Abstract
Daily life stressors are a major environmental factor contributing to precipitation and exacerbation of mental illness. Animal models using repeated homotypic stress induce anxious and depressive phenotypes and are used to study the pathophysiology of affective disorders. Here we discuss data demonstrating that repeated homotypic stress produces temporally and anatomically distinct changes in endocannabinoid signaling components within stress-responsive brain regions. We also present evidence describing the neural and behavioral correlates of these adaptations in endocannabinoid signaling. These data support a role for endocannabinoid signaling in the CNS response to chronic, homotypic stress; specifically in the process of stress-response habituation. The clinical implications of these findings for the pathophysiology and treatment of affective disorders are discussed.
Keywords: restraint, anandamide, 2-arachidonoylglycerol, depression, anxiety, marijuana, CB1 cannabinoid receptor, mouse, N-arachidonylethanolamine, fatty acid amide hydrolase, rimonabant
INTRODUCTION
One of the environmental factors that consistently precipitates and exacerbates mental illnesses, including depression and anxiety, is repeated life stress (Dinan, 2005). Importantly, homotypic stressors that occur on a daily basis, such as marital problems, medical problems, work stress, and poverty, are associated with increased depressive symptoms (Caspi et al., 2003; Hammen et al., 2004; Hammen, 2005; Southwick et al., 2005; Keller et al., 2007; Robertson Blackmore et al., 2007). The development of animal models that recapitulate anxious and depressive symptoms, and associated physiological changes, has allowed for experimental investigations into the contributions of stress to mental illness and the mechanisms underlying stress-adaptation (Pittenger & Duman, 2007). One of these experimental models is repeated restraint stress. Repeated application (between 5 and 21 consecutive days, from 30 minutes to 8 hours daily depending on the paradigm) of physical restraint to rodents has been shown to induce depressive and anxious phenotypes (Vyas & Chattarji, 2004; Mitra et al., 2005; Kim & Han, 2006), immunosuppression (Sheridan et al., 1994; Raison & Miller, 2001; Shi et al., 2003) and cognitive impairments (Radecki et al., 2005; Trofimiuk et al., 2005; Walesiuk et al., 2005; Lupien et al., 2007; Wright & Conrad, 2007); symptoms often experienced in depressive illness (Mathews & MacLeod, 2005; Schneiderman et al., 2005; Irwin & Miller, 2007). Repeated restraint also increases plasma glucocorticoid concentrations, causes adrenal gland hypertrophy, and reduces body weight (Melia et al., 1994; Kim & Han, 2006).
Behavioral and hormonal responses to repeated and predictable exposure to homotypic stressors (such as restraint) exhibit habituation. Habituation is a progressive decrease in the expression of stress responses after repeated applications of the same stressor. Habituation is stressor specific (Kant et al., 1985; Lachuer et al., 1994; Melia et al., 1994), dependent upon the inter-stimulus duration (De Boer et al., 1990), and initial stressor intensity (Natelson et al., 1988). Much of the data regarding the mechanisms of stress habituation have focused on the role of glucocorticoids (Kant et al., 1985; Melia et al., 1994; Cole et al., 2000; Jaferi et al., 2003; Jaferi & Bhatnagar, 2006). However, recent studies have also implicated the endocannabinoid signaling system in this process (Patel et al., 2005b; Kamprath et al., 2006).
ENDOCANNABINOID SIGNALING
The endocannabinoids, N-arachidonylethanolamine (anandamide; AEA) and 2-arachidonoylglycerol (2-AG), are neuroactive lipids that are produced within the brain by neurons and glial cells and function primarily as interneuronal signaling molecules (Freund et al., 2003; Piomelli, 2003; Bisogno et al., 2005). The endocannabinoids are not stored or released vesicularly, but are synthesized and/or released in response to altered neuronal activity. The syntheses of AEA and 2-AG occur via separate enzymatic cascades and are evoked by neuronal depolarization, elevations in intracellular calcium, and activation of several metabotropic and excitatory ionotropic neurotransmitter receptors (DiMarzo et al., 1994; Sugiura et al., 2002; Freund et al., 2003; Jung et al., 2005; Liu et al., 2006). The predominant endocannabinoid receptor found within the central nervous system is the G-protein coupled receptor, CB1, whose subcellular localization is primarily axonal terminals (Piomelli, 2003; Chevaleyre et al., 2006)). CB1 receptors are present at high density within limbic brain structures including the amygdala, hippocampus, and prefrontal cortex (Tsou et al., 1998; Egertova et al., 2003; Katona et al., 2006). Activation of CB1 receptors on axon terminals results in inhibition of neurotransmitter release via Gi/o-coupled intracellular signaling pathways (Chevaleyre et al., 2006). Considerable evidence supports the hypothesis that endocannabinoids function as activity-dependent, retrograde inhibitors of neurotransmitter release. In particular, endocannabinoid/CB1 signaling subserves several forms of short and long-term neuronal plasticity, including depolarization-induced suppression, and long-term depression of excitatory and inhibitory neurotransmission within stress responsive brain regions including the prefrontal cortex (PFC) (Auclair et al., 2000; Leforcade et al., 2007), amygdala (Marsicano et al., 2002; Azad et al., 2004), and nucleus accumbens (Robbe et al., 2002; Mato et al., 2007). 2-AG is primarily, but not exclusively, degraded by monoacylglycerol lipase (MGL), which is located within presynaptic terminals (Dinh et al., 2002). In contrast, AEA is degraded by an intracellular serine hydrolase, fatty acid amide hydrolase (FAAH), that is found predominantly in neurons postsynaptic to axon terminals expressing the CB1 receptor (Tsou et al., 1998b).
