Abstract
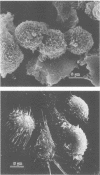
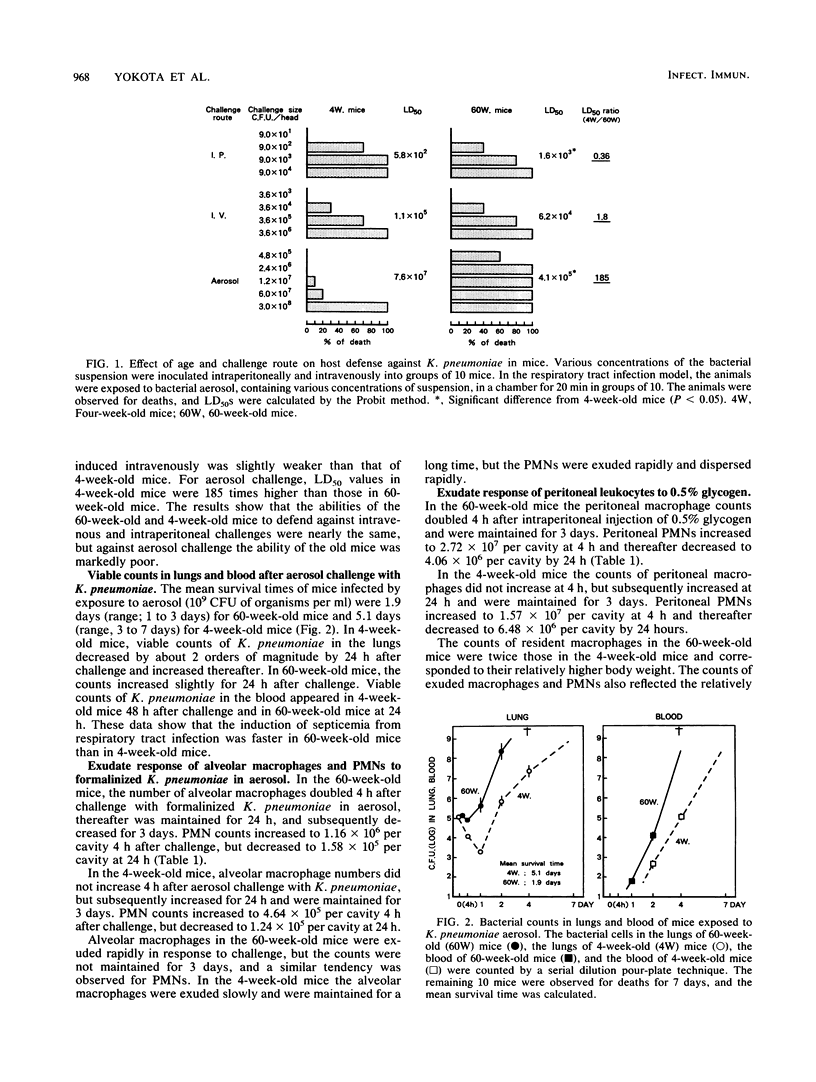
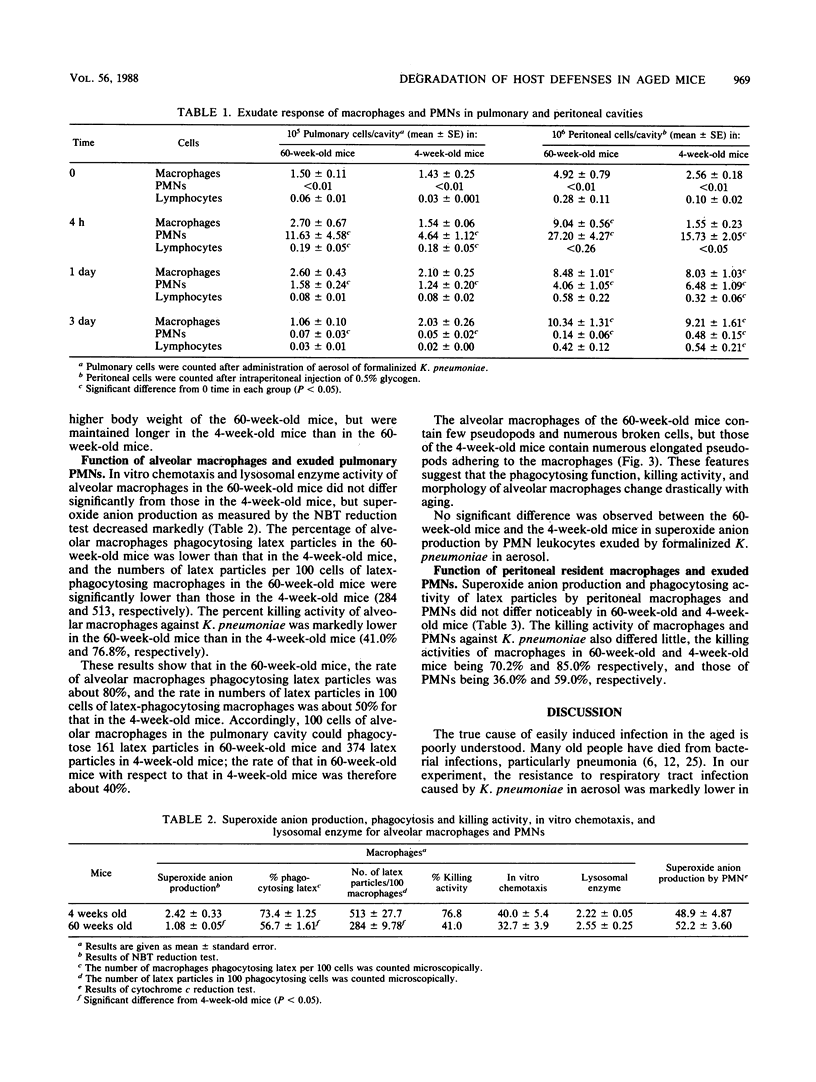
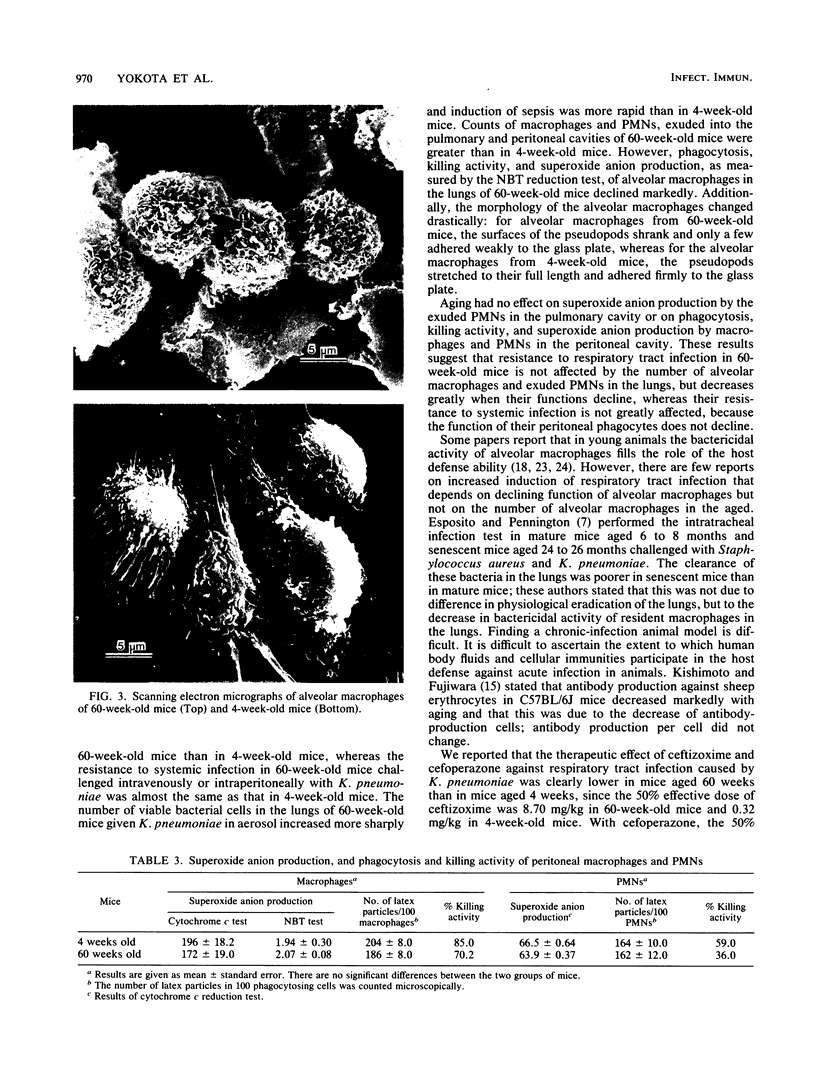
The host defense against respiratory tract infection with Klebsiella pneumoniae was much weaker in 60-week-old mice than in 4-week-old mice, but the resistance against systemic infection by intravenous and intraperitoneal challenge with K. pneumoniae in 60-week-old mice did not differ from that in 4-week-old mice. The number of alveolar macrophages at the resting stage in 60-week-old mice was the same as in 4-week-old mice, but the number of macrophages and polymorphonuclear leukocytes in the pulmonary cavity 4 h after challenge with formalinized K. pneumoniae in aerosol doubled in parallel with body weight. Phagocytosis and killing activities and superoxide anion production as measured by the Nitro Blue Tetrazolium reduction test of alveolar macrophages in 60-week-old mice were significantly weaker than in 4-week-old mice. The surfaces of the alveolar macrophages of the 60-week-old mice shrunk and a few adhered weekly to the glass plate, but the alveolar macrophages of the 4-week-old mice stretched to their full length and adhered firmly to the glass plate. These functions of alveolar macrophages clearly differed from those of peritoneal macrophages in 60-week-old mice, but those of the peritoneal phagocytes did not differ between 60-week-old and 4-week-old mice. The results suggest that the susceptibility to respiratory tract infection in 60-week-old mice is affected by a decline in the functions of alveolar macrophages rather than by the number of alveolar macrophages and exudated polymorphonuclear leukocytes in the lungs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIOZZI G., BENACERRAF B., HALPERN B. N. Quantitative study of the granulopectic activity of the reticulo-endothelial system. II. A study of the kinetics of the R. E. S. in relation to the dose of carbon injected; relationship between the weight of the organs and their activity. Br J Exp Pathol. 