Abstract
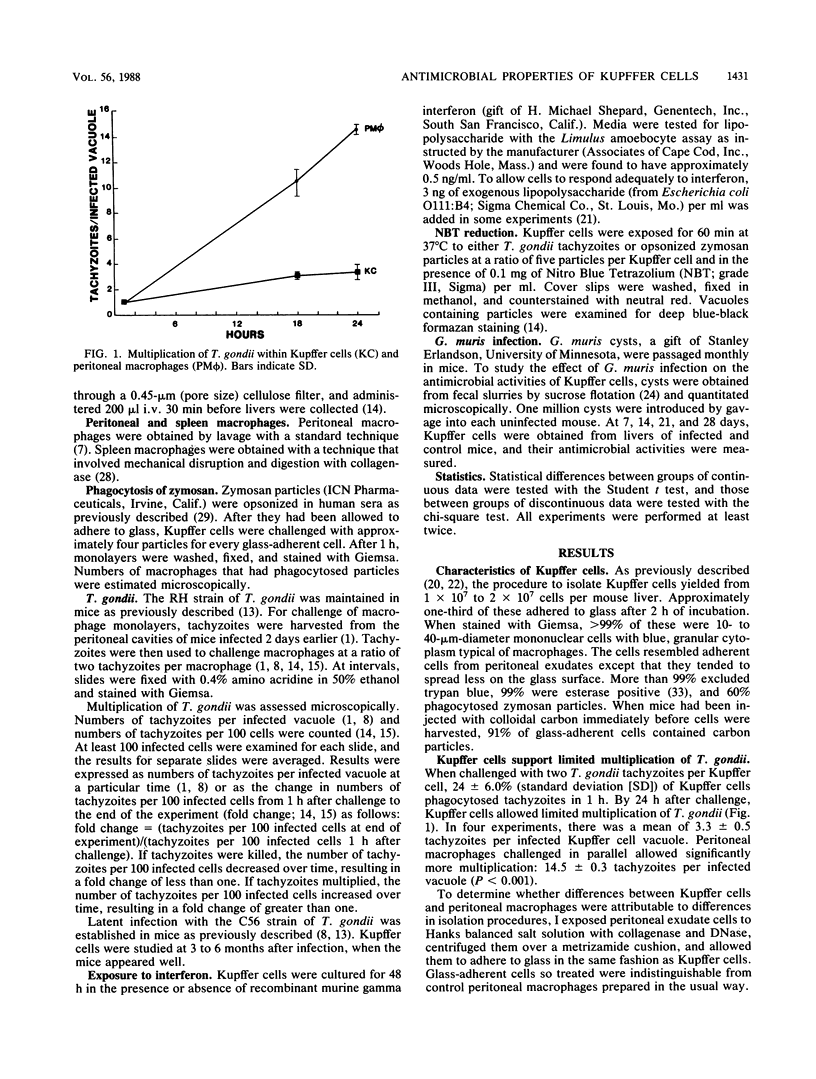
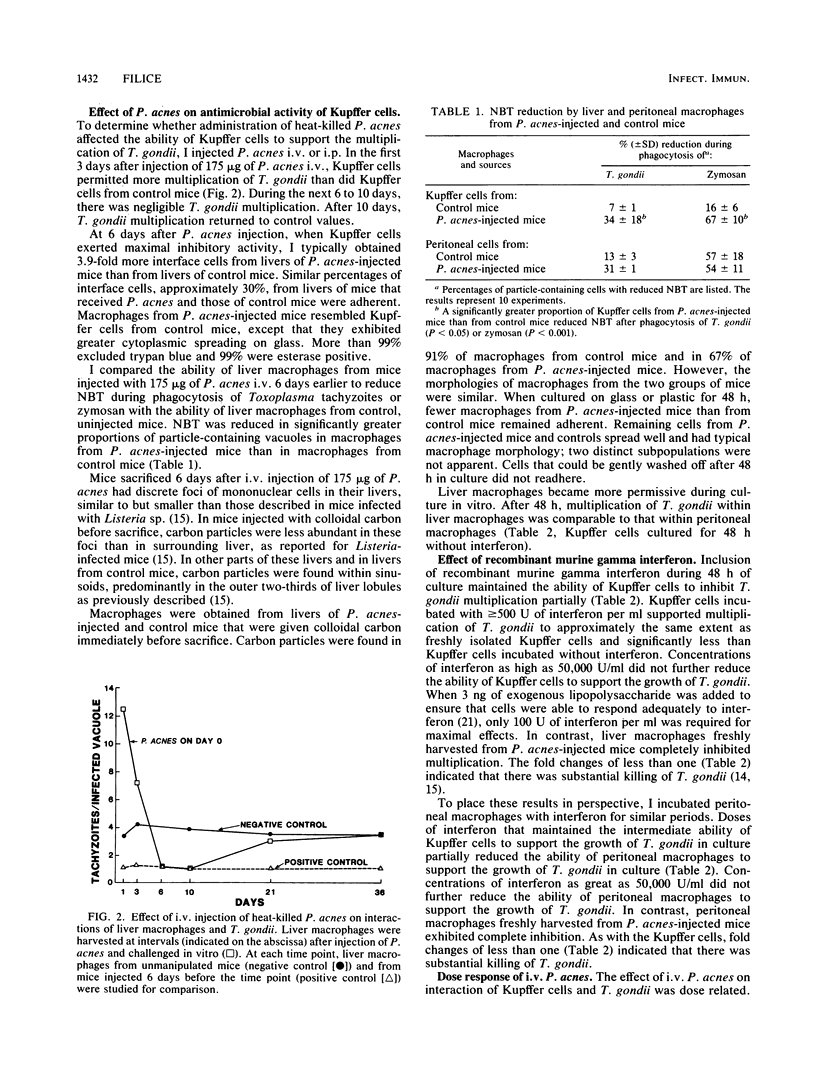
To characterize the antimicrobial activities of Kupffer cells, I harvested macrophages from livers with a technique involving perfusion with collagenase and DNase. Ninety-nine percent of glass-adherent cells had typical macrophage morphology, 99% were esterase positive, and 60% phagocytosed opsonized zymosan when challenged with four particles per macrophage. Toxoplasma gondii multiplied within Kupffer cells from unmanipulated mice, but multiplication was intermediate between that observed in highly permissive peritoneal macrophages and highly activated macrophages. Intravenous injection of heat-killed Propionibacterium acnes, a stimulus known to activate macrophages in other compartments, resulted in a uniform, highly activated population of liver macrophages. Kupffer cells from P. acnes-injected mice were capable of generating reactive oxygen intermediates as shown by reduction of Nitro Blue Tetrazolium during phagocytosis of T. gondii or opsonized zymosan. In contrast, intravenous P. acnes injection did not activate spleen macrophages. Intravenous injection of P. acnes into athymic mice activated Kupffer cells, which suggested that T cells were not essential for this response. Kupffer cells were not activated in mice with latent Toxoplasma infection or during acute Giardia muris infection. Ordinarily, Kupffer cells became highly permissive for T. gondii during 48 h in culture, but inclusion of recombinant murine gamma interferon maintained their moderate inhibitory activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. E., Jr, Remington J. S. Effect of normal and activated human macrophages on Toxoplasma gondii. J Exp Med. 1974 May 1;139(5):1154–1174. doi: 10.1084/jem.139.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur M. J., Kowalski-Saunders P., Wright R. Corynebacterium parvum-elicited hepatic macrophages demonstrate enhanced respiratory burst activity compared with resident Kupffer cells in the rat. Gastroenterology. 1986 Jul;91(1):174–181. doi: 10.1016/0016-5085(86)90455-5. [DOI] [PubMed] [Google Scholar]
- Bhatnagar R., Schirmer R., Ernst M., Decker K. Superoxide release by zymosan-stimulated rat Kupffer cells in vitro. Eur J Biochem. 1981 Sep;119(1):171–175. doi: 10.1111/j.1432-1033.1981.tb05590.x. [DOI] [PubMed] [Google Scholar]