Analysis of brain regional concentrations of the endocannabinoids by mass spectrometry has led the observation that they exhibit dynamic and often divergent changes in response to a variety of behavioral and pharmacological stimuli. Recent studies have utilized this technique, in combination with behavioral and physiological studies, to explore the effects of repeated, homotypic stress on the endocannabinoid system. We summarize these findings below and discuss their relevance to the pathophysiology and treatment of affective disorders.
REPEATED EXPOSURE TO RESTRAINT STRESS INDUCES CHANGES IN ENDOCANNABINOID SIGNALING COMPONENTS
We have examined the effects of repeated restraint stress, 30 minutes daily for varying durations, on endocannabinoid contents in different brain regions of mice (Patel et al., 2004; Patel et al., 2005b; Rademacher et al., 2008). Several consistent patterns have been observed for both 2-AG and AEA in response to restraint stress. These data indicate temporally dynamic and regionally divergent (cortical vs. striatal) effects of repeated restraint stress on these two endocannabinoid ligands (see Figure 1). A single application of 30 minute restraint does not alter the content of 2-AG within the limbic forebrain, medial prefrontal cortex (mPFC), ventral striatum, hippocampus*, amygdala, or cerebellum ((Patel et al., 2005b) and *Patel and Hillard, unpublished data), but decreases 2-AG content within the hypothalamus (Patel et al. 2004). In contrast, increasing the number of consecutive daily restraint episodes to 5, 7 and 10 results in a progressive increase in 2-AG content within the mPFC, limbic forebrain, amygdala, hippocampus, and hypothalamus immediately after the last restraint episode (Patel et al., 2005b, Rademacher et al., 2008). Interestingly, chronic treatment of rats with corticosterone also results in increased 2-AG content in the amygdala (Hill et al. 2005), suggesting that the effect of repeated restraint on amygdalar 2-AG content could be secondary to a persistent increase in glucocorticoid signaling. In contrast to these brain regions, a significant decrease in ventral striatal 2-AG content was seen after the 7th restraint (Rademacher et al., 2008).
Figure 1.
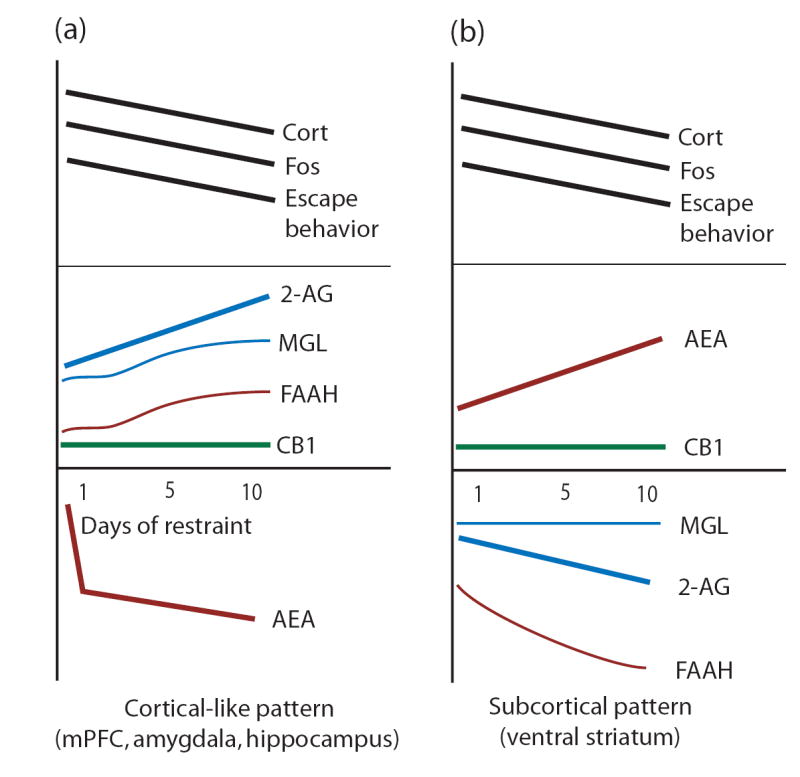
Diagramatic representation of adaptations in endocannabinoid signaling components in response to repeated restraint stress. Top panels (black) represent the habituation of neuroendocrine, neuronal and behavioral habituation in response to repeated restraint. Bottom panels show the differential patterns of changes in endocannabinoid signaling components, AEA and 2-AG levels, CB1 receptor density, FAAH and MGL activity. The cortical pattern is shown in (a), whereas the sub-cortical pattern is shown in (b).
Taken together, these data indicate that multiple exposures to the same stressor, in this case restraint, recruits or activates 2-AG-mediated signaling within cortical-like brain regions (mPFC, amygdala, hippocampus) and the hypothalamus. This effect can be considered a form of sensitization since a single episode of restraint did not increase 2-AG contents in these regions. Sensitization of the 2-AG response could occur via several possible mechanisms: first, 2-AG degradation could be reduced; second, an increase in the release of a neurotransmitter (such as glutamate) in response to stress could activate 2-AG synthetic enzymes phospholipase C (PLC) and/or diacylglycerol lipase (DAGL); or third, repeated stress exposure could up-regulate the 2-AG synthetic enzymes. With regard to the first possibility, 2-acylglycerol hydrolysis is not decreased ex vivo in cytosolic preparations from the amygdala, ventral striatum or mPFC of mice exposed to 10 episodes of restraint (Rademacher et al., 2008). With regard to the second possibility, glutamate efflux within the PFC is robust during the first exposure to a stressor, but shows habituation such that by the third exposure glutamate efflux is significantly reduced compared to the first exposure (see Moghaddam 2002). This suggests that increase in 2-AG content observed after repeated restraint stress occurs in the face of decreasing stimulatory drive (i.e. glutamate release), and is thus likely mediated by the third possibility, an increase in 2-AG synthetic capacity. While Dwivedi and colleagues reported that PLCß expression is decreased after repeated stress in the frontal cortex (Dwivedi et al., 2005), the effects of repeated restraint on the activity and expression of both other PLC isoforms and DAGL have not been studied. Interestingly, these data suggest the habituated glutamate response to repeated stress within the PFC could be mediated by increased 2-AG signaling, since 2-AG can decrease presynaptic glutamate release within the PFC ((Auclair et al., 2000;Lafourcade et al. 2007) and see below). In other words, the directionality of the interaction between 2-AG and glutamate could be 2-AG affecting glutamate release rather than glutamate release acting as the primary driving force for 2-AG release under these conditions.
