Abstract
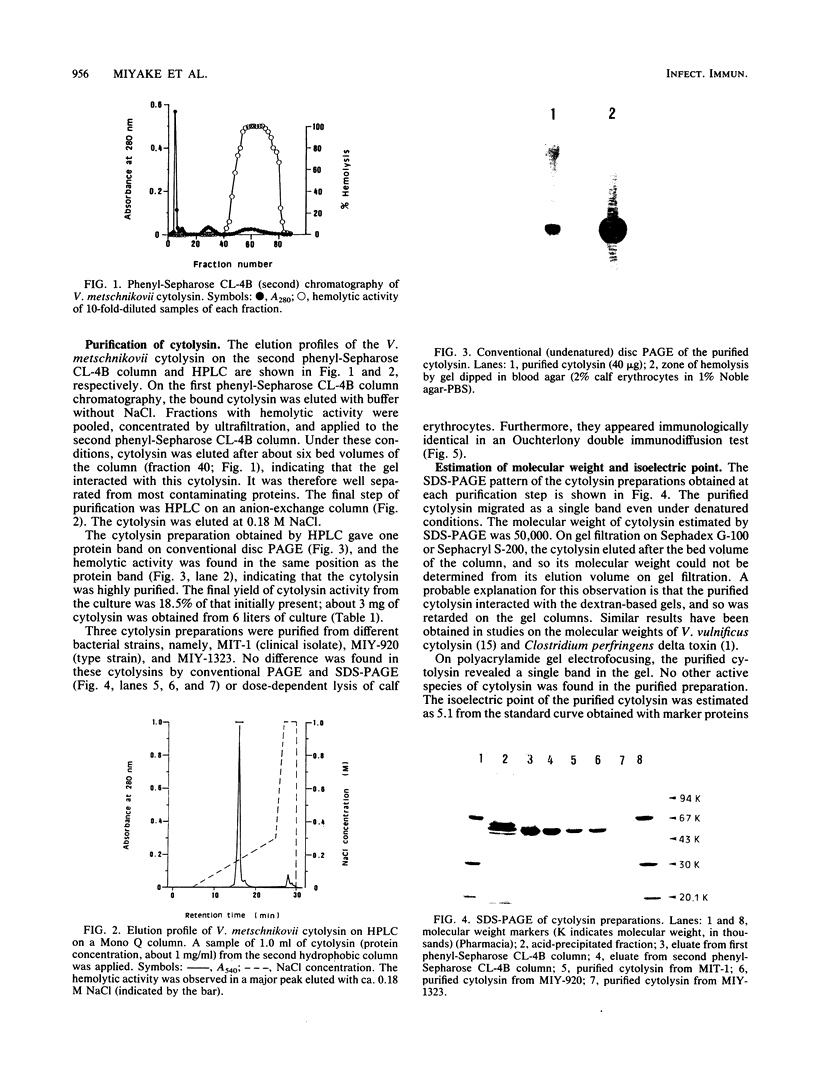
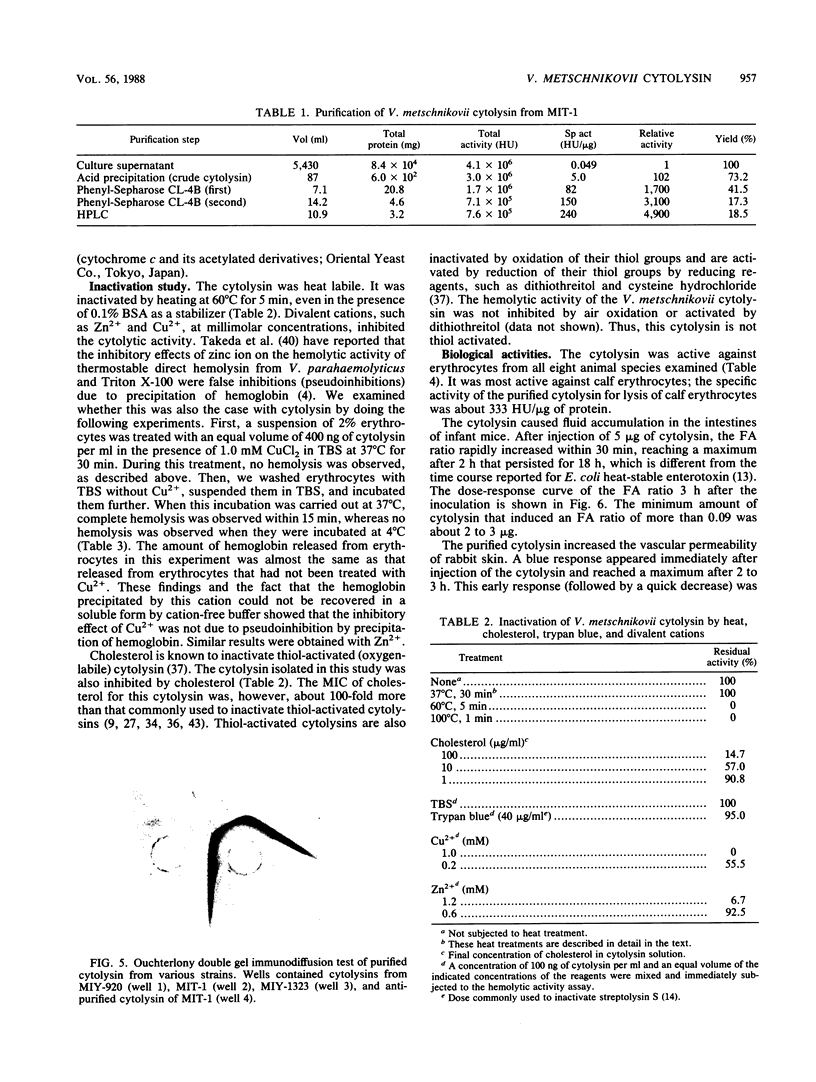
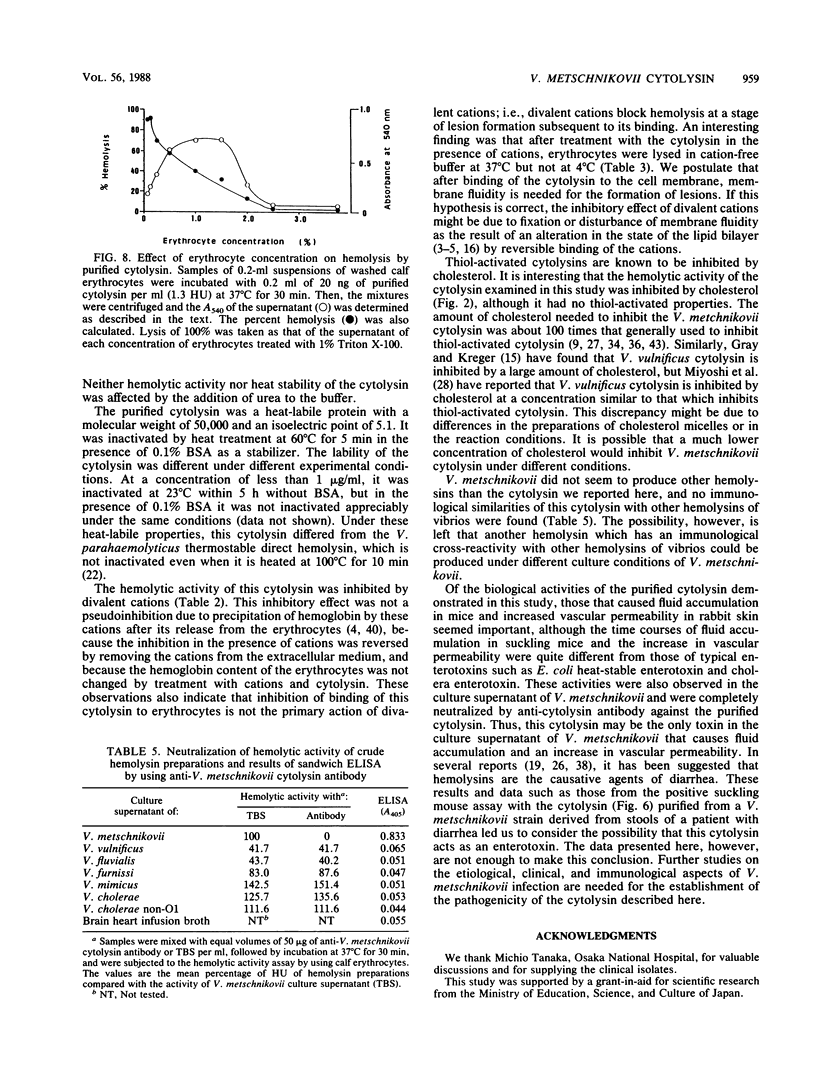
An extracellular cytolysin produced by Vibrio metschnikovii was purified by acid precipitation, phenyl-Sepharose CL-4B chromatography, and rechromatography on a phenyl-Sepharose CL-4B column and high-performance liquid chromatography on a Mono Q (anion-exchange) column. The purified cytolysin had a molecular weight of 50,000 and an isoelectric point of 5.1. It was inactivated by heating at 60 degrees C for 5 min and was inhibited by Zn2+, Cu2+, and high concentrations of cholesterol. Lysis of calf erythrocytes by cytolysin was temperature dependent and occurred only above 18 degrees C. Moreover, no lysis was observed at high concentrations of erythrocytes, suggesting that the cytolysin lyses erythrocytes by a multihit mechanism. This cytolysin had no immunological cross-reactivities with hemolysins from other Vibrio species tested, indicating that it is a new cytolysin. V. metschnikovii cytolysin lysed erythrocytes from several animal species (calf, rabbit, guinea pig, mouse, human, sheep, chicken, and horse) and cultured cells (Vero and Chinese hamster ovary), caused fluid accumulation in the intestines of infant mice, and increased vascular permeability in rabbit skin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alouf J. E., Jolivet-Reynaud C. Purification and characterization of Clostridium perfringens delta-toxin. Infect Immun. 1981 Feb;31(2):536–546. doi: 10.1128/iai.31.2.536-546.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asao T., Kinoshita Y., Kozaki S., Uemura T., Sakaguchi G. Purification and some properties of Aeromonas hydrophila hemolysin. Infect Immun. 1984 Oct;46(1):122–127. doi: 10.1128/iai.46.1.122-127.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avigad L. S., Bernheimer A. W. Inhibition by zinc of hemolysis induced by bacterial and other cytolytic agents. Infect Immun. 1976 May;13(5):1378–1381. doi: 10.1128/iai.13.5.1378-1381.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avigad L. S., Bernheimer A. W. Inhibition of hemolysis by zinc and its reversal by L-histidine. Infect Immun. 