- Campbell P. A., Czuprynski C. J., Cook J. L. Differential expression of macrophage effector functions: bactericidal versus tumoricidal activities. J Leukoc Biol. 1984 Sep;36(3):293–306. doi: 10.1002/jlb.36.3.293. [DOI] [PubMed] [Google Scholar]
- Crocker P. R., Blackwell J. M., Bradley D. J. Expression of the natural resistance gene Lsh in resident liver macrophages. Infect Immun. 1984 Mar;43(3):1033–1040. doi: 10.1128/iai.43.3.1033-1040.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofton R. W., Diesselhoff-den Dulk M. M., van Furth R. The origin, kinetics, and characteristics of the Kupffer cells in the normal steady state. J Exp Med. 1978 Jul 1;148(1):1–17. doi: 10.1084/jem.148.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filice G. A., Beaman B. L., Remington J. S. Effects of activated macrophages on Nacardia asteroides. Infect Immun. 1980 Feb;27(2):643–649. doi: 10.1128/iai.27.2.643-649.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filice G. A., Remington J. S. Coumarin or warfarin treatment of mice does not increase the microbicidal or tumoricidal capacities of macrophages. Br J Exp Pathol. 1981 Apr;62(2):131–135. [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S., Nomoto K., Matsuzaki T., Yokokura T., Mutai M. Oxygen radical production by peritoneal macrophages and Kupffer cells elicited with Lactobacillus casei. Infect Immun. 1984 Apr;44(1):61–67. doi: 10.1128/iai.44.1.61-67.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S., Seyama Y., Yokokura T., Mutai M. Cytotoxic factor production by Kupffer cells elicited with Lactobacillus casei and Corynebacterium parvum. Cancer Immunol Immunother. 1985;20(2):117–121. doi: 10.1007/BF00205677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskill S., Becker S. Disappearance and reappearance of resident macrophages: importance in C. parvum-induced tumoricidal activity. Cell Immunol. 1985 Jan;90(1):179–189. doi: 10.1016/0008-8749(85)90179-0. [DOI] [PubMed] [Google Scholar]
- Keller R., Keist R., Van der Meide P. H., Groscurth P., Aguet M., Leist T. P. Induction, maintenance, and reinduction of tumoricidal activity in bone marrow-derived mononuclear phagocytes by Corynebacterium parvum. Evidence for the involvement of a T cell- and interferon-gamma-independent pathway of macrophage activation. J Immunol. 1987 Apr 1;138(7):2366–2371. [PubMed] [Google Scholar]
