Abstract
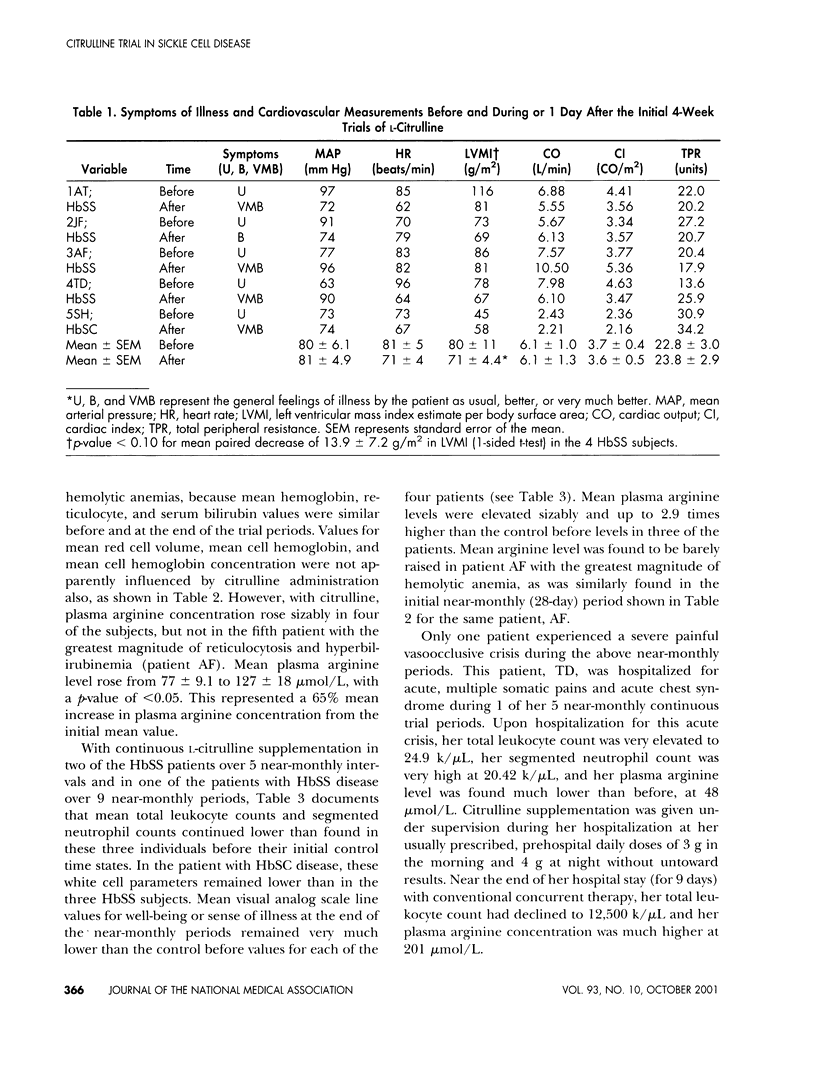
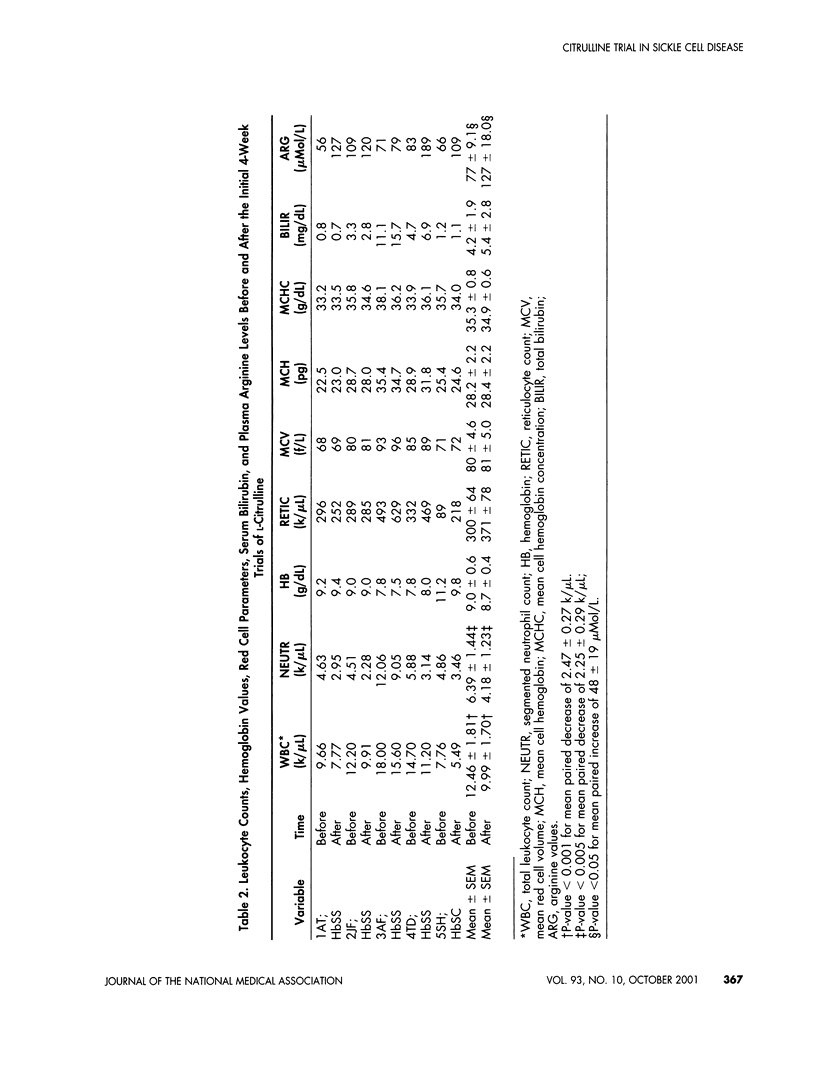
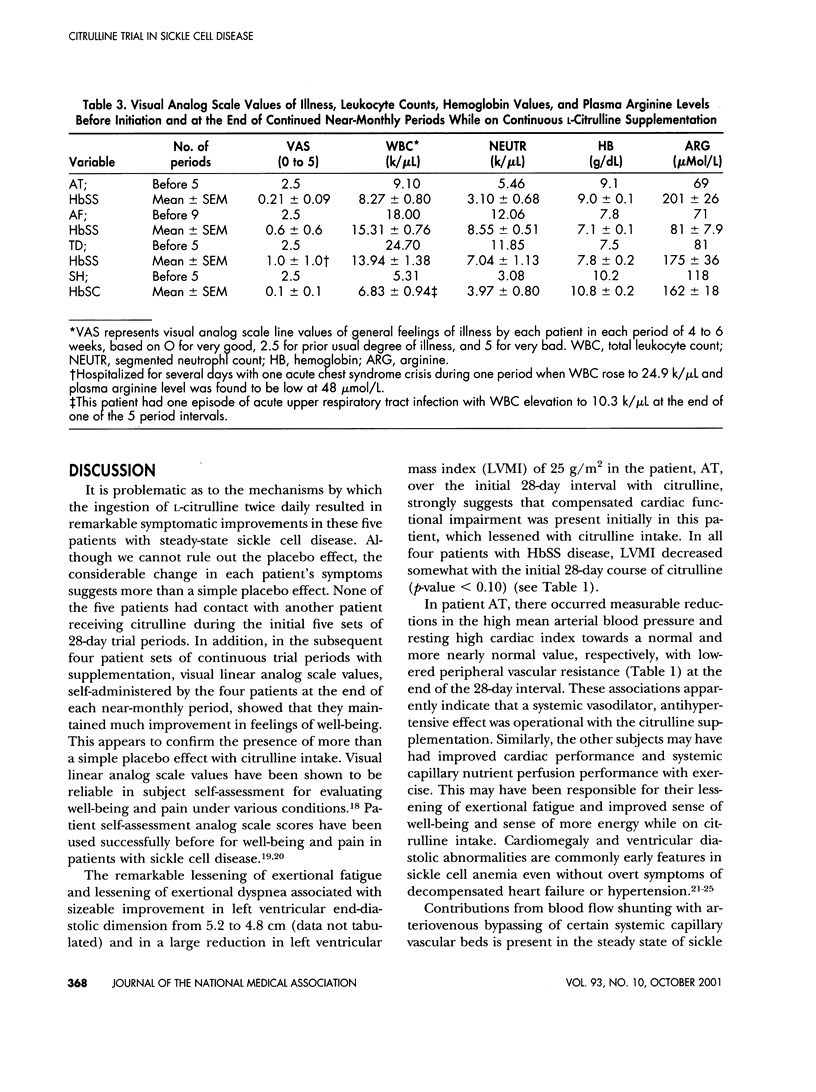
L-Arginine may be a conditionally essential amino acid in children and adolescents with sickle cell disease, particularly as required substrate in the arginine-nitric oxide pathway for endogenous nitrovasodilation and vasoprotection. Vasoprotection by arginine is mediated partly by nitric oxide-induced inhibition of endothelial damage and inhibition of adhesion and activation of leukocytes. Activated leukocytes may trigger many of the complications, including vasoocclusive events and intimal hyperplasias. High blood leukocyte counts during steady states in the absence of infection are significant laboratory risk factors for adverse complications. L-Citrulline as precursor amino acid was given orally twice daily in daily doses of approximately 0.1 g/kg in a pilot Phase II clinical trial during steady states in four homozygous sickle cell disease subjects and one sickle cell-hemoglobin C disease patient (ages 10-18). There soon resulted dramatic improvements in symptoms of well-being, raised plasma arginine levels, and reductions in high total leukocyte and high segmented neutrophil counts toward or to within normal limits. Continued L-citrulline supplementation in compliant subjects continued to lessen symptomatology, to maintain plasma arginine concentrations greater than control levels, and to maintain nearly normal total leukocyte and neutrophil counts. Side effects or toxicity from citrulline were not experienced. Oral L-citrulline may portend very useful for palliative therapy in sickle cell disease. Placebo-controlled, long-term trials are now indicated.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akinola N. O., Stevens S. M., Franklin I. M., Nash G. B., Stuart J. Subclinical ischaemic episodes during the steady state of sickle cell anaemia. J Clin Pathol. 1992 Oct;45(10):902–906. doi: 10.1136/jcp.45.10.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annane D., Sanquer S., Sébille V., Faye A., Djuranovic D., Raphaël J. C., Gajdos P., Bellissant E. Compartmentalised inducible nitric-oxide synthase activity in septic shock. Lancet. 2000 Apr 1;355(9210):1143–1148. doi: 10.1016/S0140-6736(00)02063-8. [DOI] [PubMed] [Google Scholar]
