significant organ damage caused by the chronic CPA treatment (data not shown).
Figure 6.
Inhibition of serotonin synthesis decreases tumor growth in an in vivo xenograft model of cholangiocarcinoma. Mz-ChA-1 cells were injected into the flank of athymic mice. After tumors were established, mice were treated with 150 mg/kg/day (ip) CPA, three days per week for 62 days and tumor volume assessed (A). Tumors were excised and photographed prior to histological analysis (B). Tumor latency was assessed as the time taken for the tumor to grow to 150% of the original size (C). Data are expressed as average latency (days ± SEM) and the asterix denotes significance (p<0.05) from vehicle-treated tumors.
Histological analysis of the excised tumors revealed that all cells within tumors from CPA-treated and vehicle-treated animals were CK-7 positive, indicating cholangiocyte phenotype (Supplemental Figure 4). CPA treatment significantly decreases the area of tumor necrosis (Supplemental Figure 4) and semi-quantitative analysis fibrosis shows that CPA increased fibrosis within the tumor (Supplemental Figure 4). Furthermore, the xenograft tumors also expressed Chromagranin A and NSE, in both vehicle- and CPA-treated animals (Supplemental Figure 5).
DISCUSSION
The major findings of this study relate to the dysregulation of serotonin metabolism in cholangiocarcinoma. We showed that 1) expression of the enzyme responsible for serotonin synthesis in the gastrointestinal tract, TPH1, is upregulated in cholangiocarcinoma; 2) the enzyme responsible for serotonin degradation, MAO A, is markedly decreased in cholangiocarcinoma samples and 3) that this results in an overall increase in serotonin secretion from cholangiocarcinoma cells and in the bile from cholangiocarcinoma patients. This increase in serotonin production and secretion resulted in an increased cholangiocyte proliferation in vitro, and inhibition of serotonin synthesis by CPA decrease cell proliferation in vitro and in an in vivo xenograft model of cholangiocarcinoma. These data suggest that the dysregulation of serotonin metabolism may be a key feature associated with the progression of cholangiocarcinoma and modulation of this metabolic pathway may result in the development of an effective adjunct therapy to treat this deadly disease.
Consistent with our findings that serotonin metabolism is dysregulated in cholangiocarcinoma, increased serotonin secretion is the defining feature of a number of other neuroendocrine tumors classified as carcinoid tumors (19–21). Within these tumors, serotonin is thought to stimulate cell proliferation (40, 41), and cause symptoms of the carcinoid syndrome, including diarrhea, and damage to the valves of the heart (42). Here we show that cholangiocarcinoma, while not strictly classified as carcinoid tumor, not only secreted serotonin, but displayed features of a neuroendocrine phenotype such as chromagranin A and NSE expression (43).
The expression of TPH1 has been shown to be strongly expressed in midgut carcinoid tissue (44). The authors postulate that the expression of TPH and other specific proteins on the cell surface can be recognized by CD8+ T cells and constitute an immune recognition of the tumors, which may be of great interest when pursuing an immunotherapeutic treatment strategy (44). The data presented here supports the idea that the expression of TPH1 may be involved in the process of tumor progression and cell proliferation.
In support of our findings that MAO A expression is suppressed in cholangiocarcinoma, many other neuroendocrine tumors that have a dysregulated serotonin synthesis and secretion often have a concomitant decrease in the expression of MAO A (45). In a normal cell, these amine oxidases exert an antiproliferative effect more than likely because the products of amine oxidation, aldehydes, hydrogen peroxide and other reactive oxygen species are somewhat cytotoxic (45–47). Because in certain tumor cells, the expression of such amine oxidases (including MAO A) are suppressed, in addition to the mitogenic effects of the accumulating serotonin, the potential cytotoxic, antiproliferative effects of amine oxidation are bypassed (45, 46). The mechanism of this decrease in MAO A expression is unclear. However, researchers have shown that the promoter region of MAO A can be hypermethylated under certain conditions (48). Because hypermethylation and subsequent silencing of genes such as tumor suppressor genes are a common event in the malignant transformation of cholangiocarcinoma (49), it is therefore conceivable that hypermethylation of the MAO A promoter contributes to the suppression of expression. Indeed, treatment of cholangiocarcinoma cell lines with a DNA methyltransferase inhibitor restores the expression of MAO A (DeMorrow et al unpublished observation). This is a topic of ongoing research in our laboratory.
