Abstract
Disorders of the basal ganglia such as Parkinson’s disease (PD) and Huntington’s disease are commonly thought of primarily as motor disorders; however, the cognitive symptoms of these diseases such as executive dysfunction, learning, memory and attention deficits are prominent and often more disabling than the hallmark motor symptoms. Cognitive features of PD are often neglected in preclinical studies of PD, likely due to the lack of available animal models to study them. ak mice, which are deficient in the transcription factor Pitx3, model the selective nigrostriatal DA loss in PD. Here we report that ak mice are impaired in striatum-dependent cognitive tasks including rotarod learning, t-maze and inhibitory avoidance tasks, but not the striatum-independent social transmission of food preference task. These results suggest that some neuropsychiatric symptoms in PD are related to the pathophysiology of the disease rather than stress associated with disease burden, or medications used to treat PD. Furthermore aphakia mice may be used as a novel model of non-motor symptoms in PD.
Keywords: cognition, Parkinson’s disease, pitx3, dopamine, striatum
Parkinson’s disease is often characterized as a movement disorder, however, studies show that frequently a major complaints of patients with PD are the non-motor and neuropsychiatric symptoms such as cognitive impairment, depression, anxiety and sleep disturbances. Interestingly, these cognitive symptoms can have a greater impact on quality of life measures than the hallmark motor symptoms of PD (Lauterbach 2005). Up to 80% of patients with PD show symptoms of cognitive impairment ranging from frank dementia and executive dysfunction to more subtle memory loss (Agid, Ruberg et al. 1986; Bosboom, Stoffers et al. 2004; Zgaljardic, Foldi et al. 2004; Bronnick, Aarsland et al. 2005). Several hypotheses have been proposed to account for these non-motor neuropsychiatric symptoms which occur in PD. Briefly, these hypotheses have attributed non-motor symptoms such as cognitive impairment in PD to (1) stress related to coping with a chronic illness, (2) medications used to treat PD, or (3) events intrinsic to the pathophysiology of PD.
In the present studies we set out to test this third hypothesis. If it is the case that cognitive symptoms of PD are related to the disease process then animal models that mimic specific aspects of the pathophysiology of PD should also show impairments in cognitive function. Despite this prediction, cognitive aspects of PD have been difficult to study pre-clinically since animal models generated using toxin-based methods often have severe motor impairments which prevent the normal mobility required to perform typical cognitive tests in animals (Bove, Prou et al. 2005). Thus the three above hypotheses have not been tested in animal models. It would be of great interest to have a model of cognitive aspects of PD to test potential therapeutics and investigate the neurobiology of cognitive symptoms of PD. Ideally, such a model would recapitulate important aspects of PD, but subjects would not be severely motor impaired so as to enable them to perform common behavioral tasks.
Over the last decade several genes have been identified that may play a role in dopamine neuron development as well as the pathophysiology of PD including Nurr1, Parkin, and Pitx3. Recently, we and others have shown that aphakia (ak) mice, which are deficient in the bicoid-related, homeodomain-containing transcription factor, Pitx3, fulfill major criteria for an animal model of PD. Pitx3 is restrictively expressed in the lens and midbrain and has been shown to play a key role in dopamine neuron development (Smidt, Smits et al. 2004). Consistent with an important role of Pitx3 in dopamine neuron development, Pitx3- deficient ak mice are the first genetic model to recapitulate selective nigrostriatal (A9) dopamine neuron loss (Hwang, Ardayfio et al. 2003; Nunes, Tovmasian et al. 2003; van den Munckhof, Luk et al. 2003; Smidt, Smits et al. 2004). Greater than 90% loss of A9 dopamine neurons occurs in ak mice, whereas A10 and other areas are less affected. Further validitation of the PD-like phenotype in this genetic model is that ak mice show impaired performance on select motor tests, which is reversible with L-Dopa (Hwang, Fleming et al. 2005). Table 1 summarizes the phenotypic findings reported in ak mice and their similarity to PD. Since Pitx3 is involved in lens development, ak mice are also blind. The key feature of ak mice however, is that despite select PD-like motor impairments, ak mice can ambulate well enough to perform many behavioral tasks. This makes ak mice a unique A9 dopamine deficiency model to study neuropsychiatric and non-motor aspects of PD. We report here that Pitx3 deficiency results in impairment in striatum-dependent procedural and associative learning tasks but not a non-striatal dependent associative learning task. These findings suggest that PD related pathophysiology rather than stress or medications contribute to the cognitive impairments observed in patients with PD.
Table 1.
TH, Tyrosine Hydroxylase; DA, Dopamine; VMAT, Vesicular Monoamine Transporter; DAT, Dopamine Transporter; AADC, Aromatic Amino Acid Decarboxylase; CCK, Cholecystokinin; DR, (Dopamine Receptor); SNc, Substantia Nigra, pars compacta
| PD-like phenotypic findings in aphakia mice | References | |
|---|---|---|
| Evidence for selective loss of DA containing neurons in SNc | ||
| • | Loss or reduction of DA neuron markers in nigrostriatal circuit: TH, VMAT, AADC, Drd1a, Drd3, Drd4, DAT, CCK, NTR-1, alpha-synuclein, Nurr1, D2R | Hwang-2003, Nunes-2003, Smidt-2003, Singh-2007, van den Munckhof-2003, van den Munckhof-2006, Kas-2008 |
| • | Loss of nissl staining in SNc | Hwang-2003, Nunes-2003, Smidt-2003, van den Munckhof-2003 |
| • | Loss of DA content in dorsal striatum as measured by HPLC/voltammetry | Hwang-2003, van den Munckhof-2003, Nunes-2003, Kas-2008 |
| • | Loss of retrograde labeling when fluorogold is injected into dorsal striatum | Hwang-2003, Nunes-2003, Smidt-2003, |
| • | Increase in enkephalin mRNA expression, decrease in neurotensin mRNA expression | van den Munckhof-2006 |
| Evidence for PD-like motor deficits in aphakia mice | ||
| • | Impairments in rotarod, balance beam, pole test, string test, cotton shred, hind-limb stepping, spontaneous locomotor activity | Hwang-2005, Singh-2007, van den Munckhof-2006, |
| Evidence for responsivity to L-Dopa | ||
| • | Reversal of motor deficits by acute L-Dopa | Hwang-2005, van den Munckhof-2006 |
| • | dyskinesia-like behavioral and biochemical alterations in response to chronic L-Dopa | Ding 2007 |
| • | Increase in c-fos in the dorsal striatum in response to acute L-Dopa (indicative of denervations supersensitivity) | Hwang-2005 |
Materials and Methods
Animals
Adult male ak mice used in this study were originally from The Jackson Laboratory (Bar Harbor, ME) (strain B6 _ C57BLKS-ak; JR942). They were outcrossed several times to C57BL/6 mice and maintained in the C57BL/6 background. Several breeding pairs were transferred, expanded, and maintained at the Animal Care Facility at McLean Hospital. Wild-type (wt) C57BL/6 mice were obtained from The Jackson Laboratory and used as control. Mice homozygous for retinal degeneration 1 (rd1 or Pde6brd1) mutation (B6.C3-Pde6brd1; The Jackson Laboratory) (Pittler and Baehr 1991) were also used as a blinded mouse control to assure that any potential changes that we observed were not due to the lack of vision in ak mice, but rather were related to the genotype differences in basal ganglia function. The rd1 mice are also in the C57BL/6 background. Animal use was in accordance with Institutional Animal Care and Use Committee of McLean Hospital and followed National Institutes of Health guidelines. Tests were conducted in separate groups of animals in order to avoid confounding effects of previous tests.