- Balkaran B., Char G., Morris J. S., Thomas P. W., Serjeant B. E., Serjeant G. R. Stroke in a cohort of patients with homozygous sickle cell disease. J Pediatr. 1992 Mar;120(3):360–366. doi: 10.1016/s0022-3476(05)80897-2. [DOI] [PubMed] [Google Scholar]
- Ballas S. K., Delengowski A. Pain measurement in hospitalized adults with sickle cell painful episodes. Ann Clin Lab Sci. 1993 Sep-Oct;23(5):358–361. [PubMed] [Google Scholar]
- Boggs D. R., Hyde F., Srodes C. An unusual pattern of neutrophil kinetics in sickle cell anemia. Blood. 1973 Jan;41(1):59–65. [PubMed] [Google Scholar]
- Buchanan G. R., Glader B. E. Leukocyte counts in children with sickle cell disease. Comparative values in the steady state, vaso-occlusive crisis, and bacterial infection. Am J Dis Child. 1978 Apr;132(4):396–398. doi: 10.1001/archpedi.1978.02120290068013. [DOI] [PubMed] [Google Scholar]
- Carpenter T. O., Levy H. L., Holtrop M. E., Shih V. E., Anast C. S. Lysinuric protein intolerance presenting as childhood osteoporosis. Clinical and skeletal response to citrulline therapy. N Engl J Med. 1985 Jan 31;312(5):290–294. doi: 10.1056/NEJM198501313120506. [DOI] [PubMed] [Google Scholar]
- Castro O., Brambilla D. J., Thorington B., Reindorf C. A., Scott R. B., Gillette P., Vera J. C., Levy P. S. The acute chest syndrome in sickle cell disease: incidence and risk factors. The Cooperative Study of Sickle Cell Disease. Blood. 1994 Jul 15;84(2):643–649. [PubMed] [Google Scholar]
- Charache S., Barton F. B., Moore R. D., Terrin M. L., Steinberg M. H., Dover G. J., Ballas S. K., McMahon R. P., Castro O., Orringer E. P. Hydroxyurea and sickle cell anemia. Clinical utility of a myelosuppressive "switching" agent. The Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Medicine (Baltimore) 1996 Nov;75(6):300–326. doi: 10.1097/00005792-199611000-00002. [DOI] [PubMed] [Google Scholar]