AEA contents are also affected by stress, but the pattern is different from that seen with 2-AG. In the mPFC and amygdala, restraint stress decreases AEA content regardless of the number of prior exposures (Patel et al. 2005b, Rademacher et al. 2008). In fact, in both regions, the reduction in AEA tends to become more pronounced as the number of restraint episodes increases, although this has not been a consistent finding. FAAH is up-regulated in the amygdala and mPFC (as measured by an increase in its Vmax) after 10 episodes of restraint. This change is consistent with a greater effect of stress on AEA after multiple exposures but does not explain the effect of the first restraint. It is our current hypothesis that AEA synthesis is tonically “on” in these brain regions when the mice are in a calm, normal state. The application of stress results in a reduction of AEA synthesis through a mechanism that does not habituate or sensitize to a significant degree. Alternatively, it is possible that acute restraint results in the rapid release and subsequent degradation of a limited supply of preformed AEA, which would be measured as a decrease in tissue content if stores of AEA have not had time to be replenished. This hypothesis suggests a limited capacity for “on-demand synthesis”, and could be tested by analyzing AEA precursor content together with the activity of AEA biosynthetic enzymes in response to acute restraint stress.
Interestingly, the contents of AEA and two other N-acylethanolamines (palmitoylethanolamide and oleoylethanolamide) are significantly increased in the ventral striatum after 10 episodes of restraint. These changes are accompanied by a decrease in the Vmax for FAAH, which suggests that the increased content within this region is due to decreased catabolism of this family of lipids (Rademacher et al. 2008).
We have also determined whether repeated restraint stress alters CB1 receptor density or affinity for the agonist, CP55940. Neither the Kd nor Bmax for CP55940 binding to the CB1 receptor were altered by acute or repeated restraint stress within any brain region examined (Rademacher et al., 2008). These data suggest that the major changes in the endocannabinoid system in response to repeated restraint stress occur at the level of the endogenous ligands AEA and 2-AG rather than at the level of their cognate receptor (see Figure 1), however changes in signaling efficacy through the CB1 receptor remain an open possibility.
Several important questions remain to be answered regarding the endocannabinoid response to repeated restraint stress. First, are the long-term changes in AEA and/or 2-AG dependent upon application of the last restraint per se, or are they adaptive changes that are dependent upon previous restraint stress exposure only? Examination of AEA and 2-AG tissue contents a longer time after the termination of the last restraint episode would answer this question. Second, are these sensitized adaptations in 2-AG signaling specific to the stressor (i.e. restraint), or do they generalize to any subsequent aversive experience? Determining if the sensitized 2-AG response is stressor specific would strengthen the argument for a role in habituation, since habituation is stressor specific (i.e. requires repeated exposure to the same stressor). Examination of 2-AG content after application of a novel stressor subsequent to the repeated restraint protocol would differentiate between these two possibilities. Third, do the changes in tissue endocannabinoids reflect changes in the endocannabinoids that are available to activate the CB1 receptor? This is an important issue and caveat of the data presented thus far. Recent data from Parsons and colleagues (Caille et al. 2007) suggest that dialyzable 2-AG content is a fraction of the content measured in tissue pieces. Therefore, it will be important to confirm the changes in endocannabinoid content using techniques, such as microdialysis, that can sample a pool that is more likely to be involved in signaling. Although these and other questions remain, our data support the hypothesis that robust and reproducible changes in endocannabinoid content occur in response to repeated, homotypic stress and suggest a prominent role for this signaling system in the adaptive responses to stress, some of which are described below.
ENDOCANNABINOID SIGNALING MODULATES LIMBIC NEURONAL RESPONSES TO REPEATED STRESS
Since the endocannabinoid 2-AG has been implicated in the inhibition of glutamate release within the PFC and other brain regions (Lafourcade et al. 2007), and repeated restraint stress increases 2-AG content within limbic brain structures, we explored the role of stress-induced activation of endocannabinoid signaling on neuronal activation using Fos expression as a marker of neuronal excitation (Patel et al., 2005b). Consistent with previous data, a single episode of restraint stress increased Fos expression within limbic structures including the mPFC, lateral septum, and the medial amygdala. All of these responses were significantly reduced during the 5th restraint episode, a time at which limbic forebrain 2-AG content was increased. This lead us to test the hypothesis that the habituation of neuronal activation by stress in these regions was due to an increase in 2-AG content. To test this hypothesis, we administered the CB1 receptor antagonist, rimonabant, prior to the 5th restraint application to block the effects of 2-AG on CB1 receptor signaling. Administration of rimonabant prior to the 5th restraint episode blocked the habituation and returned Fos expression to levels observed during the first restraint application in the mPFC and lateral septum (Patel et al., 2005b). We interpret these data to indicate that the progressive increase in 2-AG observed after repeated restraint serves to dampen neuronal activation induced by restraint in these brain regions and thereby contributes to the habituation of limbic neuronal responses that occurs in response to repeated homotypic stress application.