1953 Aug;34(4):441–457. [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Nathan D. G. Quantitative nitroblue tetrazolium test in chronic granulomatous disease. N Engl J Med. 1968 May 2;278(18):971–976. doi: 10.1056/NEJM196805022781801. [DOI] [PubMed] [Google Scholar]
- Bjornson A. B., Michael J. G. Contribution of humoral and cellular factors to the resistance to experimental infection by Pseudomonas aeruginosa in mice. I. Interaction between immunoglobulins, heat-labile serum factors, and phagocytic cells in the killing of bacteria. Infect Immun. 1971 Oct;4(4):462–467. doi: 10.1128/iai.4.4.462-467.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson A. B., Michael J. G. Contribution of humoral and cellular factors to the resistance to experimental infection by Pseudomonas aeruginosa in mice. II. Opsonic, agglutinative, and protective capacities of immunoglobulin G anti-Pseudomonas antibodies. Infect Immun. 1972 May;5(5):775–782. doi: 10.1128/iai.5.5.775-782.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar S., Shastri S. R., Lenora R. A. Aging and the respiratory system. Med Clin North Am. 1976;60(6):1121–1139. doi: 10.1016/s0025-7125(16)31871-5. [DOI] [PubMed] [Google Scholar]
- Dunn D. L., Barke R. A., Knight N. B., Humphrey E. W., Simmons R. L. Role of resident macrophages, peripheral neutrophils, and translymphatic absorption in bacterial clearance from the peritoneal cavity. Infect Immun. 1985 Aug;49(2):257–264. doi: 10.1128/iai.49.2.257-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebright J. R., Rytel M. W. Bacterial pneumonia in the elderly. J Am Geriatr Soc. 1980 May;28(5):220–223. doi: 10.1111/j.1532-5415.1980.