- Covitz W., Espeland M., Gallagher D., Hellenbrand W., Leff S., Talner N. The heart in sickle cell anemia. The Cooperative Study of Sickle Cell Disease (CSSCD). Chest. 1995 Nov;108(5):1214–1219. doi: 10.1378/chest.108.5.1214. [DOI] [PubMed] [Google Scholar]
- Dalakas M. C., Mock V., Hawkins M. J. Fatigue: definitions, mechanisms, and paradigms for study. Semin Oncol. 1998 Feb;25(1 Suppl 1):48–53. [PubMed] [Google Scholar]
- De Caterina R., Libby P., Peng H. B., Thannickal V. J., Rajavashisth T. B., Gimbrone M. A., Jr, Shin W. S., Liao J. K. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995 Jul;96(1):60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrade G., Poitrineau O., Bernasconi F., Garnier D., Donatien Y. Fonction ventriculaire gauche et drépanocytose. Etude échocardiographique. Arch Mal Coeur Vaiss. 1989 Dec;82(12):1975–1981. [PubMed] [Google Scholar]
- Graido-Gonzalez E., Doherty J. C., Bergreen E. W., Organ G., Telfer M., McMillen M. A. Plasma endothelin-1, cytokine, and prostaglandin E2 levels in sickle cell disease and acute vaso-occlusive sickle crisis. Blood. 1998 Oct 1;92(7):2551–2555. [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Hebbel R. P., Vercellotti G. M. The endothelial biology of sickle cell disease. J Lab Clin Med. 1997 Mar;129(3):288–293. doi: 10.1016/s0022-2143(97)90176-1. [DOI] [PubMed] [Google Scholar]
- Hofstra T. C., Kalra V. K., Meiselman H. J., Coates T. D. Sickle erythrocytes adhere to polymorphonuclear neutrophils and activate the neutrophil respiratory burst. Blood. 1996 May 15;87(10):4440–4447. [PubMed] [Google Scholar]
- Kuvibidila S., Gardner R., Ode D., Yu L., Lane G., Warrier R. P. Tumor necrosis factor alpha in children with sickle cell disease in stable condition. J Natl Med Assoc. 1997 Sep;89(9):609–615. [PMC free article] [PubMed] [Google Scholar]
- Lard L. R., Mul F. P., de Haas M., Roos D., Duits A. J. Neutrophil activation in sickle cell disease. J Leukoc Biol. 1999 Sep;66(3):411–415. doi: 10.1002/jlb.66.3.411. [DOI] [PubMed] [Google Scholar]