Procedures
In order to probe cognition in ak mice, learning and memory tests were chosen that employed the striatum, but also had varying sensory and motor requirements. Although our previous studies indicate little or no gross neurological impairments in ak mice, we utilized a variety of distinct tasks in order to avoid obtaining false positives due to genotype differences in locomotor activity, vision or other sensory modalities. Three distinct tasks were chosen to assess implicit memory (e.g. motor or procedural memory) and two tasks for explicit memory (e.g. associative memory) since both types of deficits have been reported in PD. Additionally, utilizing multiple tasks would further strengthen the case that any potential impairments observed in ak mice are likely to be attributed to the disrupted nigrostriatal pathway. The tasks chosen in this study, rotarod learning, inhibitory avoidance and the swimming and dry t-maze, are tasks that have been shown to be disrupted by neurochemical, electrolytic or genetic lesions of the striatum and furthermore they can be performed by blind mice (Caston, Hilber et al. 2003; Van Raamsdonk, Pearson et al. 2005).
Implicit-Procedural Learning
Rotarod
Mice were placed on an accelerating rotarod at the same time daily for 3 consecutive days. Mice received 3 consecutive trials per day. The rotarod accelerated from 4 to 40 revolutions per minute (RPMs) over 4 minutes. The latency to fall off was recorded. The increase in latency over 3 consecutive days of testing is the measure of procedural learning
Swimming T-Maze
A swimming T-maze test was used to assess procedural learning. In this test, mice were placed in the base of an opaque, milk-filled T-maze with an escape platform located in the right or left arm of the maze (T-maze dimensions: arms, 38 × 14 cm; water depth, 7 cm; platform, 10 × 14 cm). Mice had to learn to turn right or left after reaching the top of the T to reach the platform directly. The platform was in the same location for each mouse and counter balanced on the left or right side across the mice. Since the task is performed in the dark and the platform is under water, mice must rely on egocentric navigation to remember the correct location of the platform. The time to reach the platform and the number of errors were recorded. An error was recorded each time the mouse swam down an arm that did not contain the platform. Mice received 3 consecutive trials per day for 5 days. Normal mice rapidly learn to locate the platform as demonstrated by a decrease in latency and errors over successive days.
Dry T-maze
The swimming T-maze described above is physically demanding and thus exhaustion or motor impairments could potentially influence or confound performance in this task. To complement the swimming T-maze and control for any motor or exhaustion deficits among groups, we compared wt, ak, and rd1 mice on a dry version of the swimming T-maze that utilized food as a reinforcer. This task requires egocentric navigation but is not physically demanding and thus should not be markedly influenced by motor impairments or fatigue.
Food Restriction
Four days before pretraining and until the completion of the experiment, access to food was restricted to one hour per day.
Behavioral Testing
Mice were tested in a transparent plexiglas T-maze that consisted of three identical arms: a left arm, a right arm and a central arm that was perpendicular to the left and right arm. Each arm was 38 cm long and 14 cm wide. A sliding door separated the left and right arm from the central arm. A food cup, 0.5 cm deep and 1cm in diameter, was inserted in the floor at the distal end of either the left or right arm. The maze was elevated 40 cm above the floor.
Pretraining
Prior to training, two consecutive sessions of pre-training were conducted to help animals’ habituate to the maze and food reward. On the first day of pre-training, mice were released from the south arm and, for 10 minutes, allowed to explore the dry T-maze. On the second day of pre-training, food pellets were scattered on the floor of the T-maze and mice were allowed to explore and eat in the maze for 10 minutes.
Training
On the next day, mice were released from the central arm and trained to collect a food pellet placed at the end of the left arm. After entering the left arm (correct response) or the right arm (incorrect response) the mouse was retained for 15 s in the chosen arm. After 15 s mice were removed from the maze and placed in a holding cage for the 30 s inter-trial interval. Each mouse ran 5 trials per day for 5 consecutive days.
Explicit-Associative Learning
Inhibitory Avoidance
(Dyer, Hammond et al. 1975; Farr, Banks et al. 2002). Inhibitory avoidance was conducted in the Gemini Avoidance System from San Diego Instruments. This apparatus consists of a control computer, shocker and a test enclosure with two chambers (shock compartment and start compartment) and a grid floor that can deliver shock. The start compartment contained a plastic start box which was separated from the shock compartment by an automatic gate. Blinders were placed on the window in each compartment so that the task was conducted in complete darkness. Even though it has been shown that blind mice can perform the inhibitory avoidance task, we wanted to additionally assure that control animals were not using visual cues to perform the task. Mice were placed in the acrylic start box on the right hand side. After a 30 second habituation period the gate went up and mice could enter the shock compartment by stepping down onto the grid floor of the shock compartment. When all 4 limbs were in the shock compartment the gate closed and the mice received a 0.15 mA shock, 2 seconds in duration. The latency to enter the shock compartment was recorded (acquisition). Mice were removed from the shock compartment after 10 seconds and returned to the home cage. Twenty-four hrs later the procedure is repeated except this time no shock was given. Increased latency to enter the shock compartment on day 2 (retention) is taken as the measure of memory. Change in behavior due to the shock (i.e. learning and memory) is measured as the difference between day 1 and day 2 and is altered in mice with striatal lesions.