A synaptic mechanism subserving this phenomenon can be hypothesized based on recent data from Manzoni and colleagues who demonstrated that CB1 receptors are located on excitatory presynaptic terminals in the mPFC and the 2-AG synthetic enzyme, DAGL, is located within dendrites of PFC pyramidal neurons (Lafourcade et al., 2007). Furthermore, activation of CB1 receptors results in a decrease in excitatory transmission onto PFC pyramidal neurons (Lafourcade et al., 2007). More interestingly, tetanic stimulation-induced long-term depression of excitatory transmission is dependent upon CB1 receptor activation by 2-AG (Lafourcade et al., 2007). Based on these data, we suggest the following model for the role of 2-AG in the habituation of neuronal activation in response to repeated restraint stress (Figure 2). Acute restraint stress activates glutamatergic inputs to the mPFC which initiates both the release of 2-AG and Fos protein expression. 2-AG, in turn, activates CB1 receptors on the glutamatergic terminals resulting in decreased glutamate release; however, this does not occur robustly since rimonabant had no effect on Fos expression following a single restraint in these regions. Upon exposure to the 5th restraint, stress-related afferent input patterns to the mPFC result in synthesis of larger amounts of 2-AG (possibly via an activity-dependent, up-regulation of postsynaptic 2-AG synthetic enzymes), resulting in a strong reduction in glutamate release and downstream Fos expression. The net result is a habituated Fos response to repeated restraint, and is consistent with the habituated PFC glutamate efflux observed by microdialysis after repeated stress exposure (Moghaddam 2002). However, when the CB1 receptor antagonist is administered prior to the 5th restraint, the 2-AG-CB1 receptor-dependent synaptic suppression is blocked, resulting in loss of habituation. Determining whether CB1 receptor antagonist administration prior to the 5th restraint episode also reverses the habituated glutamate efflux would further validate this model.
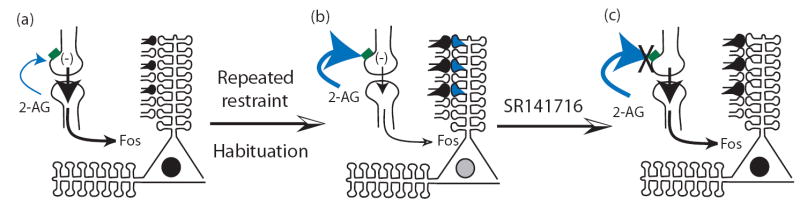
Figure 2.
Proposed mechanism by which 2-AG signaling, activated by repeated restraint stress, could contribute to the habituated neuronal responses. Initial exposure to stress (a) increases Fos expression (black nuclei) in limbic brain regions via activation of specific afferent synaptic activity patterns (black presynaptic terminals), this activation causes a small increases 2-AG signaling that serves to decrease presynaptic glutamatergic transmission onto mPFC pyramidal neurons via activation of CB1 receptors (green). After repeated stress application (b), an activity dependent up-regulation of 2-AG synthetic enzymes (blue post-synaptic dendritic spines) results in a large increase in 2-AG synthesis in response to stress. The large increase in 2-AG activates presynaptic CB1 receptors which decreases presynaptic glutamate release, post-synaptic depolarization and, thereby, reduces Fos expression (grey nulei). When rimonabant (SR141716) is administered after habituation has been established (c), 2-AG can no longer inhibit presynaptic glutamate release, and Fos expression is reinstated to pre-habituation levels.
ENDOCANNABINOID SIGNALING MODULATES BEHAVIORAL RESPONSES TO REPEATED STRESS
When restrained in plastic conical tubes, mice initially exhibit escape behaviors including biting, turning and chewing. These behaviors subside during the course of the 30 minute restraint episode, and show significant habituation during the 5th restraint episode (Patel et al., 2005b). During the 5th restraint episode, when 2-AG is elevated in limbic brain regions, administration of rimonabant re-instates the escape behaviors, although not to the extent that was observed during the first restraint episode (Patel et al., 2005b). These data suggest a partial role for 2-AG signaling in behavioral habituation during repeated restraint exposure. Similarly, Kamprath et al. (2006) have provided evidence that the CB1 receptor is required for the habituation of innate fear behaviors in mice. In this paradigm, presentation of the tone stimulus used in fear conditioning paradigms (preceded by a sensitizing shock) results in freezing behavior that habituates following repeated presentations; this represents a non-associative component of extinction conditioned fear behavior (Kamprath & Wotjak, 2004). Mice lacking CB1 receptors do not show habituation of these innate fear responses after repeated tone presentation (Kamprath et al., 2006). These authors suggest that the impairments in extinction of conditioned fear behavior observed in CB1 null mice, and after CB1 receptor blockade, are a result of an impaired “habituation component” of the extinction process (Kamprath et al., 2006). Taken together, these data support the hypothesis that the endocannabinoid system is involved in behavioral habituation to repeated, homotypic stress exposure.
We have also explored the role of the endocannabinoid system in the anhedonia induced by repeated stress exposure (Rademacher & Hillard, 2007). Anhedonia, a prominent symptom of depression in humans, is seen as a reduction in sucrose preference in mice exposed to repeated stress. This reduction in sucrose preference is present between the 1st and 7th restraint episodes and was reversed by pretreatment of the mice with inhibitors of FAAH and enhanced by blockade of the CB1 receptor. These findings are consistent with a stress-buffering role for endocannabinoid signaling. However, these data also suggest a growing reliance on endocannabinoid signaling as the number of restraint exposures increases since administration of rimonabant prior to the 10th restraint episode severely reduced sucrose preference (Rademacher & Hillard, 2007). Therefore, although the repeated exposure to restraint did not habituate with regard to its effect on sucrose consumption, the endocannabinoid system was nonetheless progressively recruited and acted to reverse the effects of the stress on reward-motivated behavior. These data, together with the increase in cortical 2-AG content and ventral striatal AEA content observed during repeated restraint exposure, support the hypothesis that one or both of these signaling systems functions as a neuroadaptive mechanism to counteract the anhedonic phenotype induced by repeated restraint stress. This hypothesis is further supported by our finding that direct and indirect CB1 agonists reverse restraint stress-induced decreases in sucrose preference prior to the 10th restraint exposure (Rademacher & Hillard, 2007), and the data of others that low levels of CB1 receptor activation facilitate operant responding for food and drugs of abuse in non-stressed animals (Higgs et al., 2005; Solinas & Goldberg, 2005; Solinas et al., 2005). Taken together, these data support the hypothesis that the endocannabinoid system facilitates motivated behavior in a generalized fashion and thereby counteracts stress-induced anhedonia.