As mentioned previously, the consequences of increased serotonin production on tumor growth depend upon the tumor type in question and perhaps the predominant serotonin receptor subtype present (9). On one hand, serotonin is known as a growth factor for several types of non-tumoral cells (10, 11), and it has been proposed to take part in the autocrine loops of growth factors contributing to cell proliferation in aggressive tumors such as small cell lung carcinoma (12), choriocarcinoma (50), and bladder cancer (15). Depending on the tumor type, either serotonin type 2 or serotonin type 1 receptor antagonists have been found to inhibit the serotonin-induced increase in tumor growth (13–15, 50). In contrast, several studies have also reported that serotonin and a serotonin type 2 receptor agonist can inhibit tumor growth (18). This effect may be related with the specific vasoconstrictive effect of serotonin and a serotonin type 2 receptor agonist on the vessels irrigating the tumor (16–18). Here, we show a proliferative effect of serotonin on cholangiocarcinoma growth and the inhibition of serotonin production effectively inhibits tumor growth. Furthermore, we could show that inhibition of the serotonin receptors 5HTR 1A, 5HTR 2A, 5HTR 2B, 5HTR 4 and 5HTR 6 effectively blocked the growth promoting effects of serotonin. The effects of increased serotonin accumulation on other aspects of cholangiocarcinoma tumorogenesis, such as malignant transformation, metastatic activity and angiogenesis are also being studied in our laboratory.
In conclusion, the data presented here indicate a dysregulated serotonin metabolic pathway in cholangiocarcinoma compared to non-malignant cholangiocytes. Specifically, there is an increase in the expression of the enzyme responsible for serotonin synthesis, TPH1, and a decreased expression of MAO A, the enzyme responsible for serotonin degradation. This leads to an increased accumulation and secretion of serotonin from cholangiocarcinoma and an increase in serotonin in the bile from cholangiocarcinoma patients. Specific inhibition of serotonin production leads to a suppression of tumor growth in a xenograft model of cholangiocarcinoma, which suggests that agents that modulate the metabolism of serotonin may be useful therapeutic tools for the treatment of this devastating cancer.
Acknowledgments
We acknowledge Glen Cryer of the Scott & White Hospital, Grants Administration Office for his assistance with proof reading and the Scott & White Hospital animal facility staff for assistance with animal surgical models.
Grant support: This work was supported by an NIH K01 grant award (DK078532) to Dr DeMorrow and by a VA Merit Award, a VA Research Scholar Award and the NIH grant DK062975 and DK58411 to Dr. Alpini, by MIUR grant 2005067975_004 to Dr. Marzioni; by MIUR grant 2006068958_001 to the Dept. of Gastroenterology, Università Politecnica delle Marche, Ancona, Italy. E. Gaudio was supported by MIUR grants (PRIN 2005) and Faculty funds, and D. Alvaro was supported by MIUR grants: 2005: 2005067975_002.
Abbreviations
- BDL
bile duct ligation
- CPA
chlorophenylalanine
- MAO
monoamine oxidase
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt cell proliferation assays
- TPH1
tryptophan hydroxylase 1
Footnotes
Financial disclosure: The authors of this manuscript have no financial arrangements to disclose.
References
- 1.Alpini G, McGill JM, LaRusso NF. The pathobiology of biliary epithelia. Hepatology. 2002;35:1256–68. doi: 10.1053/jhep.2002.33541. [DOI] [PubMed] [Google Scholar]
- 2.Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5–15. doi: 10.1002/hep.20537. [DOI] [PubMed] [Google Scholar]
- 3.Diksic M, Young SN. Study of the brain serotonergic system with labeled alpha-methyl-L-tryptophan. J Neurochem. 2001;78:1185–200. doi: 10.1046/j.1471-4159.2001.00536.x. [DOI] [PubMed] [Google Scholar]