Social Transmission of Food Preference
After 18 h food deprivation, mice designated as demonstrators were given 1 hour access to powdered chow flavored with either cocoa or cinnamon. Each demonstrator was then placed into a cage with another mouse designated the observer for 30 min. Observers interacted with the demonstrator, including sniffing the scent around the muzzle and the breath of the demonstrator mouse. After the interaction the observer was subsequently food deprived for 22 h, then given a 1 hour two-choice flavor preference test. Powdered chow mixed with the familiar flavor previously eaten by the demonstrator and cued by the demonstrator’s breath odor is one choice. The other choice is powdered chow containing the uncued novel flavor; both are available in separate jars throughout the choice session. Consumption of each food is measured by weighing the food jars before and after the 1 hour choice test. Subjects that remember the flavor cued 24 hours earlier will show a bias for that flavor when simultaneously presented with a choice between chow containing the cued flavor and an uncued flavor.
Results
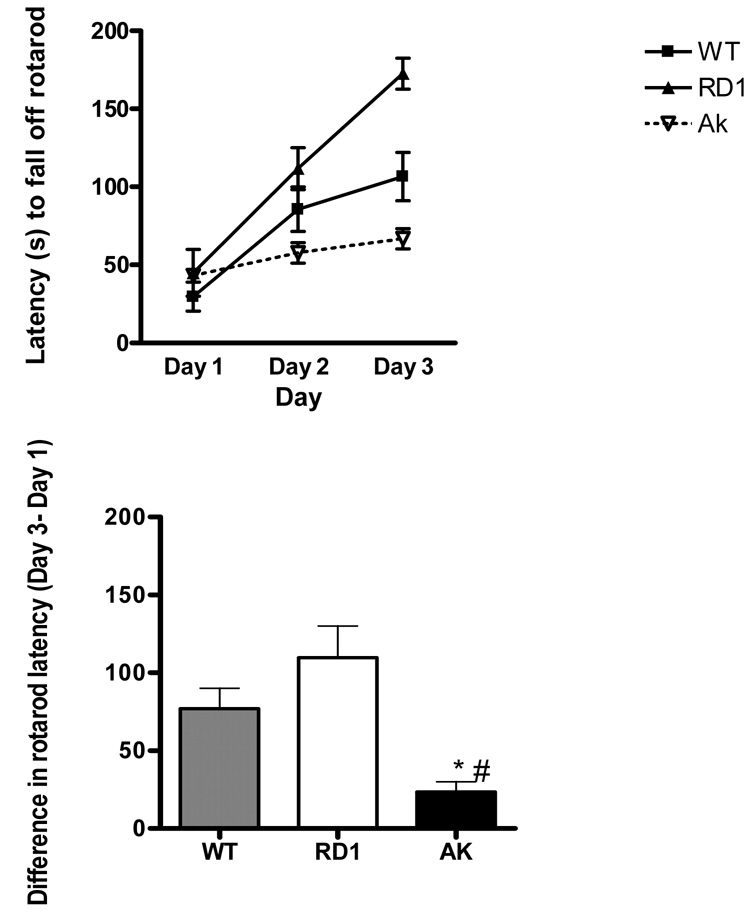
The rotatord is used to measure balance, coordination, motor function and motor learning. Lesions of the striatum and cerebellum can impair rotarod performance and/or learning. As shown in Figure 1 performance on the rotarod was similar across all three groups on day 1 which suggests lack of gross motor impairment in ak mice. Whereas wt and rd1 mice improved their performance over the next two days the learning curve for ak mice was nearly flat (Figure 1, Top Panel). Analysis of the learning curves demonstrated a main effect of day F(2,62)=53.91, p<0.0001 and genotype F(2,62) = 10.35, p<0.0004 and an interaction between day and genotype F(4, 62) = 8.69, p <0.0001. To further assess the magnitude of rotarod learning across days we compared the difference between rotarod latency on day 1 and rotarod latency on day 3 between all three groups. ak mice demonstrated a smaller improvement (Figure 1, Bottom Panel) over the 3 days compared to wt (p<0.05) and rd1 mice (p<0.01), consistent with impaired motor learning. Closer analysis of the rotarod performance over the 3 days indicates that most of the genotype difference in learning occurred between day 1 and day 2. Specifically, wt and rd1 mice increased their latency by 788% and 740% respectively. In contrast ak mice only improved their performance by 57% from day 1 to day 2 which was significantly lower than wt (p<0.05) and rd1 (p<0.05) groups (Kruskall-Wallis test). From day 2 to day 3 wt, rd1 and ak mice showed only slight improvement of 41%, 90% and 22% respectively which is markedly reduced compared to the improvement observed from day 1 to day 2.
Figure 1. ak mice are impaired on motor learning in the rotarod.
(n=8–9 mice per group). (Top Panel) Mean latency to fall off the rotarod during three consecutive trials per day for three days in wt (checkered bar), rd1 (white bar) and ak (black bar) mice. Change in latency to fall off of rotarod over 3 days, reported as difference in latency from day 1 to day 3. *p<0.05 compared to wt mice. #p<0.001 compared to rd1 mice(Kruskall-Wallis test, Dunn’s Multiple Comparison Test).