ENDOCANNABINOID SIGNALING MODULATES NEUROENDOCRINE RESPONSES TO REPEATED STRESS
Endocannabinoid signaling also plays a role in adaptation of the hypothalamic-pituitary-adrenal (HPA) axis to repeated restraint stress. Repeated activation of the HPA axis by restraint stress also demonstrates habituation as measured by a progressive decrease in plasma corticosterone with increasing numbers of restraint episodes (Patel et al., 2004). As mentioned above, 2-AG is decreased in the hypothalamus after the first restraint exposure, while AEA is unchanged. However, 2-AG is increased after the 5th restraint application, at which time the corticosterone response shows habituation (Patel et al., 2004). This finding, combined with the data that low doses of direct CB1 agonists and indirect CB1 agonists can inhibit stress-induced corticosterone release, lead us to hypothesize that 2-AG synthesis is increased upon repeated restraint application and that this change contributes to habituated HPA-axis response to repeated restraint stress (Patel et al., 2004).
CONCLUSIONS AND CLINICAL IMPLICATIONS
The data reviewed above suggests a role for endocannabinoid signaling in the adaptation to repeated homotypic stress. Alterations in endocannabinoid signaling in response to repeated homotypic stress appear to be at the level of endogenous ligands rather than changes in CB1 receptor density or agonist affinity, although it is possible that changes in signal transduction efficacy have occurred. Alterations in endocannabinoid content appear to be selective for stress-sensitive brain regions since there were no changes observed within the cerebellum, a brain region mainly involved in motor coordination in rodents (although the cerebellum has been implicated in cognitive functioning in humans (see (Thach, 2007)). Within brain regions that exhibited changes in AEA and 2-AG content with repeated restraint stress, two general patterns were observed. A cortical-like pattern was observed within the mPFC, amygdala, and hippocampus (Figure 2). Although not a cortical structure, the hypothalamus showed a similar pattern, suggesting similar mechanisms could regulate endocannabinoid signaling in all of these regions. A different pattern was observed within the ventral striatum. These differential patterns could have implications for the pathophysiology and treatment of stress-related psychiatric disorders including depression, anxiety and post-traumatic stress disorder (PTSD).
In the brain regions exhibiting the cortical pattern of endocannabinoid changes, long-term decreases in tissue content of AEA are likely mediated by increased degradation. These data suggest that repeated stress induces an AEA deficiency in these cortical regions and that pharmacological augmentation or normalization of AEA signaling could represent a promising approach to the treatment of anxiety and depression. In support of this hypothesis, FAAH inhibitors (that increase brain AEA levels) have anti-anxiety and anti-depressant properties (Kathuria et al., 2003; Gobbi et al., 2005; Patel & Hillard, 2006; Bortolato et al., 2007; Naidu et al., 2007), and viral over-expression of FAAH within the PFC decreases AEA levels and induces anxiety behaviors in rats (Rubino et al., 2007). In addition, we have found that women suffering from minor depression have decreased plasma AEA levels (Hill et al., 2007), however another study did not detect changes in AEA content in the cerebrospinal fluid from patients with affective disorders compared to controls (Giuffrida et al., 2004).
In contrast to AEA, 2-AG increases in cortical-like structures, and exhibits a sensitized and robust response to repeated restraint stress. We suggest this progressive increase contributes to the habituation of neuronal, endocrine, and behavioral responses to stress. The contribution of 2-AG-CB1 signaling to the expression of habituation, which is an important contributor to the extinction of conditioned fear responses, suggests that pharmacological augmentation of 2-AG signaling could have therapeutic potential for treatment of anxiety disorders including PTSD (Marsicano et al., 2002; Suzuki et al., 2004; Chhatwal et al., 2005; Kamprath et al., 2006).
Within subcortical regions such as the ventral striatum, our data indicate that repeated restraint results in a 2-AG deficit together with enhanced AEA signaling. Ventral striatal AEA signaling increases the hedonic impact of rewarding stimuli (Mahler et al., 2007), increases mesolimbic dopamine activity (Solinas et al., 2006), and likely counteracts stress-induced decreases in sucrose preference (Rademacher & Hillard, 2007). Together with observations that injections of 2-AG or compounds that increase 2-AG levels into the ventral striatum increase feeding behavior (Kirkham et al., 2002; Soria-Gomez et al., 2007), these data suggest that pharmacological augmentation of either AEA or 2-AG signaling could be used to treat anhedonic symptoms of depression.