1978 Mar;19(3):1101–1103. doi: 10.1128/iai.19.3.1101-1103.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashford C. L., Alder G. M., Menestrina G., Micklem K. J., Murphy J. J., Pasternak C. A. Membrane damage by hemolytic viruses, toxins, complement, and other cytotoxic agents. A common mechanism blocked by divalent cations. J Biol Chem. 1986 Jul 15;261(20):9300–9308. [PubMed] [Google Scholar]
- Batzri S., Korn E. D. Single bilayer liposomes prepared without sonication. Biochim Biophys Acta. 1973 Apr 16;298(4):1015–1019. doi: 10.1016/0005-2736(73)90408-2. [DOI] [PubMed] [Google Scholar]
- Blake P. A., Weaver R. E., Hollis D. G. Diseases of humans (other than cholera) caused by vibrios. Annu Rev Microbiol. 1980;34:341–367. doi: 10.1146/annurev.mi.34.100180.002013. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dean A. G., Ching Y. C., Williams R. G., Harden L. B. Test for Escherichia coli enterotoxin using infant mice: application in a study of diarrhea in children in Honolulu. J Infect Dis. 1972 Apr;125(4):407–411. doi: 10.1093/infdis/125.4.407. [DOI] [PubMed] [Google Scholar]
- Giannella R. A. Suckling mouse model for detection of heat-stable Escherichia coli enterotoxin: characteristics of the model. Infect Immun. 1976 Jul;14(1):95–99. doi: 10.1128/iai.14.1.95-99.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray L. D., Kreger A. S. Purification and characterization of an extracellular cytolysin produced by Vibrio vulnificus. Infect Immun. 1985 Apr;48(1):62–72. doi: 10.1128/iai.48.1.62-72.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshman S., Sugg N. Effect of calcium ions on staphylococcal alpha-toxin-induced hemolysis of rabbit erythrocytes. Infect Immun. 1985 Jan;47(1):37–40. doi: 10.1128/iai.47.1.37-40.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T., Sato M., Kongmuang U., Miwatani T. Identification of enterotoxigenic Escherichia coli-producing heat-labile enterotoxin by an enzyme-linked immunosorbent assay on single colonies grown on MacConkey agar plates. J Diarrhoeal Dis Res. 1984 Dec;2(4):223–227. [PubMed] [Google Scholar]