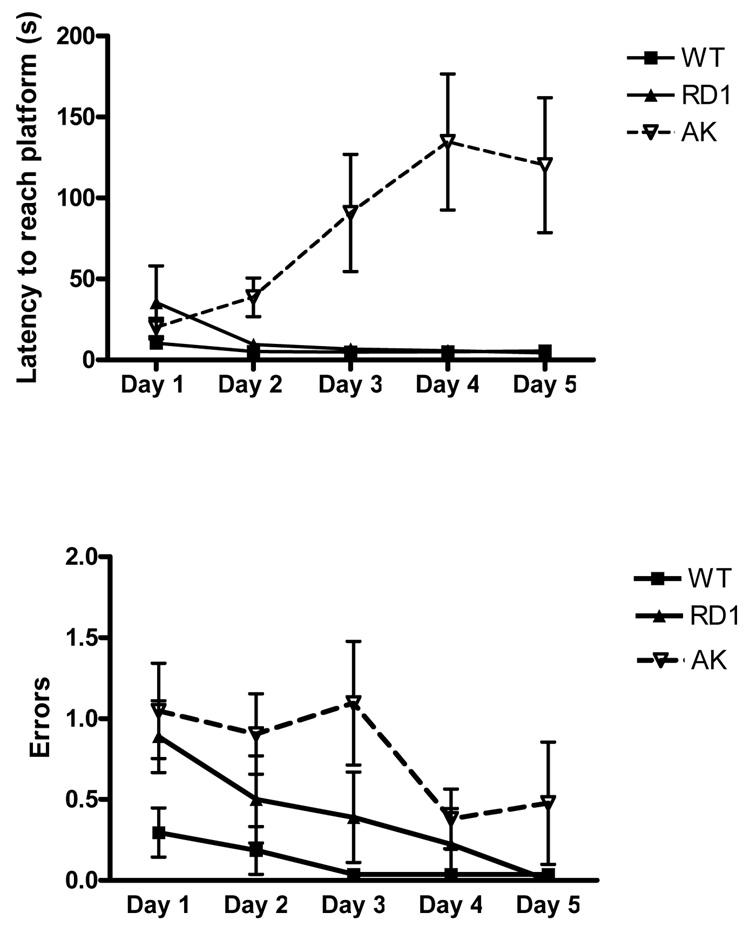
The swimming t-maze is another procedural memory task; however unlike the rotarod it requires egocentric navigation for mice to find an escape platform. This task can be performed by mice with retinal degeneration and is sensitive to striatal damage (Van Raamsdonk, Pearson et al. 2005). As shown in Figure 2, Top Panel, wt and rd1 mice over the 5 days became increasingly faster at finding the platform. In stark contrast to the decrease in latency in the wt and rd1 mice, ak mice actually increased their latency to reach the platform over the 5 days. Analysis of the learning curve showed a main effect of day F(4,76)=3.10, p=0.02 and genotype F(2,76)=8.88, p=0.0019 and a significant interaction between day and genotype F(8,76)=6.19, p<0.0001 in the latency to reach the platform. Since the ak mice swam with similar speed to wt and rd1 on day 1, the genotype effect is likely related more to impairment of procedural learning in ak mice, rather than impaired motor function per se. If ak mice were unable to swim, day 1 latencies would likely have been higher than wt or rd1 mice. Consistent with this idea there was a main effect of genotype on mean number of errors (Figure 2, Bottom Panel) committed F(2,76)=8.28, p=0.0026 and day F(4.76)=4.46, p=0.00026. Interestingly, by the third day of testing most wt and rd1 mice learned the swim task well enough to run most trials without any errors. ak mice in contrast continued to make errors. Our criterion for successfully learning the task was 3 consecutive days (9 trials) without an error. The number of mice that achieved this criterion was lower (p=0.0319) in ak mice (1 out of 7) than in the wt (7 out 9) or rd1 (4 out of 6) groups.
Figure 2. ak mice are impaired on the swimming t maze.
(n=6–9 mice per group). (Top Panel) Latency to swim to platform in seconds. (Main effect of day F(4,76)=3.10, p=0.02 and genotype F(2,76)=8.88, p=0.0019. Day × genotype interaction (F(8,76)=6.19, p<0.0001). (Bottom panel) Mean number of errors made in swimming t maze over 5 days (mean of 3 trials per day per animal). Main effect of genotype F(2,76)=8.28, p=0.0026 and day F(4.76)=4.46, p=0.00026. Criterion was 3 consecutive days without errors (9 consecutive trials). The number of mice that achieved this criterion was lower (p=0.0319) in ak mice (1 out of 7) than in the wt (7 out 9) or rd1 (4 out of 6) groups.
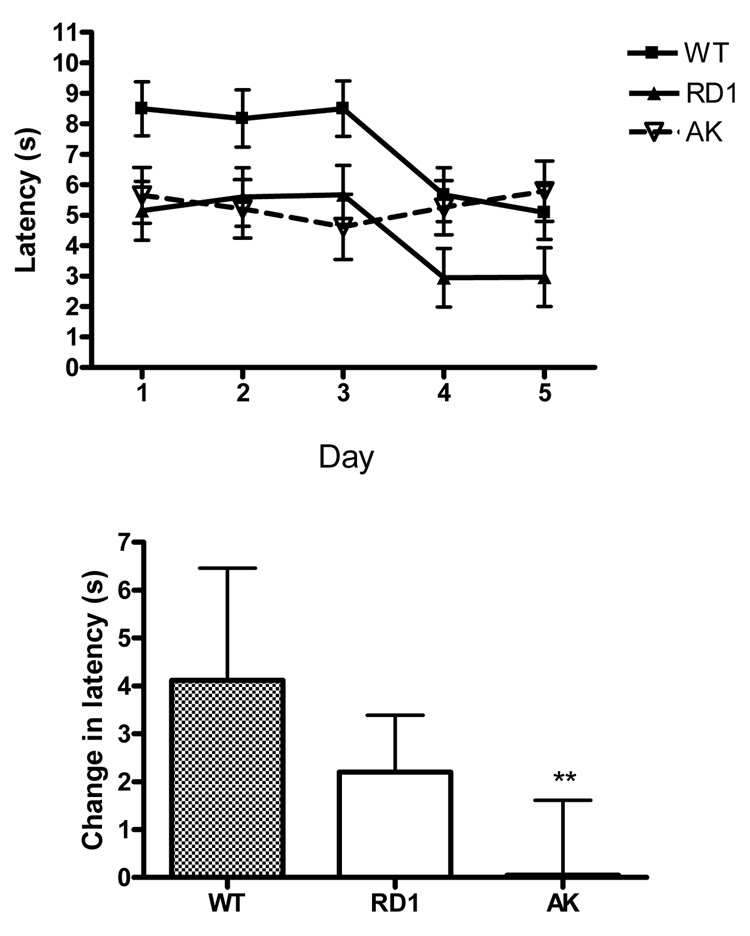
Due to the strenuous nature of the swimming T-maze we examined the behavior of ak mice on a dry, food motivated T-maze in order to rule out the effects of stress and fatigue. As shown in Figure 3 wt and rd1 mice showed robust decrease in latency between day 3 and 4, which is indicative of procedural learning and memory. The apparently elevated latency in wt animals compared to rd1 and ak mice is likely due to the observation that the sighted wt mice were engaging in more visual exploration of the apparatus. The improvement between day 3 and 4 was not observed in ak mice. Rather the curve for the ak mouse was nearly flat, similar to their performance in the rotarod. (F(10,560) = 2.33, p = 0.01). In comparing the change in latency between day 1 and day 5 ak mice had a significantly less difference score than wt or rd1 mice, indicative of impaired procedural memory (F(2,28.377) = 8.3, p = 0.003). In contrast to the latency, errors in the dry T-maze however did not significantly differ. Together the data from the rotarod swim and dry T-maze suggests procedural memory impairments in ak mice.