The above hypotheses regarding the potential utility of indirect cannabinoid agonists in the treatment of affective disorders is consistent with the epidemiological data that the most common reasons given for continued cannabis use include the desire for its anxiolytic and antidepressant effects. However, paradoxical anxiety and panic are well known side effects of cannabis intoxication, especially at high doses and in stressful environmental contexts (Gregg et al., 1976; Naliboff et al., 1976; Szuster et al., 1988; Thomas, 1996; Hall & Solowij, 1998; Reilly et al., 1998). In this regard, we have shown that, in contrast to direct CB1 receptor agonists, a FAAH inhibitor does not have biphasic dose dependent effects, and does not synergize with stress to active brain structures involved in the generation of anxiety, an effect that does occur with direct agonists (Patel et al., 2004; Patel et al., 2005a; Patel & Hillard, 2006). In summary, these data suggest the endocannabinoid system is an important mediator of stress habituation, and pharmacological augmentation of endocannabinoid signaling represents a mechanistically-sound target for the development of pharmacological agents for the treatment of affective disorders that could be devoid of the adverse side-effects of direct CB1 agonists.
Acknowledgments
The authors were supported by Research for a Healthier Tomorrow, a component of the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin and NIH grants R01 DA016967 (CJH) and F30 DA15575 (SP).
Abbreviations
- AEA
N-arachidonylethanolamine
- FAAH
fatty acid amide hydrolase
- HPA
hypothalamic-pituitary-adrenal
- PFC
prefrontal cortex
- mPFC
medial prefrontal cortex
- MGL
monoacylglycerol lipase
- PTSD
post-traumatic stress disorder
- CB1
type-1 cannabinoid receptor
- 2-AG
2-arachidonoylglycerol
- PLC
phospholipase C
- DAGL
diacylglycerol lipase
References
- Auclair N, Otani S, Soubrie P, Crepel F. Cannabinoids modulate synaptic strength and plasticity at glutamatergic synapses of rat prefrontal cortex pyramidal neurons. J Neurophysiol. 2000;83:3287–3293. doi: 10.1152/jn.2000.83.6.3287. [DOI] [PubMed] [Google Scholar]
- Azad SC, Monory K, Marsicano G, Cravatt BF, Lutz B, Zieglgansberger W, Rammes G. Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. J Neurosci. 2004;24:9953–9961. doi: 10.1523/JNEUROSCI.2134-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Ligresti A, Di Marzo V. The endocannabinoid signaling system: biochemical aspects. Pharmacol Biochem Behav. 2005;81:224–238. doi: 10.1016/j.pbb.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol Psychiatry. 2007;62:1103–10. doi: 10.1016/j.biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Caille S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations in extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine-self administration. J Neurosci. 2007;27:3695–702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology. 2005;30:516–524. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- Cole MA, Kalman BA, Pace TW, Topczewski F, Lowrey MJ, Spencer RL. Selective blockade of the mineralocorticoid receptor impairs hypothalamic-pituitary-adrenal axis expression of habituation. J Neuroendocrinol. 2000;12:1034–1042. doi: 10.1046/j.1365-2826.2000.00555.x. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Koopmans SJ, Slangen JL, Van der Gugten J. Plasma catecholamine, corticosterone and glucose responses to repeated stress in rats: effect of interstressor interval length. Physiol Behav. 1990;47:1117–1124. doi: 10.1016/0031-9384(90)90361-7. [DOI] [PubMed] [Google Scholar]
- DiMarzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Dinan TG. Stress: the shared common component in major mental illnesses. Eur Psychiatry. 2005;20(Suppl 3):S326–328. doi: 10.1016/s0924-9338(05)80184-1. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Freund TF, Piomelli D. A role for monoglyceride lipase in 2-arachidonoylglycerol inactivation. Chem Phys Lipids. 2002;121:149–158. doi: 10.1016/s0009-3084(02)00150-0. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Mondal AC, Rizavi HS, Shukla PK, Pandey GN. Single and repeated stress-induced modulation of phospholipase C catalytic activity and expression: role in LH behavior. Neuropsychopharmacology. 2005;30:473–83. doi: 10.1038/sj.npp.1300605. [DOI] [PubMed] [Google Scholar]
- Egertova M, Cravatt BF, Elphick MR. Comparative analysis of fatty acid amide hydrolase and CB(1) cannabinoid receptor expression in the mouse brain: evidence of a widespread role for fatty acid amide hydrolase in regulation of endocannabinoid signaling. Neuroscience. 2003;119:481–496. doi: 10.1016/s0306-4522(03)00145-3. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Leweke FM, Gerth CW, Schreiber D, Koethe D, Faulhaber J, Klosterkotter J, Piomelli D. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology. 2004;29:2108–14. doi: 10.1038/sj.npp.1300558. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg JM, Small EW, Moore R, Raft D, Toomey TC. Emotional response to intravenous delta9tetrahydrocannabinol during oral surgery. J Oral Surg. 1976;34:301–313. [PubMed] [Google Scholar]
- Hall W, Solowij N. Adverse effects of cannabis. Lancet. 1998;352:1611–1616. doi: 10.1016/S0140-6736(98)05021-1. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annu Rev Clin Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hammen C, Brennan PA, Shih JH. Family discord and stress predictors of depression and other disorders in adolescent children of depressed and nondepressed women. J Am Acad Child Adolesc Psychiatry. 2004;43:994–1002. doi: 10.1097/01.chi.0000127588.57468.f6. [DOI] [PubMed] [Google Scholar]
- Higgs S, Barber DJ, Cooper AJ, Terry P. Differential effects of two cannabinoid receptor agonists on progressive ratio responding for food and free-feeding in rats. Behav Pharmacol. 2005;16:389–393. doi: 10.1097/00008877-200509000-00011. [DOI] [PubMed] [Google Scholar]