- Ichinose Y., Yamamoto K., Nakasone N., Tanabe M. J., Takeda T., Miwatani T., Iwanaga M. Enterotoxicity of El Tor-like hemolysin of non-O1 Vibrio cholerae. Infect Immun. 1987 May;55(5):1090–1093. doi: 10.1128/iai.55.5.1090-1093.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Akiyama Y., Kinoshita T., Higashi Y., Amano T. Evidence for a one-hit theory in the immune bactericidal reaction and demonstration of a multi-hit response for hemolysis by streptolysin O and Clostridium perfringens theta-toxin. Infect Immun. 1976 Feb;13(2):337–344. doi: 10.1128/iai.13.2.337-344.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Jacques W., Rajashekaraiah K. R., Farmer J. J., 3rd, Hickman F. W., Morris J. G., Kallick C. A. Vibrio metschnikovii bacteremia in a patient with cholecystitis. J Clin Microbiol. 1981 Dec;14(6):711–712. doi: 10.1128/jcm.14.6.711-712.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. W., Colwell R. R., Kaper J. B. Vibrio parahaemolyticus and related halophilic Vibrios. Crit Rev Microbiol. 1982;10(1):77–124. doi: 10.3109/10408418209113506. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCardell B. A., Madden J. M., Shah D. B. Isolation and characterization of a cytolysin produced by Vibrio cholerae serogroup non-O1. Can J Microbiol. 1985 Aug;31(8):711–720. doi: 10.1139/m85-135. [DOI] [PubMed] [Google Scholar]
- Mitsui N., Mitsui K., Hase J. Purification and some properties of tetanolysin. Microbiol Immunol. 1980;24(7):575–584. doi: 10.1111/j.1348-0421.1980.tb02860.x. [DOI] [PubMed] [Google Scholar]
- Morris J. G., Jr, Black R. E. Cholera and other vibrioses in the United States. N Engl J Med. 1985 Feb 7;312(6):343–350. doi: 10.1056/NEJM198502073120604. [DOI] [PubMed] [Google Scholar]
- Morris J. G., Jr, Miller H. G., Wilson R., Tacket C. O., Hollis D. G., Hickman F. W., Weaver R. E., Blake P. A. Illness caused by Vibrio damsela and Vibrio hollisae. Lancet. 1982 Jun 5;1(8284):1294–1297. doi: 10.1016/s0140-6736(82)92853-7. [DOI] [PubMed] [Google Scholar]
- Oberley T. D., Duncan J. L. Characteristics of streptolysin O action. Infect Immun. 1971 Dec;4(6):683–687. doi: 10.1128/iai.4.6.683-687.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS H. J., TERRYBERRY J. E. Counting actively metabolizing tissue cultured cells. Exp Cell Res. 1957 Oct;13(2):341–347. doi: 10.1016/0014-4827(57)90013-7. [DOI] [PubMed] [Google Scholar]