Figure 3. ak mice are impaired on the dry t-maze.
(n=6 mice per group) (Top Panel) Latency to reach distal end of arm over 5 days in wt, rd1 and ak mice. (Bottom Panel) Difference between day 1 and day 5 latency(s). ak mice show reduced difference score (F(2,28.377) = 8.3, p = 0.003).
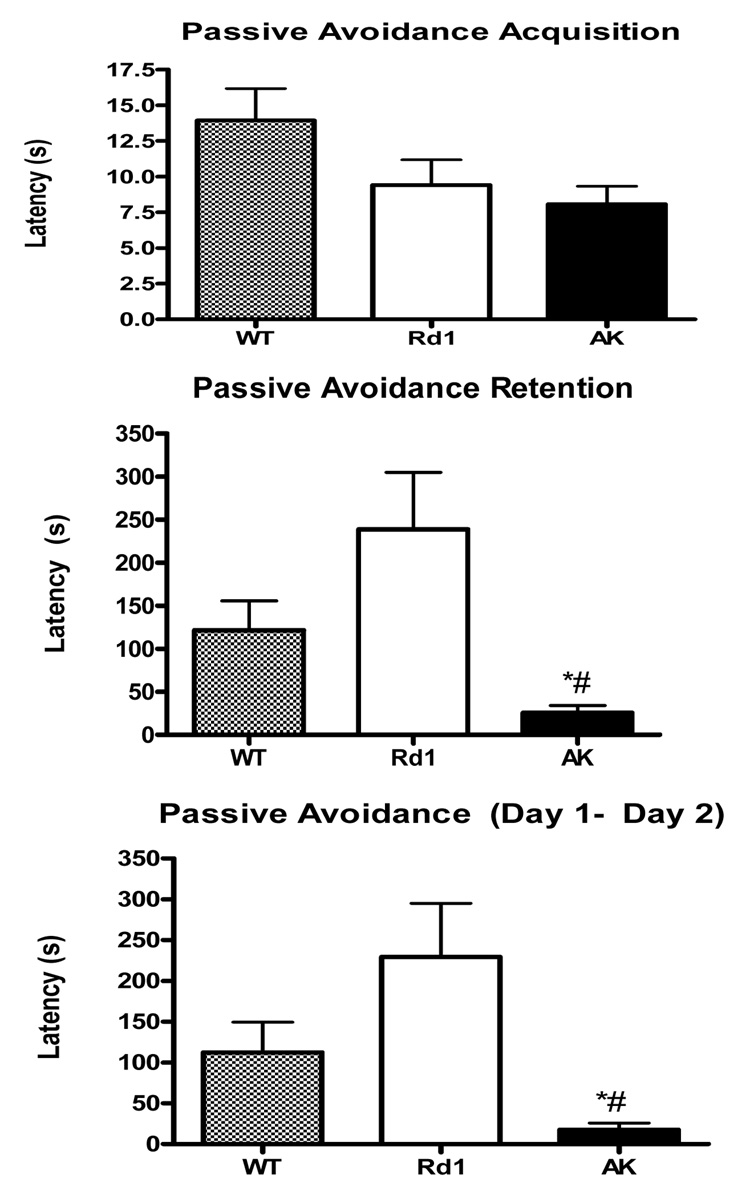
Inhibitory avoidance learning is an operant associative learning task that blind mice can readily perform (Dyer, Hammond et al. 1975; Farr, Banks et al. 2002). In addition inhibitory avoidance is altered by lesions of the caudate nucleus as well as manipulations of the prefrontal cortex, hippocampus and amygdala. These brain regions may be involved in cognitive dysfunction in PD. In the acquisition trial of the inhibitory avoidance task there was no difference between wt, rd1 and ak mice in the latency step into the shock paired side (Figure 4, Top Panel). The day 1 latencies can be used as a rough measure of baseline locomotor activity, since long or short latencies can indicate motor impairment or hyperactivity respectively. Thus the lack of difference across the groups on day 1 latencies is important because it indicates that ak mice show no evidence of gross locomotor activity impairment or hyperactivity on this task. As evidence of learning on day two (Figure 4, Middle Panel), 24 hrs after receiving the shock, wt and rd1 mice showed increased latencies, often more than 10 fold greater than the latencies on day 1. In contrast the latencies of ak mice increased only slightly if at all on day 2, indicating impaired memory for the shock presented 24hrs earlier. When comparing all three groups on the change in inhibitory avoidance, which is the change in latency due to learning and which factors in baseline latency, ak mice were significantly different from wt (p<0.05) and rd1 (p<0.001, One-Way ANOVA, Tukey’s Post-hoc) mice (Figure 4, Bottom Panel). Wild-type and rd1 mice did not differ on any measures. To rule out differences in shock sensitivity we measured vocalizations (>2s) and found no statistical differences in vocalizations (wt = 2.5, rd1 = 1.3, ak = 2.2).
Figure 4. ak mice are impaired on passive avoidance learning.