- Hill MN, Ho WS, Meier SE, Gorzalka BB, Hillard CJ. Chronic corticosterone treatment increases the endocannabinoid 2-arachidonylglycerol in rat amygdala. Eur J Pharmacol. 2005;528:99–102. doi: 10.1016/j.ejphar.2005.10.058. [DOI] [PubMed] [Google Scholar]
- Hillard CJ. Biochemistry and pharmacology of the endocannabinoids arachidonylethanolamide and 2-arachidonylglycerol. Prostaglandins Other Lipid Mediat. 2000;61:3–18. doi: 10.1016/s0090-6980(00)00051-4. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Jarrahian A. Accumulation of anandamide: Evidence for cellular diversity. Neuropharmacology. 2005;48:1072–1078. doi: 10.1016/j.neuropharm.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Jaferi A, Bhatnagar S. Corticosterone can act at the posterior paraventricular thalamus to inhibit hypothalamic-pituitary-adrenal activity in animals that habituate to repeated stress. Endocrinology. 2006;147:4917–4930. doi: 10.1210/en.2005-1393. [DOI] [PubMed] [Google Scholar]
- Jaferi A, Nowak N, Bhatnagar S. Negative feedback functions in chronically stressed rats: role of the posterior paraventricular thalamus. Physiol Behav. 2003;78:365–373. doi: 10.1016/s0031-9384(03)00014-3. [DOI] [PubMed] [Google Scholar]
- Jung KM, Mangieri R, Stapleton C, Kim J, Fegley D, Wallace M, Mackie K, Piomelli D. Stimulation of endocannabinoid formation in brain slice cultures through activation of group I metabotropic glutamate receptors. Mol Pharmacol. 2005;68:1196–202. doi: 10.1124/mol.105.013961. [DOI] [PubMed] [Google Scholar]
- Kamprath K, Marsicano G, Tang J, Monory K, Bisogno T, Di Marzo V, Lutz B, Wotjak CT. Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. J Neurosci. 2006;26:6677–6686. doi: 10.1523/JNEUROSCI.0153-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamprath K, Wotjak CT. Nonassociative learning processes determine expression and extinction of conditioned fear in mice. Learn Mem. 2004;11:770–786. doi: 10.1101/lm.86104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant GJ, Eggleston T, Landman-Roberts L, Kenion CC, Driver GC, Meyerhoff JL. Habituation to repeated stress is stressor specific. Pharmacol Biochem Behav. 1985;22:631–634. doi: 10.1016/0091-3057(85)90286-2. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MC, Neale MC, Kendler KS. Association of different adverse life events with distinct patterns of depressive symptoms. Am J Psychiatry. 2007;164:1521–1529. doi: 10.1176/appi.ajp.2007.06091564. [DOI] [PubMed] [Google Scholar]
- Kim KS, Han PL. Optimization of chronic stress paradigms using anxiety-and depression-like behavioral parameters. J Neurosci Res. 2006;83:497–507. doi: 10.1002/jnr.20754. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachuer J, Delton I, Buda M, Tappaz M. The habituation of brainstem catecholaminergic groups to chronic daily restraint stress is stress specific like that of the hypothalamo-pituitary-adrenal axis. Brain Res. 1994;638:196–202. doi: 10.1016/0006-8993(94)90650-5. [DOI] [PubMed] [Google Scholar]
- Lafourcade M, Elezgarai I, Mato S, Bakiri Y, Grandes P, Manzoni OJ. Molecular components and functions of the endocannabinoid system in mouse prefrontal cortex. PLoS ONE. 2007;2:e709. doi: 10.1371/journal.pone.0000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, Chan AC, Zhou Z, Huang BX, Kim HY, Kunos G. A biosynthetic pathway for anandamide. Proc Natl Acad Sci U S A. 2006;103:13345–13350. doi: 10.1073/pnas.0601832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn. 2007;65:209–37. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: Anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology. 2007;32:2267–78. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annu Rev Clin Psychol. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Mato S, Lafourcade M, Robbe D, Bakiri Y, Manzoni OJ. Role of the cyclic-AMP/PKA cascade and of P/Q-type Ca(++) channels in endocannabinoid-mediated long-term depression in the nucleus accumbens. Neuropharmacology. 2007;54:87–94. doi: 10.1016/j.neuropharm.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Melia KR, Ryabinin AE, Schroeder R, Bloom FE, Wilson MC. Induction and habituation of immediate early gene expression in rat brain by acute and repeated restraint stress. J Neurosci. 1994;14:5929–5938. doi: 10.1523/JNEUROSCI.14-10-05929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol Psychiatry. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Varvel SA, Ahn K, Cravatt BF, Martin BR, Lichtman AH. Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology (Berl) 2007;192:61–70. doi: 10.1007/s00213-006-0689-4. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Rickles WH, Cohen MJ, Naimark RS. Interactions of marijuana and induced stress: forearm blood flow, heart rate, and skin conductance. Psychophysiology. 1976;13:517–522. doi: 10.1111/j.1469-8986.1976.tb00871.x. [DOI] [PubMed] [Google Scholar]
- Natelson BH, Ottenweller JE, Cook JA, Pitman D, McCarty R, Tapp WN. Effect of stressor intensity on habituation of the adrenocortical stress response. Physiol Behav. 1988;43:41–46. doi: 10.1016/0031-9384(88)90096-0. [DOI] [PubMed] [Google Scholar]