- Krahenbuhl J. L., Ruskin J., Remington J. S. The use of killed vaccines in immunization against an intracellular parasite: Toxoplasma gondii. J Immunol. 1972 Feb;108(2):425–431. [PubMed] [Google Scholar]
- Lepay D. A., Nathan C. F., Steinman R. M., Murray H. W., Cohn Z. A. Murine Kupffer cells. Mononuclear phagocytes deficient in the generation of reactive oxygen intermediates. J Exp Med. 1985 May 1;161(5):1079–1096. doi: 10.1084/jem.161.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepay D. A., Steinman R. M., Nathan C. F., Murray H. W., Cohn Z. A. Liver macrophages in murine listeriosis. Cell-mediated immunity is correlated with an influx of macrophages capable of generating reactive oxygen intermediates. J Exp Med. 1985 Jun 1;161(6):1503–1512. doi: 10.1084/jem.161.6.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo S., Nakagawara A., Ikeda K., Mitsuyama M., Nomoto K. Enhanced release of reactive oxygen intermediates by immunologically activated rat Kupffer cells. Clin Exp Immunol. 1985 Jan;59(1):203–209. [PMC free article] [PubMed] [Google Scholar]
- McLeod R., Remington J. S. Studies on the specificity of killing of intracellular pathogens by macrophages. Cell Immunol. 1977 Nov;34(1):156–174. doi: 10.1016/0008-8749(77)90238-6. [DOI] [PubMed] [Google Scholar]
- Miyata H., Himeno K., Nomoto K. Mechanisms of the potentiation of specific antitumor immunity by intratumor injection of Corynebacterium parvum. Cancer Res. 1983 Oct;43(10):4670–4675. [PubMed] [Google Scholar]
- Nacy C. A., Fortier A. H., Meltzer M. S., Buchmeier N. A., Schreiber R. D. Macrophage activation to kill Leishmania major: activation of macrophages for intracellular destruction of amastigotes can be induced by both recombinant interferon-gamma and non-interferon lymphokines. J Immunol. 1985 Nov;135(5):3505–3511. [PubMed] [Google Scholar]
- Nadler P. I., Klingenstein R. J., Richman L. K., Ahmann G. B. The murine Kupffer cell. II. Accessory cell function in in vitro primary antibody responses, mitogen-induced proliferation, and stimulation of mixed lymphocyte responses. J Immunol. 1980 Dec;125(6):2521–2525. [PubMed] [Google Scholar]
- Pace J. L., Russell S. W., Torres B. A., Johnson H. M., Gray P. W. Recombinant mouse gamma interferon induces the priming step in macrophage activation for tumor cell killing. J Immunol. 1983 May;130(5):2011–2013. [PubMed] [Google Scholar]