(wt, checkered bar, n=11; rd1, white bar, n=5; ak, black bar, n=14). (Top panel) No difference in latency (in seconds) to enter the shock paired compartment on day 1 between the three groups. (Middle Panel) ak mice do not show an enhanced latency to enter shock paired side 24 hrs after receiving shock indicating impaired memory (retention). (Bottom Panel) Passive avoidance, the change in latency due to learning (Day 2-Day 1) is reduced in ak mice. *p<0.05, compared to wt mice, # p<0.01 compared to rd1 mice, One-way ANOVA, Tukeys post-hoc test
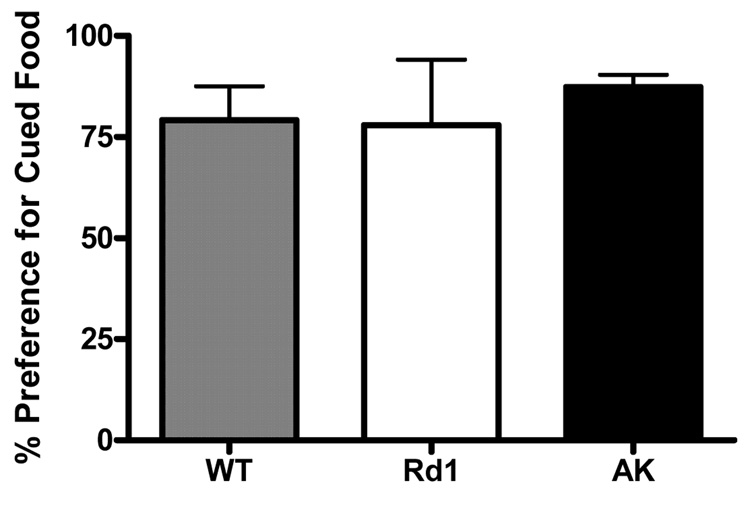
To determine the specificity of the impairments observed in the striatum-dependent tasks described above we assessed the behavior of ak mice on an associative learning task that is not thought to be heavily dependent on the striatum or DA neurotransmission. Social Transmission of Food Preference (STFP) is a non-spatial, associative learning and memory task thought to preferentially involve cholinergic circuits in the prefrontal cortex. In contrast to the above results using the inhibitory avoidance paradigm, ak mice were indistinguishable from wt, and rd1 mice in performing the STFP task (Figure 4). All 3 groups demonstrated greater than 75% preference for the chow with the cued flavor indicating intact memory for the social interaction that occurred 24 hours earlier.
Discussion
The major finding reported here is that deficiency of the transcription factor Pitx3 in mice results in striatum-dependent cognitive impairments. Importantly, the type of impairment we have observed in ak mice overlaps categorically with that observed in human PD since PD has been shown to involve both procedural (Allain, Lieury et al. 1995; Thomas, Reymann et al. 1996) and associative learning impairments (Vriezen and Moscovitch 1990; Sprengelmeyer, Canavan et al. 1995). These novel findings are interesting for several reasons: First our results suggests that at least some of the cognitive impairments observed in PD are the result of pathophysiological changes intrinsic to PD. Other hypotheses are that cognitive impairments are due to the stress of living with a chronic disease or DA pharmacotherapy. These three hypotheses, (1) stress, (2) medications, and (3) pathophysiology are discussed briefly.
Given the well known detrimental effects of stress on mood and cognition, one potential cause of cognitive impairments and neuropsychiatric symptoms in PD is the psychological burden or stress of living with a chronic medical illness (Duff, Mold et al. 2007). Under this premise, PD-related cognitive symptoms are not specific to PD but would be expected to occur in any disabling chronic medical illness. The second potential cause of cognitive symptoms in PD is the medication used to treat PD (Papapetropoulos, Argyriou et al. 2005; Cools 2006) . Dopamine (DA) pharmacotherapy could potentially impair cognition given the important role of DA in executive function, and the harmful effects of excess DA on cognition (Papapetropoulos, Argyriou et al. 2005). Anti-cholinergics are another class of PD treatment that could feasibly cause the cognitive impairment observed in PD. The last hypothesis regarding the etiology of cognitive symptoms in PD is that it could be a result of specific pathophysiological events intrinsic to the disease, such as loss of nigrostriatal dopamine or other neurotransmitters altered in PD (Zgaljardic, Foldi et al. 2004). Indeed, PD involves disruption of several key signaling molecules that are important in learning and memory including DA, acetylcholine, norepinephrine and glutamate (Guttman 1987; Di Chiara and Morelli 1993). It cannot be ruled out that behavioral differences observed in ak mice are related to disruption of neurotransmitter systems other than DA, however given the preponderance of evidence suggesting DA is important in striatum-dependent cognition and the strength of the DA related phenotype in ak mice, the DA system seems a likely candidate.
Secondly this provides to our knowledge the first genetic model of cognitive impairment in PD. The major advantages of this model are the capacity to probe cognition and other non-motor aspects of DA function in severely DA-deficient (>90% DA loss) subjects. Such testing is difficult to conduct in other PD DA-deficiency models since similar bilateral lesions of DA using toxins such as 6-OHDA results in severely motor impaired, unmotivated and unhealthy animals. Furthermore, though the myriad DA receptor knockout mice have provided invaluable information on the function of DA receptors, there are few pathway specific models that can be used to dissociate A9 vs. A10 related contributions. As is the case with all experimental animal models, ak mice cannot be said to model all aspects of PD. In fact, ak mice do not appear to model the progressive neurodegeneration . They do however recapitulate severe nigrostriatal DA loss (Hwang, Ardayfio et al. 2003), which is a hallmark feature of PD and likely responsible for much of the behavioral symptoms in PD. Additionally, we have previously reported that ak mice mimic other key features of PD such as L-Dopa responsivity, nigrostriatal motor defect and dopamine supersensitivity (Hwang, Fleming et al. 2005). Our recent analysis also showed that chronic treatment with L-DOPA and dopamine receptor agonists induces novel dyskinetic behaviors in ak mice, which was attenuated by anti-dyskinetic agents (Ding, Restrepo et al. 2007). Altogether the ak mice can be said to model an impressive number of features of PD.
We favor the view that the differences reported here are cognition related, however, another potential interpretation of these data is that the impairments that we observe in ak mice are artifacts of genotype differences in locomotor activity or sensory capacity. There is little support for this view however, since ak mice showed no evidence of global neurological deficits or severe motor disturbance in any of the paradigms tested here, which is consistent with our previous studies. ak mice did not differ from wt or rd1 mice on day 1 baseline performance of any of the tasks tested. Instead the main difference observed between ak mice and the wt and rd1 mice is that ak mice failed to demonstrate experience-dependent change in behavior. This is consistent with the idea of select striatum-dependent learning and memory impairment in ak mice.