- Patel S, Cravatt BF, Hillard CJ. Synergistic interactions between cannabinoids and environmental stress in the activation of the central amygdala. Neuropsychopharmacology. 2005a;30:497–507. doi: 10.1038/sj.npp.1300535. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther. 2006;318:304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145:5431–5438. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Hillard CJ. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signaling. Eur J Neurosci. 2005b;21:1057–1069. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signaling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: A convergence of mechanisms. Neuropsychopharmacology. 2007;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Radecki DT, Brown LM, Martinez J, Teyler TJ. BDNF protects against stress-induced impairments in spatial learning and memory and LTP. Hippocampus. 2005;15:246–253. doi: 10.1002/hipo.20048. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Hillard CJ. Interactions between endocannabinoids and stress-induced decreased sensitivity to natural reward. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:633–641. doi: 10.1016/j.pnpbp.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher DJ, Meier SE, Shi L, Vanessa Ho WS, Jarrahian A, Hillard CJ. Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology. 2007;54:108–16. doi: 10.1016/j.neuropharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. The neuroimmunology of stress and depression. Semin Clin Neuropsychiatry. 2001;6:277–294. doi: 10.1053/scnp.2001.0060277. [DOI] [PubMed] [Google Scholar]
- Reilly D, Didcott P, Swift W, Hall W. Long-term cannabis use: characteristics of users in an Australian rural area. Addiction. 1998;93:837–846. doi: 10.1046/j.1360-0443.1998.9368375.x. [DOI] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson Blackmore EK, Stansfeld SA, Weller I, Munce S, Zagorski BM, Stewart DE. Major depressive episodes and work stress: Results from a national population survey. Am J Public Health. 2007;97:2088–93. doi: 10.2105/AJPH.2006.104406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Realini N, Castigliono C, Guidali C, Vigano D, Marras E, Petrosino S, Perletti G, Maccarrone M, Di Marzo V, Parolaro D. Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex . Cerebral Cortex. 2007 doi: 10.1093/cercor/bhm161. in press. [DOI] [PubMed] [Google Scholar]
- Schneiderman N, Ironson G, Siegel SD. Stress and health: psychological, behavioral, and biological determinants. Annu Rev Clin Psychol. 2005;1:607–628. doi: 10.1146/annurev.clinpsy.1.102803.144141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan JF, Dobbs C, Brown D, Zwilling B. Psychoneuroimmunology: stress effects on pathogenesis and immunity during infection. Clin Microbiol Rev. 1994;7:200–212. doi: 10.1128/cmr.7.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Devadas S, Greeneltch KM, Yin D, Allan Mufson R, Zhou JN. Stressed to death: implication of lymphocyte apoptosis for psychoneuroimmunology. Brain Behav Immun. 2003;17(Suppl 1):S18–26. doi: 10.1016/s0889-1591(02)00062-4. [DOI] [PubMed] [Google Scholar]
- Solinas M, Goldberg SR. Motivational effects of cannabinoids and opioids on food reinforcement depend on simultaneous activation of cannabinoid and opioid systems. Neuropsychopharmacology. 2005;30:2035–2045. doi: 10.1038/sj.npp.1300720. [DOI] [PubMed] [Google Scholar]
- Solinas M, Justinova Z, Goldberg SR, Tanda G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. J Neurochem. 2006;98:408–419. doi: 10.1111/j.1471-4159.2006.03880.x. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Tanda G, Makriyannis A, Matthews SA, Goldberg SR. Cannabinoid agonists but not inhibitors of endogenous cannabinoid transport or metabolism enhance the reinforcing efficacy of heroin in rats. Neuropsychopharmacology. 2005;30:2046–2057. doi: 10.1038/sj.npp.1300754. [DOI] [PubMed] [Google Scholar]
- Soria-Gomez E, Matias I, Rueda-Orozco PE, Cisneros M, Petrosino S, Navarro L, Di Marzo V, Prospero-Garcia O. Pharmacological enhancement of the endocannabinoid system in the nucleus accumbens shell stimulates food intake and increases c-Fos expression in the hypothalamus. Br J Pharmacol. 2007;151:1109–1116. doi: 10.1038/sj.bjp.0707313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick SM, Vythilingam M, Charney DS. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu Rev Clin Psychol. 2005;1:255–291. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kobayashi Y, Oka S, Waku K. Biosynthesis and degradation of anandamide and 2-arachidonoylglycerol and their possible physiological significance. Prostaglandins Leukot Essent Fatty Acids. 2002;66:173–192. doi: 10.1054/plef.2001.0356. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szuster RR, Pontius EB, Campos PE. Marijuana sensitivity and panic anxiety. J Clin Psychiatry. 1988;49:427–429. [PubMed] [Google Scholar]
- Thach WT. On the mechanism of cerebellar contributions to cognition. Cerebellum. 2007;6:163–167. doi: 10.1080/14734220701373530. [DOI] [PubMed] [Google Scholar]
- Thomas H. A community survey of adverse effects of cannabis use. Drug Alcohol Depend. 1996;42:201–207. doi: 10.1016/s0376-8716(96)01277-x. [DOI] [PubMed] [Google Scholar]
- Trofimiuk E, Walesiuk A, Braszko JJ. St John’s wort (Hypericum perforatum) diminishes cognitive impairment caused by the chronic restraint stress in rats. Pharmacol Res. 2005;51:239–246. doi: 10.1016/j.phrs.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Tsou K, Tsou K, Nogueron MI, Muthian S, Sañudo-Pena MC, Hillard CJ, Deutsch DG, Walker JM. Fatty acid amide hydrolase is located preferentially in large neurons in the rat central nervous system as revealed by immunohistochemistry. Neurosci Lett. 1998b;254:137–40. doi: 10.1016/s0304-3940(98)00700-9. [DOI] [PubMed] [Google Scholar]
- Vyas A, Chattarji S. Modulation of different states of anxiety-like behavior by chronic stress. Behav Neurosci. 2004;118:1450–1454. doi: 10.1037/0735-7044.118.6.1450. [DOI] [PubMed] [Google Scholar]
- Walesiuk A, Trofimiuk E, Braszko JJ. Gingko biloba extract diminishes stress-induced memory deficits in rats. Pharmacol Rep. 2005;57:176–187. [PubMed] [Google Scholar]
- Wright RL, Conrad CD. Enriched environment prevents chronic stress-induced spatial learning and memory deficits. Behav Brain Res. 2007;187:41–7. doi: 10.1016/j.bbr.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]