Abstract
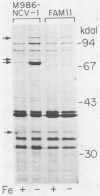
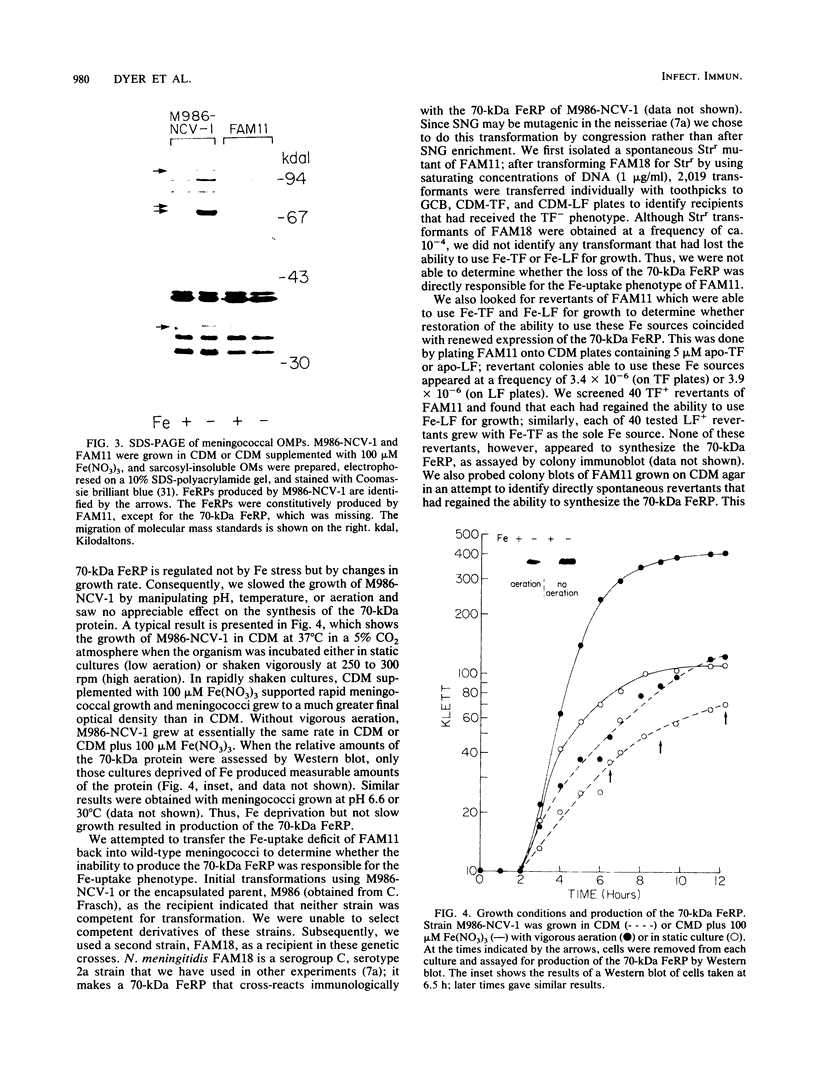
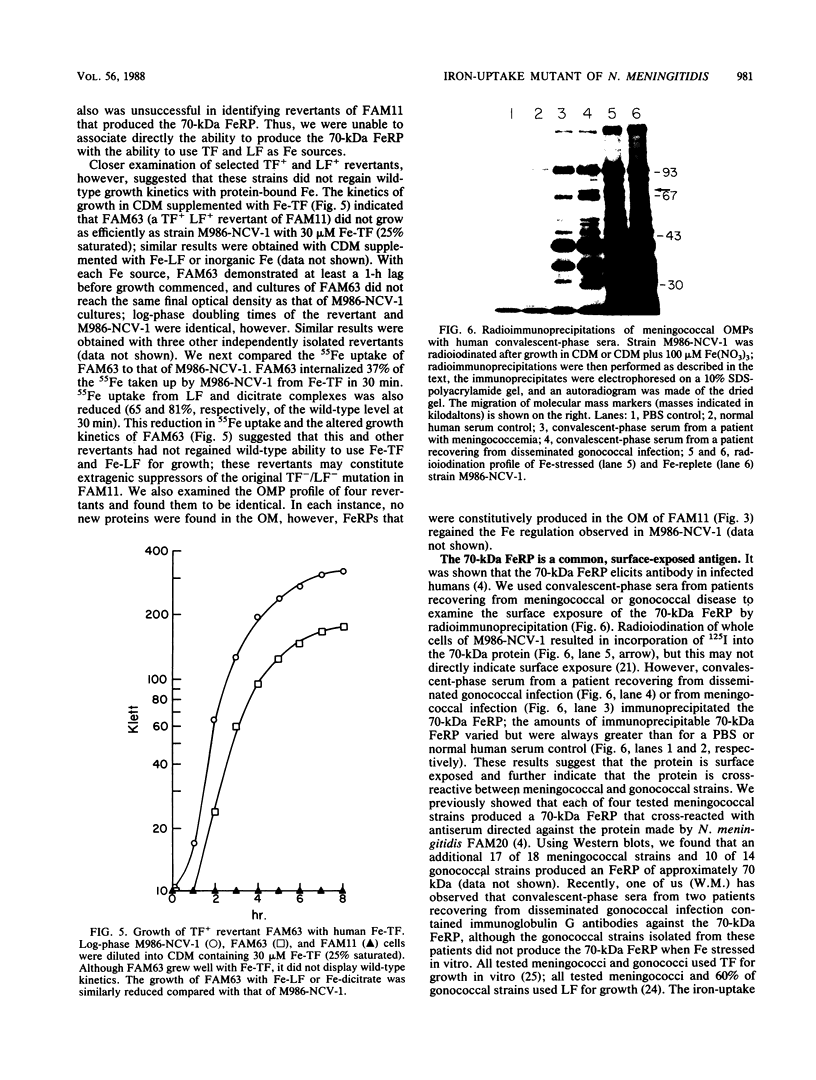
We isolated an iron-uptake mutant of Neisseria meningitidis M986-NCV-1 that was severely limited in the ability to use several sources of iron in the form of Fe3+. This mutant, FAM11, grew poorly or not at all with human transferrin (TF) or lactoferrin (LF) as the sole iron source in a defined medium but grew as well as wild-type meningococci with hemin or hemoglobin. Uptake of 55Fe bound to TF, LF, dicitrate complexes, aerobactin, or nitrilotriacetate was reduced to 0 to 4% of the wild-type level. FAM11 did not produce an iron-repressible outer membrane protein (FeRP) of 70 kilodaltons (kDa) found in membranes of iron-stressed M986-NCV-1. Western blot (immunoblot) analysis using rabbit antiserum against this protein revealed that at least 17 of 18 meningococcal and 10 of 14 gonococcal strains produced an FeRP of ca. 70 kDa. The 70-kDa FeRP was shown to be surface exposed by radioimmunoprecipitation with human immune sera. These data suggest that the 70-kDa FeRP is somehow involved in Fe uptake from TF and LF. However, we were unable to transform the iron-uptake phenotype from FAM11 into wild-type meningococci to confirm this. Revertants of FAM11 that grew with TF and LF did not regain the ability to make the 70-kDa FeRP but also did not completely regain the Fe-uptake phenotype of M986-NCV-1.