Our finding of cognitive impairment in ak mice adds to the growing literature implicating an important role for the basal ganglia in cognition and neuropsychiatric symptoms. Deficits of procedural and associative learning have similarly been observed with other mouse models of striatal dysfunction including the YAC128 and R6/2 mouse models of Huntington’s disease (Lione, Carter et al. 1999; Van Raamsdonk, Pearson et al. 2005). For example YAC128 which have reduced dopamine in the striatum are impaired in the swimming t-maze (Van Raamsdonk, Pearson et al. 2005). It is interesting that in contrast to Parkinson’s disease, the cognitive impairment and neuropsychiatric features of Huntington’s disease are well recognized and an active area of both clinical and pre-clinical research. Furthermore, a recent elegant study showed that the heterozygous mice of Nurr1, another key transcription factor for dopamine neuron development (Wallen and Perlmann 2003; Smidt and Burbach 2007), display a behavioral pattern indicative of potential relevance for symptoms of schizophrenia (Rojas, Joodmardi et al. 2007).
Cognitive symptoms in PD cause significant burden to the patient, care-givers and society (Schrag, Morley et al. 2004; Schrag, Hovris et al. 2006). Currently there are few practical methods to investigate cognitive features of PD, which precludes important preclinical studies that could potentially elucidate mechanisms and identify therapeutics. We have found that cognitive aspects of PD can be studied in a novel, readily available genetic model of PD, which does not require the variability or labor intensiveness of toxin based models, nor the severe motor impairments. We propose that the ak mouse can facilitate important studies of cognition and neuropsychiatric symptoms in PD and a move away from the singular focus on motor aspects of PD. These studies should not only elucidate mechanisms of cognitive dysfunction in PD, but more generally provide a readily available tool to study important neuropsychiatric functions of the basal ganglia and DA. As an example of this depression, which occurs in greater than 40% of patients with PD and executive dysfunction are common and disabling features of PD which are not well understood (Uekermann, Daum et al. 2003; Lemke, Fuchs et al. 2004; Weintraub, Moberg et al. 2005). Without gross motor impairment as a potential confound ak mice are well suited to be used as a DA-deficiency model to study these and other important neuropsychiatric features of PD.
Figure 5. ak mice perform normally on social transmission of food preference (STFP).
Data represents percent preference for a previously cued food over a novel food in wt (checkered bar), rd1 (white bar) and ak (black bar). No difference in preference for cued food in ak mice. (n=4–6 mice per group) p>0.05 (One-way ANOVA).
Acknowledgements
This work was supported by NIH grants MH48866 and DC006501, and an International Grant from Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology, the Republic of Korea. The authors are grateful to Jessie Kang for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agid Y, Ruberg M, et al. Parkinson's disease and dementia. Clin Neuropharmacol. 1986;9 Suppl 2:S22–S36. [PubMed] [Google Scholar]
- Allain H, Lieury A, et al. Procedural memory and Parkinson's disease. Dementia. 1995;6(3):174–178. doi: 10.1159/000106942. [DOI] [PubMed] [Google Scholar]
- Bosboom JL, Stoffers D, et al. Cognitive dysfunction and dementia in Parkinson's disease. J Neural Transm. 2004;111(10–11):1303–1315. doi: 10.1007/s00702-004-0168-1. [DOI] [PubMed] [Google Scholar]
- Bove J, Prou D, et al. Toxin-induced models of Parkinson's disease. NeuroRx. 2005;2(3):484–494. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronnick K, Aarsland D, et al. Neuropsychiatric disturbances in Parkinson's disease clusters in five groups with different prevalence of dementia. Acta Psychiatr Scand. 2005;112(3):201–207. doi: 10.1111/j.1600-0447.2005.00562.x. [DOI] [PubMed] [Google Scholar]
- Caston J, Hilber P, et al. Effect of training on motor abilities of heterozygous staggerer mutant (Rora(+)/Rora(sg)) mice during aging. Behav Brain Res. 2003;141(1):35–42. doi: 10.1016/s0166-4328(02)00319-4. [DOI] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for l-DOPA treatment in Parkinson's disease. Neurosci Biobehav Rev. 2006;30(1):1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Morelli M. Dopamine-acetylcholine-glutamate interactions in the striatum. A working hypothesis. Adv Neurol. 1993;60:102–106. [PubMed] [Google Scholar]
- Ding Y, Restrepo J, et al. Chronic 3,4-dihydroxyphenylalanine treatment induces dyskinesia in aphakia mice, a novel genetic model of Parkinson's disease. Neurobiol Dis. 2007;27(1):11–23. doi: 10.1016/j.nbd.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Mold JW, et al. Medical burden and cognition in older patients in primary care: selective deficits in attention. Arch Clin Neuropsychol. 2007;22(5):569–575. doi: 10.1016/j.acn.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Dujardin K, Laurent B. Dysfunction of the human memory systems: role of the dopaminergic transmission. Curr Opin Neurol. 2003;16 Suppl 2:S11–S16. doi: 10.1097/00019052-200312002-00003. [DOI] [PubMed] [Google Scholar]
- Dyer RS, Hammond MA, et al. Influence of enucleation upon two-way avoidance behavior of rats, hamsters, chinchillas and BALB/cJ mice. Physiol Behav. 1975;14(2):211–216. doi: 10.1016/0031-9384(75)90168-7. [DOI] [PubMed] [Google Scholar]
- Farr SA, Banks WA, et al. Blind mice are not impaired in T-maze footshock avoidance acquisition and retention. Physiol Behav. 2002;76(4–5):531–538. doi: 10.1016/s0031-9384(02)00749-7. [DOI] [PubMed] [Google Scholar]
- Guttman M. Receptors in the basal ganglia. Can J Neurol Sci. 1987;14(3 Suppl):395–401. doi: 10.1017/s0317167100037793. [DOI] [PubMed] [Google Scholar]