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Esposito A. L., Pennington J. E. Effects of aging on antibacterial mechanisms in experimental pneumonia. Am Rev Respir Dis. 1983 Oct;128(4):662–667. doi: 10.1164/arrd.1983.128.4.662. [DOI] [PubMed] [Google Scholar]
- Fisher M. W. A polyvalent human gamma-globulin immune to Pseudomonas aeruginosa: passive protection of mice against lethal infection. J Infect Dis. 1977 Aug;136 (Suppl):S181–S185. doi: 10.1093/infdis/136.supplement.s181. [DOI] [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. THE ROLE OF THE ALVEOLAR MACROPHAGE IN THE CLEARANCE OF BACTERIA FROM THE LUNG. J Exp Med. 1964 Jan 1;119:167–176. doi: 10.1084/jem.119.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner I. D., Remington J. S. Age-related decline in the resistance of mice to infection with intracellular pathogens. Infect Immun. 1977 May;16(2):593–598. doi: 10.1128/iai.16.2.593-598.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Keele B. B., Jr, Misra H. P., Lehmeyer J. E., Webb L. S., Baehner R. L., RaJagopalan K. V. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest. 1975 Jun;55(6):1357–1372. doi: 10.1172/JCI108055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konen T. G., Smith G. S., Walford R. L. Decline in mixed lymphocyte reactivity of spleen cells from aged mice of a long-lived strain. J Immunol. 1973 May;110(5):1216–1221. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaForce F. M. Effect of alveolar lining material on phagocytic and bactericidal activity of lung macrophages against Staphylococcus aureau. J Lab Clin Med. 1976 Nov;88(5):691–699. [PubMed] [Google Scholar]