- 4.Ormsbee HS, 3rd, Fondacaro JD. Action of serotonin on the gastrointestinal tract. Proc Soc Exp Biol Med. 1985;178:333–8. doi: 10.3181/00379727-178-42016. [DOI] [PubMed] [Google Scholar]
- 5.Kroeze WK, Kristiansen K, Roth BL. Molecular biology of serotonin receptors structure and function at the molecular level. Curr Top Med Chem. 2002;2:507–28. doi: 10.2174/1568026023393796. [DOI] [PubMed] [Google Scholar]
- 6.Martel F. Recent advances on the importance of the serotonin transporter SERT in the rat intestine. Pharmacol Res. 2006;54:73–6. doi: 10.1016/j.phrs.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Shih JC. Cloning, after cloning, knock-out mice, and physiological functions of MAO A and B. Neurotoxicology. 2004;25:21–30. doi: 10.1016/S0161-813X(03)00112-8. [DOI] [PubMed] [Google Scholar]
- 8.Ekstedt B. Substrate specificity of monoamine oxidase in pig liver mitochondria. Med Biol. 1979;57:220–3. [PubMed] [Google Scholar]
- 9.Vicaut E, Laemmel E, Stucker O. Impact of serotonin on tumour growth. Ann Med. 2000;32:187–94. doi: 10.3109/07853890008998826. [DOI] [PubMed] [Google Scholar]
- 10.Nocito A, Georgiev P, Dahm F, et al. Platelets and platelet-derived serotonin promote tissue repair after normothermic hepatic ischemia in mice. Hepatology. 2007;45:369–76. doi: 10.1002/hep.21516. [DOI] [PubMed] [Google Scholar]
- 11.Yang M, Li K, Ng PC, et al. Promoting Effects of Serotonin on Hematopoiesis: Ex Vivo Expansion of Cord Blood CD34+ Stem/Progenitor Cells, Proliferation of Bone Marrow Stromal Cells and Anti-Apoptosis. Stem Cells. 2007 doi: 10.1634/stemcells.2007-0048. [DOI] [PubMed] [Google Scholar]
- 12.Vicentini LM, Cattaneo MG, Fesce R. Evidence for receptor subtype cross-talk in the mitogenic action of serotonin on human small-cell lung carcinoma cells. Eur J Pharmacol. 1996;318:497–504. doi: 10.1016/s0014-2999(96)00812-6. [DOI] [PubMed] [Google Scholar]
- 13.Siddiqui EJ, Shabbir M, Mikhailidis DP, Thompson CS, Mumtaz FH. The role of serotonin (5-hydroxytryptamine1A and 1B) receptors in prostate cancer cell proliferation. J Urol. 2006;176:1648–53. doi: 10.1016/j.juro.2006.06.087. [DOI] [PubMed] [Google Scholar]
- 14.Sonier B, Arseneault M, Lavigne C, Ouellette RJ, Vaillancourt C. The 5-HT2A serotoninergic receptor is expressed in the MCF-7 human breast cancer cell line and reveals a mitogenic effect of serotonin. Biochem Biophys Res Commun. 2006;343:1053–9. doi: 10.1016/j.bbrc.2006.03.080. [DOI] [PubMed] [Google Scholar]
- 15.Siddiqui EJ, Shabbir MA, Mikhailidis DP, Mumtaz FH, Thompson CS. The effect of serotonin and serotonin antagonists on bladder cancer cell proliferation. BJU Int. 2006;97:634–9. doi: 10.1111/j.1464-410X.2006.06056.x. [DOI] [PubMed] [Google Scholar]
- 16.Huhnt W, Lubbe AS. Venules and arterioles in xenotransplanted human colon adenocarcinoma critically constrict with hyperthermia and serotonin. J Cancer Res Clin Oncol. 1995;121:267–74. doi: 10.1007/BF01209592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huhnt W, Lubbe AS. Growth, microvessel density and tumor cell invasion of human colon adenocarcinoma under repeated treatment with hyperthermia and serotonin. J Cancer Res Clin Oncol. 1995;121:423–8. doi: 10.1007/BF01212950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stucker O, Vicaut E, Teisseire B. Specific response to 5-HT2 agonists by arterioles linked to Meth A tumors in mice. Am J Physiol. 1992;262:H704–9. doi: 10.1152/ajpheart.1992.262.3.H704. [DOI] [PubMed] [Google Scholar]
- 19.di Sant’Agnese PA. Neuroendocrine cells of the prostate and neuroendocrine differentiation in prostatic carcinoma: a review of morphologic aspects. Urology. 1998;51:121–4. doi: 10.1016/s0090-4295(98)00064-8. [DOI] [PubMed] [Google Scholar]