- Lewis J. F., Maron B. J., Castro O., Moosa Y. A. Left ventricular diastolic filling abnormalities identified by Doppler echocardiography in asymptomatic patients with sickle cell anemia. J Am Coll Cardiol. 1991 Jun;17(7):1473–1478. doi: 10.1016/0735-1097(91)90634-l. [DOI] [PubMed] [Google Scholar]
- Lonsdorfer J., Bogui P., Otayeck A., Bursaux E., Poyart C., Cabannes R. Cardiorespiratory adjustments in chronic sickle cell anemia. Bull Eur Physiopathol Respir. 1983 Jul-Aug;19(4):339–344. [PubMed] [Google Scholar]
- Marcus A. J., Broekman M. J. Cell-free hemoglobin as an oxygen carrier removes nitric oxide, resulting in defective thromboregulation. Circulation. 1996 Jan 15;93(2):208–209. doi: 10.1161/01.cir.93.2.208. [DOI] [PubMed] [Google Scholar]
- Moncada S., Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993 Dec 30;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Pegelow C. H., Colangelo L., Steinberg M., Wright E. C., Smith J., Phillips G., Vichinsky E. Natural history of blood pressure in sickle cell disease: risks for stroke and death associated with relative hypertension in sickle cell anemia. Am J Med. 1997 Feb;102(2):171–177. doi: 10.1016/s0002-9343(96)00407-x. [DOI] [PubMed] [Google Scholar]
- Platt O. S., Brambilla D. J., Rosse W. F., Milner P. F., Castro O., Steinberg M. H., Klug P. P. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994 Jun 9;330(23):1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- Platt O. S. The acute chest syndrome of sickle cell disease. N Engl J Med. 2000 Jun 22;342(25):1904–1907. doi: 10.1056/NEJM200006223422510. [DOI] [PubMed] [Google Scholar]
- Rees D. C., Cervi P., Grimwade D., O'Driscoll A., Hamilton M., Parker N. E., Porter J. B. The metabolites of nitric oxide in sickle-cell disease. Br J Haematol. 1995 Dec;91(4):834–837. doi: 10.1111/j.1365-2141.1995.tb05397.x. [DOI] [PubMed] [Google Scholar]
- Revill S. I., Robinson J. O., Rosen M., Hogg M. I. The reliability of a linear analogue for evaluating pain. Anaesthesia. 1976 Nov;31(9):1191–1198. doi: 10.1111/j.1365-2044.1976.tb11971.x. [DOI] [PubMed] [Google Scholar]