- Law B. J., Marks M. I. Age-related prevalence of human serum IgG and IgM antibody to the core glycolipid of Escherichia coli strain J5, as measured by ELISA. J Infect Dis. 1985 Jun;151(6):988–994. doi: 10.1093/infdis/151.6.988. [DOI] [PubMed] [Google Scholar]
- Musher D. M., Goree A., Baughn R. E., Birdsall H. H. Immunoglobulin A from bronchopulmonary secretions blocks bactericidal and opsonizing effects of antibody to nontypable Haemophilus influenzae. Infect Immun. 1984 Jul;45(1):36–40. doi: 10.1128/iai.45.1.36-40.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAVILLARD E. R. In vitro phagocytic and bactericidal ability of alveolar and peritoneal macrophages of normal rats. Aust J Exp Biol Med Sci. 1963 Jun;41:265–274. doi: 10.1038/icb.1963.26. [DOI] [PubMed] [Google Scholar]
- Patel P. J. Aging and cellular defense mechanisms: age-related changes in resistance of mice to Listeria monocytogenes. Infect Immun. 1981 May;32(2):557–562. doi: 10.1128/iai.32.2.557-562.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm S. R., Gross G. N., Pierce A. K. Early bacterial clearance from murine lungs. Species-dependent phagocyte response. J Clin Invest. 1980 Aug;66(2):194–199. doi: 10.1172/JCI109844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E. L. Infectious diseases in the elderly. Ann Intern Med. 1983 Mar;98(3):395–400. doi: 10.7326/0003-4819-98-3-395. [DOI] [PubMed] [Google Scholar]
- Shayegani M., De Courcy S. J., Jr, Mudd S. Cell-mediated immunity in mice infected with S. aureus and elicited with specific bacterial antigens. J Reticuloendothel Soc. 1973 Jul;14(1):44–51. [PubMed] [Google Scholar]
- Tomioka H., Iwamura Y., Suzuki Y., Ohtomo S., Hashimoto Y. Protective effects of S-sulfonated human gamma globulin against experimental infections in mice. Infect Immun. 1980 Nov;30(2):329–336. doi: 10.1128/iai.30.2.329-336.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]