- Prigent D., Alouf J. E. Interaction of steptolysin O with sterols. Biochim Biophys Acta. 1976 Aug 16;443(2):288–300. doi: 10.1016/0005-2736(76)90511-3. [DOI] [PubMed] [Google Scholar]
- Shandera W. X., Johnston J. M., Davis B. R., Blake P. A. Disease from infection with Vibrio mimicus, a newly recognized Vibrio species. Clinical characteristics and edipemiology. Ann Intern Med. 1983 Aug;99(2):169–171. [PubMed] [Google Scholar]
- Smyth C. J. The identification and purification of multiple forms of theta-haemolysin (theta-toxin) of Clostridium perfringens type A. J Gen Microbiol. 1975 Apr;87(2):219–238. doi: 10.1099/00221287-87-2-219. [DOI] [PubMed] [Google Scholar]
- Tacket C. O., Hickman F., Pierce G. V., Mendoza L. F. Diarrhea associated with Vibrio fluvialis in the United States. J Clin Microbiol. 1982 Nov;16(5):991–992. doi: 10.1128/jcm.16.5.991-992.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Ogiso Y., Miwatani T. Effect of zinc ion on the hemolytic activity of thermostable direct hemolysin from Vibrio parahaemolyticus, streptolysin O, and Triton X-100. Infect Immun. 1977 Aug;17(2):239–243. doi: 10.1128/iai.17.2.239-243.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson K. C., Kerr E. J. Sterol structural requirements for inhibition of streptolysin O activity. Biochem J. 1974 Apr;140(1):95–98. doi: 10.1042/bj1400095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Al-Omani M., Honda T., Takeda Y., Miwatani T. Non-O1 Vibrio cholerae hemolysin: purification, partial characterization, and immunological relatedness to El Tor hemolysin. Infect Immun. 1984 Jul;45(1):192–196. doi: 10.1128/iai.45.1.192-196.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoh M., Honda T., Miwatani T. Purification and partial characterization of a non-O1 Vibrio cholerae hemolysin that cross-reacts with thermostable direct hemolysin of Vibrio parahaemolyticus. Infect Immun. 1986 Apr;52(1):319–322. doi: 10.1128/iai.52.1.319-322.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]