Full text
PDF

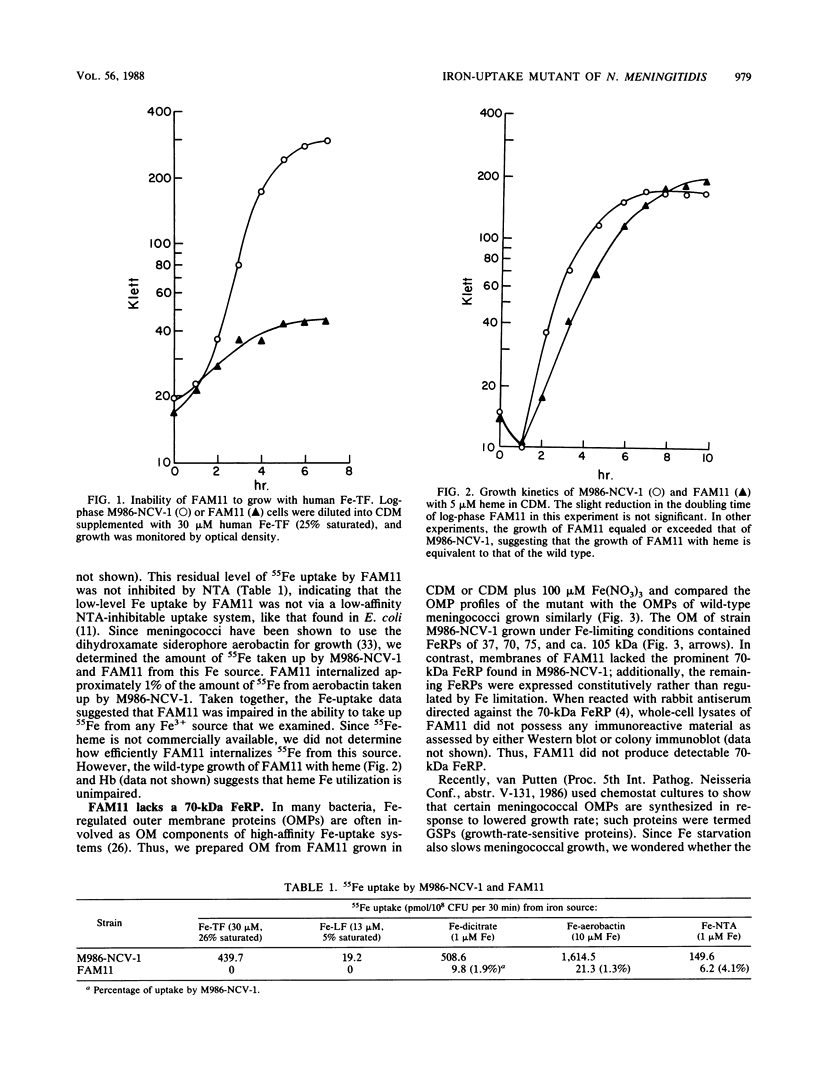


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald F. S., Simonson C., DeVoe I. W. Comparison of iron binding and uptake from FeCl3 and Fe-citrated by Neisseria meningitidis. Can J Microbiol. 1981 Oct;27(10):1066–1070. doi: 10.1139/m81-166. [DOI] [PubMed] [Google Scholar]
- Bassford P. J., Jr, Bradbeer C., Kadner R. J., Schnaitman C. A. Transport of vitamin B12 in tonB mutants of Escherichia coli. J Bacteriol. 1976 Oct;128(1):242–247. doi: 10.1128/jb.128.1.242-247.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. R., Dyer D. W., Thompson M. K., Sparling P. F. Human immune response to iron-repressible outer membrane proteins of Neisseria meningitidis. Infect Immun. 1986 Dec;54(3):710–713. doi: 10.1128/iai.54.3.710-713.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cowart R. E., Foster B. G. Differential effects of iron on the growth of Listeria monocytogenes: minimum requirements and mechanism of acquisition. J Infect Dis. 1985 Apr;151(4):721–730. doi: 10.1093/infdis/151.4.721. [DOI] [PubMed] [Google Scholar]
- Devoe I. W., Golchrist J. E. Localization of tetramethylphenylenediamine-oxidase in the outer cell wall layer of Neisseria meningitidis. J Bacteriol. 1976 Oct;128(1):144–148. doi: 10.1128/jb.128.1.144-148.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer D. W., McKenna W., Woods J. P., Sparling P. F. Isolation by streptonigrin enrichment and characterization of a transferrin-specific iron uptake mutant of Neisseria meningitidis. Microb Pathog. 1987 Nov;3(5):351–363. doi: 10.1016/0882-4010(87)90005-2. [DOI] [PubMed] [Google Scholar]
- Dyer D. W., West E. P., Sparling P. F. Effects of serum carrier proteins on the growth of pathogenic neisseriae with heme-bound iron. Infect Immun. 1987 Sep;55(9):2171–2175. doi: 10.1128/iai.55.9.2171-2175.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Sciortino C. V., McIntosh M. A. Role of iron in microbe-host interactions. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S759–S777. doi: 10.1093/clinids/5.supplement_4.s759. [DOI] [PubMed] [Google Scholar]
- Frasch C. E., Peppler M. S. Protection against group B Neisseria meningitidis disease: preparation of soluble protein and protein-polysaccharide immunogens. Infect Immun. 1982 Jul;37(1):271–280. doi: 10.1128/iai.37.1.271-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost G. E., Rosenberg H. Relationship between the tonB locus and iron transport in Escherichia coli. J Bacteriol. 1975 Nov;124(2):704–712. doi: 10.1128/jb.124.2.704-712.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost G. E., Rosenberg H. The inducible citrate-dependent iron transport system in Escherichia coli K12. Biochim Biophys Acta. 1973 Nov 30;330(1):90–101. doi: 10.1016/0005-2736(73)90287-3. [DOI] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., Johnston K. H. Detection of antibody-accessible proteins on the cell surface of Haemophilus influenzae type b. Infect Immun. 1981 Sep;33(3):950–953. doi: 10.1128/iai.33.3.950-953.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbein B. E. Differences in virulence for mice between disease and carrier strains of Neisseria meningitidis. Can J Microbiol. 1981 Jul;27(7):738–741. doi: 10.1139/m81-113. [DOI] [PubMed] [Google Scholar]