- 20.Lertprasertsuke N, Tsutsumi Y. Neuroendocrine carcinoma of the urinary bladder: case report and review of the literature. Jpn J Clin Oncol. 1991;21:203–10. [PubMed] [Google Scholar]
- 21.Memon MA, Nelson H. Gastrointestinal carcinoid tumors: current management strategies. Dis Colon Rectum. 1997;40:1101–18. doi: 10.1007/BF02050937. [DOI] [PubMed] [Google Scholar]
- 22.Jones EA, Bergasa NV. The pathogenesis and treatment of pruritus and fatigue in patients with PBC. Eur J Gastroenterol Hepatol. 1999;11:623–31. doi: 10.1097/00042737-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Swain MG, Maric M. Improvement in cholestasis-associated fatigue with a serotonin receptor agonist using a novel rat model of fatigue assessment. Hepatology. 1997;25:291–4. doi: 10.1002/hep.510250206. [DOI] [PubMed] [Google Scholar]
- 24.Marzioni M, Glaser S, Francis H, et al. Autocrine/paracrine regulation of the growth of the biliary tree by the neuroendocrine hormone serotonin. Gastroenterology. 2005;128:121–37. doi: 10.1053/j.gastro.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Knuth A, Gabbert H, Dippold W, et al. Biliary adenocarcinoma. Characterisation of three new human tumor cell lines. J Hepatol. 1985;1:579–96. doi: 10.1016/s0168-8278(85)80002-7. [DOI] [PubMed] [Google Scholar]
- 26.Kusaka Y, Muraoka A, Tokiwa T, Sato J. [Establishment and characterization of a human cholangiocellular carcinoma cell line] Hum Cell. 1988;1:92–4. [PubMed] [Google Scholar]
- 27.Saijyo S, Kudo T, Suzuki M, et al. Establishment of a new extrahepatic bile duct carcinoma cell line, TFK-1. Tohoku J Exp Med. 1995;177:61–71. doi: 10.1620/tjem.177.61. [DOI] [PubMed] [Google Scholar]
- 28.DeMorrow S, Glaser S, Francis H, et al. Opposing actions of endocannabinoids on cholangiocarcinoma growth: Recruitment of fas and fas ligand to lipid rafts. J Biol Chem. 2007;282:13098–113. doi: 10.1074/jbc.M608238200. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu Y, Demetris AJ, Gollin SM, et al. Two new human cholangiocarcinoma cell lines and their cytogenetics and responses to growth factors, hormones, cytokines or immunologic effector cells. Int J Cancer. 1992;52:252–60. doi: 10.1002/ijc.2910520217. [DOI] [PubMed] [Google Scholar]
- 30.Miyagiwa M, Ichida T, Tokiwa T, Sato J, Sasaki H. A new human cholangiocellular carcinoma cell line (HuCC-T1) producing carbohydrate antigen 19/9 in serum-free medium. In Vitro Cell Dev Biol. 1989;25:503–10. doi: 10.1007/BF02623562. [DOI] [PubMed] [Google Scholar]
- 31.Storto PD, Saidman SL, Demetris AJ, Letessier E, Whiteside TL, Gollin SM. Chromosomal breakpoints in cholangiocarcinoma cell lines. Genes Chromosomes Cancer. 1990;2:300–10. doi: 10.1002/gcc.2870020408. [DOI] [PubMed] [Google Scholar]
- 32.Grubman SA, Perrone RD, Lee DW, et al. Regulation of intracellular pH by immortalized human intrahepatic biliary epithelial cell lines. Am J Physiol. 1994;266:G1060–70. doi: 10.1152/ajpgi.1994.266.6.G1060. [DOI] [PubMed] [Google Scholar]
- 33.Kanno N, Glaser S, Chowdhury U, et al. Gastrin inhibits cholangiocarcinoma growth through increased apoptosis by activation of Ca2+-dependent protein kinase C-alpha. J Hepatol. 2001;34:284–91. doi: 10.1016/s0168-8278(00)00025-8. [DOI] [PubMed] [Google Scholar]
- 34.Liu Q, Yang Q, Sun W, et al. Discovery and Characterization of Novel Tryptophan Hydroxylase Inhibitors That Selectively Inhibit Serotonin Synthesis in the Gastrointestinal Tract. J Pharmacol Exp Ther. 2008;325:47–55. doi: 10.1124/jpet.107.132670. [DOI] [PubMed] [Google Scholar]
- 35.Fava G, Marucci L, Glaser S, et al. gamma-Aminobutyric acid inhibits cholangiocarcinoma growth by cyclic AMP-dependent regulation of the protein kinase A/extracellular signal-regulated kinase 1/2 pathway. Cancer Res. 2005;65:11437–46. doi: 10.1158/0008-5472.CAN-05-1470. [DOI] [PubMed] [Google Scholar]