- SPROULE B. J., HALDEN E. R., MILLER W. F. A study of cardiopulmonary alterations in patients with sickle cell disease and its variants. J Clin Invest. 1958 Mar;37(3):486–495. doi: 10.1172/JCI103629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovey A., Lin Y., Browne P., Choong S., Wayner E., Hebbel R. P. Circulating activated endothelial cells in sickle cell anemia. N Engl J Med. 1997 Nov 27;337(22):1584–1590. doi: 10.1056/NEJM199711273372203. [DOI] [PubMed] [Google Scholar]
- Thrall J. H., Rucknagel D. L. Increased bone marrow blood flow in sickle cell anemia demonstrated by thallium-201 and Tc-99m human albumin microspheres. Radiology. 1978 Jun;127(3):817–819. doi: 10.1148/127.3.817. [DOI] [PubMed] [Google Scholar]
- Varani J., Ginsburg I., Schuger L., Gibbs D. F., Bromberg J., Johnson K. J., Ryan U. S., Ward P. A. Endothelial cell killing by neutrophils. Synergistic interaction of oxygen products and proteases. Am J Pathol. 1989 Sep;135(3):435–438. [PMC free article] [PubMed] [Google Scholar]
- Varat M. A., Adolph R. J., Fowler N. O. Cardiovascular effects of anemia. Am Heart J. 1972 Mar;83(3):415–426. doi: 10.1016/0002-8703(72)90445-0. [DOI] [PubMed] [Google Scholar]
- Werdehoff S. G., Moore R. B., Hoff C. J., Fillingim E., Hackman A. M. Elevated plasma endothelin-1 levels in sickle cell anemia: relationships to oxygen saturation and left ventricular hypertrophy. Am J Hematol. 1998 Jul;58(3):195–199. doi: 10.1002/(sici)1096-8652(199807)58:3<195::aid-ajh6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Wright C. E., Rees D. D., Moncada S. Protective and pathological roles of nitric oxide in endotoxin shock. Cardiovasc Res. 1992 Jan;26(1):48–57. doi: 10.1093/cvr/26.1.48. [DOI] [PubMed] [Google Scholar]
- Wu G., Flynn N. E., Flynn S. P., Jolly C. A., Davis P. K. Dietary protein or arginine deficiency impairs constitutive and inducible nitric oxide synthesis by young rats. J Nutr. 1999 Jul;129(7):1347–1354. doi: 10.1093/jn/129.7.1347. [DOI] [PubMed] [Google Scholar]
- Xia Y., Dawson V. L., Dawson T. M., Snyder S. H., Zweier J. L. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci U S A. 1996 Jun 25;93(13):6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]