- Holbein B. E. Enhancement of Neisseria meningitidis infection in mice by addition of iron bound to transferrin. Infect Immun. 1981 Oct;34(1):120–125. doi: 10.1128/iai.34.1.120-125.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbein B. E. Iron-controlled infection with Neisseria meningitidis in mice. Infect Immun. 1980 Sep;29(3):886–891. doi: 10.1128/iai.29.3.886-891.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbein B. E., Jericho K. W., Likes G. C. Neisseria meningitidis infection in mice: influence of iron, variations in virulence among strains, and pathology. Infect Immun. 1979 May;24(2):545–551. doi: 10.1128/iai.24.2.545-551.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima N., Bates G. W. The reduction and release of iron from Fe3+ .transferrin.CO3(2-). J Biol Chem. 1979 Sep 25;254(18):8847–8854. [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Lactoperoxidase and Iodo-Gen-catalyzed iodination labels inner and outer membrane proteins of Haemophilus influenzae. J Bacteriol. 1983 Jul;155(1):443–446. doi: 10.1128/jb.155.1.443-446.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Mickelsen P. A., Blackman E., Sparling P. F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from lactoferrin. Infect Immun. 1982 Mar;35(3):915–920. doi: 10.1128/iai.35.3.915-920.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelsen P. A., Sparling P. F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect Immun. 1981 Aug;33(2):555–564. doi: 10.1128/iai.33.2.555-564.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Sarubbi F. A., Jr, Blackman E., Sparling P. F. Genetic mapping of linked antibiotic resistance loci in Neisseria gonorrhoeae. J Bacteriol. 1974 Dec;120(3):1284–1292. doi: 10.1128/jb.120.3.1284-1292.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson C., Brener D., DeVoe I. W. Expression of a high-affinity mechanism for acquisition of transferrin iron by Neisseria meningitidis. Infect Immun. 1982 Apr;36(1):107–113. doi: 10.1128/iai.36.1.107-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. E., Sparling P. F. Aerobactin utilization by Neisseria gonorrhoeae and cloning of a genomic DNA fragment that complements Escherichia coli fhuB mutations. J Bacteriol. 1987 Aug;169(8):3414–3421. doi: 10.1128/jb.169.8.3414-3421.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. E., Sparling P. F. Response of Neisseria gonorrhoeae to iron limitation: alterations in expression of membrane proteins without apparent siderophore production. Infect Immun. 1985 Feb;47(2):388–394. doi: 10.1128/iai.47.2.388-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey R. J., Finkelstein R. A. Assmilation of iron by pathogenic Neisseria spp. Infect Immun. 1981 May;32(2):592–599. doi: 10.1128/iai.32.2.592-599.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeowell H. N., White J. R. Iron requirement in the bactericidal mechanism of streptonigrin. Antimicrob Agents Chemother. 1982 Dec;22(6):961–968. doi: 10.1128/aac.22.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]