- 36.LeSage G, Glaser S, Ueno Y, et al. Regression of cholangiocyte proliferation after cessation of ANIT feeding is coupled with increased apoptosis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G182–90. doi: 10.1152/ajpgi.2001.281.1.G182. [DOI] [PubMed] [Google Scholar]
- 37.Carpino G, Morini S, Ginanni Corradini S, et al. Alpha-SMA expression in hepatic stellate cells and quantitative analysis of hepatic fibrosis in cirrhosis and in recurrent chronic hepatitis after liver transplantation. Dig Liver Dis. 2005;37:349–56. doi: 10.1016/j.dld.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Alvaro D, Alpini G, Onori P, et al. Estrogens stimulate proliferation of intrahepatic biliary epithelium in rats. Gastroenterology. 2000;119:1681–91. doi: 10.1053/gast.2000.20184. [DOI] [PubMed] [Google Scholar]
- 39.Gaudio E, Onori P, Pannarale L, Alvaro D. Hepatic microcirculation and peribiliary plexus in experimental biliary cirrhosis: a morphological study. Gastroenterology. 1996;111:1118–24. doi: 10.1016/s0016-5085(96)70081-1. [DOI] [PubMed] [Google Scholar]
- 40.Cattaneo MG, Palazzi E, Bondiolotti G, Vicentini LM. 5-HT1D receptor type is involved in stimulation of cell proliferation by serotonin in human small cell lung carcinoma. Eur J Pharmacol. 1994;268:425–30. doi: 10.1016/0922-4106(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 41.Codignola A, Tarroni P, Cattaneo MG, Vicentini LM, Clementi F, Sher E. Serotonin release and cell proliferation are under the control of alpha-bungarotoxin-sensitive nicotinic receptors in small-cell lung carcinoma cell lines. FEBS Lett. 1994;342:286–90. doi: 10.1016/0014-5793(94)80518-0. [DOI] [PubMed] [Google Scholar]
- 42.van der Horst-Schrivers AN, Wymenga AN, Links TP, Willemse PH, Kema IP, de Vries EG. Complications of midgut carcinoid tumors and carcinoid syndrome. Neuroendocrinology. 2004;80(Suppl 1):28–32. doi: 10.1159/000080737. [DOI] [PubMed] [Google Scholar]
- 43.Kasprzak A, Zabel M, Biczysko W. Selected markers (chromogranin A, neuron-specific enolase, synaptophysin, protein gene product 9.5) in diagnosis and prognosis of neuroendocrine pulmonary tumours. Pol J Pathol. 2007;58:23–33. [PubMed] [Google Scholar]
- 44.Vikman S, Giandomenico V, Sommaggio R, Oberg K, Essand M, Totterman TH. CD8(+) T cells against multiple tumor-associated antigens in peripheral blood of midgut carcinoid patients. Cancer Immunol Immunother. 2008;57:399–409. doi: 10.1007/s00262-007-0382-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pietrangeli P, Mondovi B. Amine oxidases and tumors. Neurotoxicology. 2004;25:317–24. doi: 10.1016/S0161-813X(03)00109-8. [DOI] [PubMed] [Google Scholar]
- 46.Toninello A, Salvi M, Pietrangeli P, Mondovi B. Biogenic amines and apoptosis: minireview article. Amino Acids. 2004;26:339–43. doi: 10.1007/s00726-004-0080-x. [DOI] [PubMed] [Google Scholar]
- 47.Ou XM, Chen K, Shih JC. Monoamine oxidase A and repressor R1 are involved in apoptotic signaling pathway. Proc Natl Acad Sci U S A. 2006;103:10923–8. doi: 10.1073/pnas.0601515103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinsonneault JK, Papp AC, Sadee W. Allelic mRNA expression of X-linked monoamine oxidase a (MAOA) in human brain: dissection of epigenetic and genetic factors. Hum Mol Genet. 2006;15:2636–49. doi: 10.1093/hmg/ddl192. [DOI] [PubMed] [Google Scholar]
- 49.Stutes M, Tran S, DeMorrow S. Genetic and epigenetic changes associated with cholangiocarcinoma: From DNA methylation to microRNAs. World J Gastroenterol. 2007;13:6465–9. doi: 10.3748/wjg.v13.i48.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sonier B, Lavigne C, Arseneault M, Ouellette R, Vaillancourt C. Expression of the 5–HT2A serotoninergic receptor in human placenta and choriocarcinoma cells: mitogenic implications of serotonin. Placenta. 2005;26:484–90. doi: 10.1016/j.placenta.2004.08.003. [DOI] [PubMed] [Google Scholar]