- Hwang DY, Ardayfio P, et al. Selective loss of dopaminergic neurons in the substantia nigra of Pitx3-deficient aphakia mice. Brain Res Mol Brain Res. 2003;114(2):123–131. doi: 10.1016/s0169-328x(03)00162-1. [DOI] [PubMed] [Google Scholar]
- Hwang DY, Fleming SM, et al. 3,4-dihydroxyphenylalanine reverses the motor deficits in Pitx3-deficient aphakia mice: behavioral characterization of a novel genetic model of Parkinson's disease. J Neurosci. 2005;25(8):2132–2137. doi: 10.1523/JNEUROSCI.3718-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterbach EC. The neuropsychiatry of Parkinson's disease. Minerva Med. 2005;96(3):155–173. [PubMed] [Google Scholar]
- Lemke MR, Fuchs G, et al. Depression and Parkinson's disease. J Neurol. 2004;251 Suppl 6:VI/24–VI/27. doi: 10.1007/s00415-004-1606-6. [DOI] [PubMed] [Google Scholar]
- Lione LA, Carter RJ, et al. Selective discrimination learning impairments in mice expressing the human Huntington's disease mutation. J Neurosci. 1999;19(23):10428–10437. doi: 10.1523/JNEUROSCI.19-23-10428.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes I, Tovmasian LT, et al. Pitx3 is required for development of substantia nigra dopaminergic neurons. Proc Natl Acad Sci U S A. 2003;100(7):4245–4250. doi: 10.1073/pnas.0230529100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapetropoulos S, Argyriou AA, et al. Factors associated with drug-induced visual hallucinations in Parkinson's disease. J Neurol. 2005;252(10):1223–1228. doi: 10.1007/s00415-005-0840-x. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Grafman J, et al. Procedural learning in Parkinson's disease and cerebellar degeneration. Ann Neurol. 1993;34(4):594–602. doi: 10.1002/ana.410340414. [DOI] [PubMed] [Google Scholar]
- Pittler SJ, Baehr W. Identification of a nonsense mutation in the rod photoreceptor cGMP phosphodiesterase beta-subunit gene of the rd mouse. Proc Natl Acad Sci U S A. 1991;88(19):8322–8326. doi: 10.1073/pnas.88.19.8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas P, Joodmardi E, et al. Adult mice with reduced Nurr1 expression: an animal model for schizophrenia. Mol Psychiatry. 2007;12(8):756–766. doi: 10.1038/sj.mp.4001993. [DOI] [PubMed] [Google Scholar]
- Roncacci S, Troisi E, et al. Implicit memory in parkinsonian patients: evidence for deficient skill learning. Eur Neurol. 1996;36(3):154–159. doi: 10.1159/000117234. [DOI] [PubMed] [Google Scholar]
- Sarazin M, Deweer B, et al. Procedural learning and striatofrontal dysfunction in Parkinson's disease. Mov Disord. 2002;17(2):265–273. doi: 10.1002/mds.10018. [DOI] [PubMed] [Google Scholar]
- Schrag A, Hovris A, et al. Caregiver-burden in parkinson's disease is closely associated with psychiatric symptoms, falls, and disability. Parkinsonism Relat Disord. 2006;12(1):35–41. doi: 10.1016/j.parkreldis.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Schrag A, Morley D, et al. Impact of Parkinson's disease on patients' adolescent and adult children. Parkinsonism Relat Disord. 2004;10(7):391–397. doi: 10.1016/j.parkreldis.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Smidt MP, Burbach JP. How to make a mesodiencephalic dopaminergic neuron. Nat Rev Neurosci. 2007;8(1):21–32. doi: 10.1038/nrn2039. [DOI] [PubMed] [Google Scholar]
- Smidt MP, Smits SM, et al. Homeobox gene Pitx3 and its role in the development of dopamine neurons of the substantia nigra. Cell Tissue Res. 2004;318(1):35–43. doi: 10.1007/s00441-004-0943-1. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Canavan AG, et al. Associative learning in degenerative neostriatal disorders: contrasts in explicit and implicit remembering between Parkinson's and Huntington's diseases. Mov Disord. 1995;10(1):51–65. doi: 10.1002/mds.870100110. [DOI] [PubMed] [Google Scholar]
- Thomas V, Reymann JM, et al. Assessment of procedural memory in Parkinson's disease. Prog Neuropsychopharmacol Biol Psychiatry. 1996;20(4):641–650. doi: 10.1016/0278-5846(96)00037-1. [DOI] [PubMed] [Google Scholar]
- Uekermann J, Daum I, et al. Depressed mood and executive dysfunction in early Parkinson's disease. Acta Neurol Scand. 2003;107(5):341–348. doi: 10.1034/j.1600-0404.2003.02155.x. [DOI] [PubMed] [Google Scholar]
- Vakil E, Herishanu-Naaman S. Declarative and procedural learning in Parkinson's disease patients having tremor or bradykinesia as the predominant symptom. Cortex. 1998;34(4):611–620. doi: 10.1016/s0010-9452(08)70518-5. [DOI] [PubMed] [Google Scholar]
- van den Munckhof P, Luk KC, et al. Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development. 2003;130(11):2535–2542. doi: 10.1242/dev.00464. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Pearson J, et al. Cognitive dysfunction precedes neuropathology and motor abnormalities in the YAC128 mouse model of Huntington's disease. J Neurosci. 2005;25(16):4169–4180. doi: 10.1523/JNEUROSCI.0590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriezen ER, Moscovitch M. Memory for temporal order and conditional associative-learning in patients with Parkinson's disease. Neuropsychologia. 1990;28(12):1283–1293. doi: 10.1016/0028-3932(90)90044-o. [DOI] [PubMed] [Google Scholar]
- Wallen A, Perlmann T. Transcriptional control of dopamine neuron development. Ann N Y Acad Sci. 2003;991:48–60. doi: 10.1111/j.1749-6632.2003.tb07462.x. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Moberg PJ, et al. Dimensions of executive function in Parkinson's disease. Dement Geriatr Cogn Disord. 2005;20(2–3):140–144. doi: 10.1159/000087043. [DOI] [PubMed] [Google Scholar]
- Zgaljardic DJ, Foldi NS, et al. Cognitive and behavioral dysfunction in Parkinson's disease: neurochemical and clinicopathological contributions. J Neural Transm. 2004;111(10–11):1287–1301. doi: 10.1007/s00702-004-0178-z. [DOI] [PubMed] [Google Scholar]