- Richman L. K., Klingenstein R. J., Richman J. A., Strober W., Berzofsky J. A. The murine Kupffer cell. I. Characterization of the cell serving accessory function in antigen-specific T cell proliferation. J Immunol. 1979 Dec;123(6):2602–2609. [PubMed] [Google Scholar]
- Richman L. R., Strober W., Berzofsky J. A. Genetic control of the immune response to myoglobin. III. Determinant-specific, two Ir gene phenotype is regulated by the genotype of reconstituting Kupffer cells. J Immunol. 1980 Feb;124(2):619–625. [PubMed] [Google Scholar]
- Roberts-Thomson I. C., Stevens D. P., Mahmoud A. A., Warren K. S. Giardiasis in the mouse: an animal model. Gastroenterology. 1976 Jul;71(1):57–61. [PubMed] [Google Scholar]
- Ruskin J., McIntosh J., Remington J. S. Studies on the mechanisms of resistance to phylogenetically diverse intracellular organisms. J Immunol. 1969 Aug;103(2):252–259. [PubMed] [Google Scholar]
- Sherwood E. R., Williams D. L., McNamee R. B., Jones E. L., Browder I. W., Di Luzio N. R. In vitro tumoricidal activity of resting and glucan-activated Kupffer cells. J Leukoc Biol. 1987 Jul;42(1):69–75. doi: 10.1002/jlb.42.1.69. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing E. J., Gardner I. D., Ryning F. W., Remington J. S. Dissociation of effector functions in populations of activated macrophages. Nature. 1977 Aug 18;268(5621):642–644. doi: 10.1038/268642a0. [DOI] [PubMed] [Google Scholar]
- Wing E. J., Remington J. S. Studies on the regulation of lymphocyte reactivity by normal and activated macrophages. Cell Immunol. 1977 Apr;30(1):108–121. doi: 10.1016/0008-8749(77)90052-1. [DOI] [PubMed] [Google Scholar]
- Woodruff M. F., Warner N. L. Effect of Corynebacterium parvum on tumor growth in normal and athymic (nude) mice. J Natl Cancer Inst. 1977 Jan;58(1):111–116. doi: 10.1093/jnci/58.1.11. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- van Dissel J. T., Stikkelbroeck J. J., Michel B. C., van den Barselaar M. T., Leijh P. C., van Furth R. Inability of recombinant interferon-gamma to activate the antibacterial activity of mouse peritoneal macrophages against Listeria monocytogenes and Salmonella typhimurium. J Immunol. 1987 Sep 1;139(5):1673–1678. [PubMed] [Google Scholar]
- van Furth R., Diesselhoff-den Dulk M. M. Dual origin of mouse spleen macrophages. J Exp Med. 1984 Nov 1;160(5):1273–1283. doi: 10.1084/jem.160.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]


