Abstract
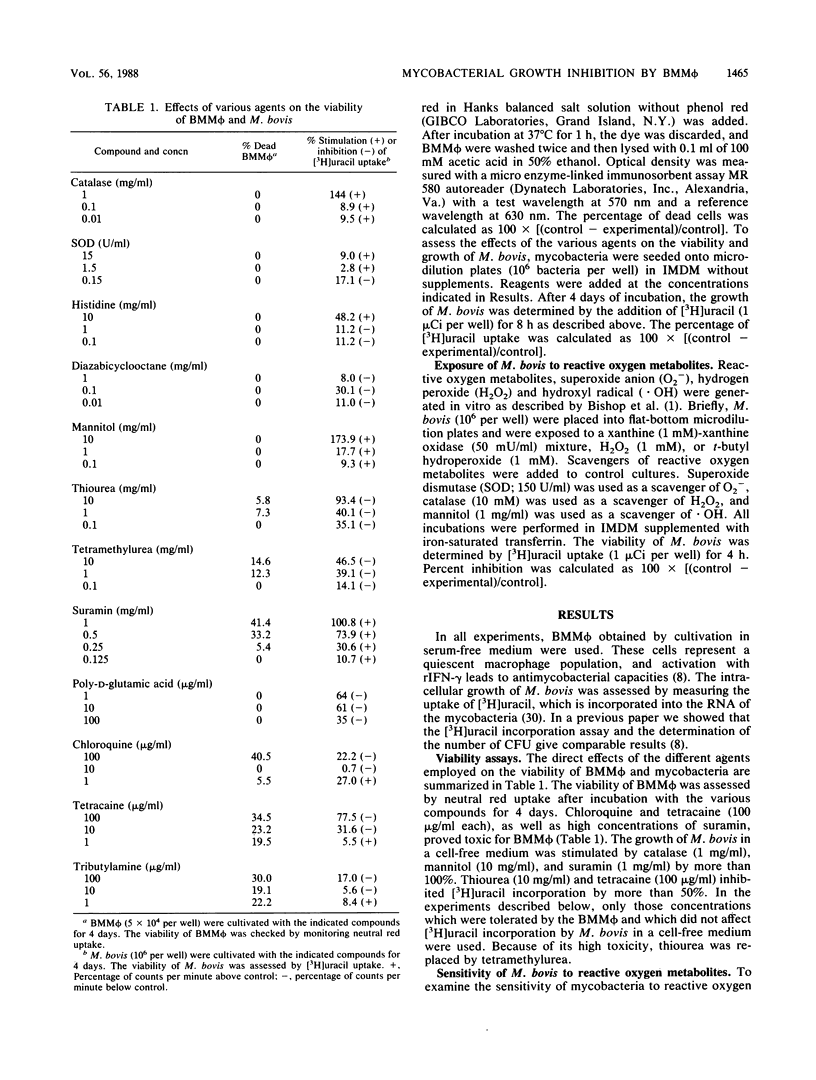
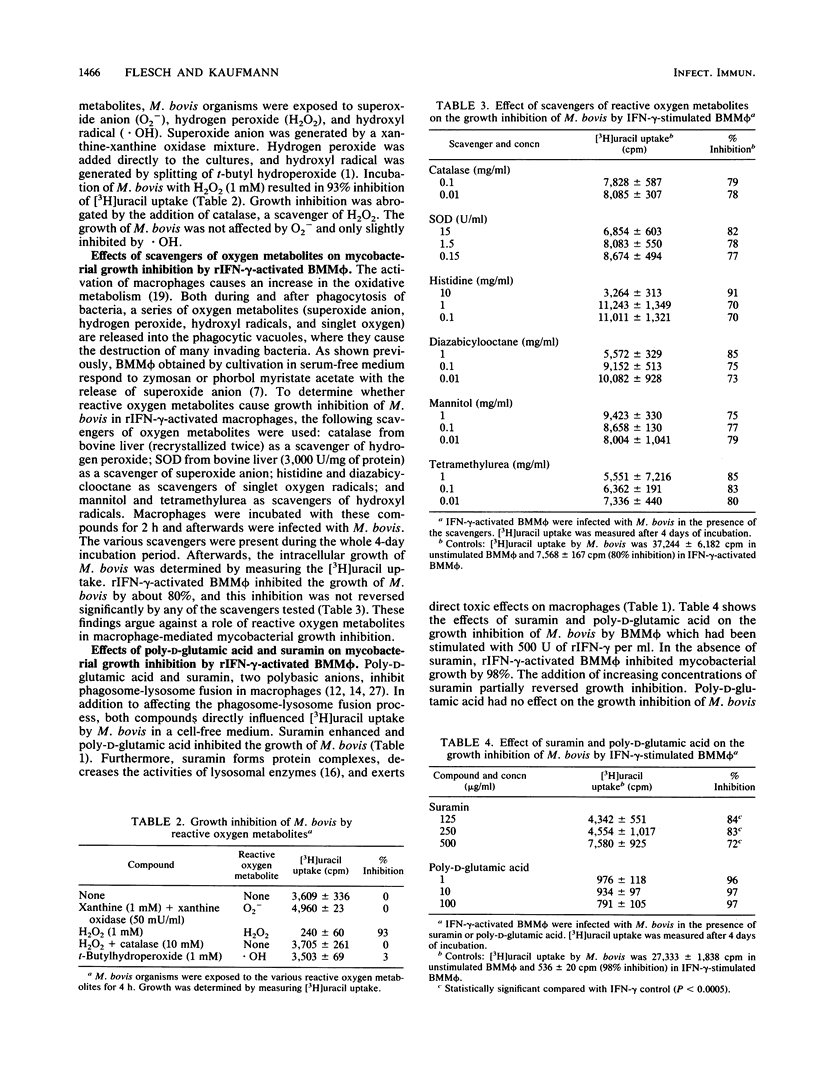
Bone marrow-derived murine macrophages are able to inhibit the growth of Mycobacterium bovis and of some strains of M. tuberculosis after stimulation with either recombinant gamma interferon (rIFN-gamma) or lymphokines from antigen-specific T-cell clones. To elucidate the mechanism(s) involved in antimycobacterial activity, macrophages were infected with M. bovis in the presence of agents thought to influence the antimicrobial effects of phagocytes. Scavengers of toxic oxygen metabolites failed to influence the capacity of IFN-gamma-activated bone marrow macrophages to inhibit the growth of M. bovis. Suramin slightly affected mycobacterial growth in IFN-gamma-activated macrophages, and chloroquine markedly induced growth inhibition of M. bovis in unstimulated macrophages. We conclude that growth inhibition of M. bovis by IFN-gamma-activated macrophages is an oxygen-independent process.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop C. J., Rzepczyk C. M., Stenzel D., Anderson K. The role of reactive oxygen metabolites in lymphocyte-mediated cytolysis. J Cell Sci. 1987 Apr;87(Pt 3):473–481. doi: 10.1242/jcs.87.3.473. [DOI] [PubMed] [Google Scholar]
- Brandely M., Lagrange P., Hurtrel B., Motta I., Truffa-Bachi P. Effects of suramin on the immune responses to sheep red blood cells in mice. I. In vivo studies. Cell Immunol. 1985 Jul;93(2):280–291. doi: 10.1016/0008-8749(85)90134-0. [DOI] [PubMed] [Google Scholar]
- Byrne G. I., Faubion C. L. Lymphokine-mediated microbistatic mechanisms restrict Chlamydia psittaci growth in macrophages. J Immunol. 1982 Jan;128(1):469–474. [PubMed] [Google Scholar]
- Catterall J. R., Sharma S. D., Remington J. S. Oxygen-independent killing by alveolar macrophages. J Exp Med. 1986 May 1;163(5):1113–1131. doi: 10.1084/jem.163.5.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'ARCY HART P., REES R. J. Enchancement of experimental tuberculosis in the mouse by suramin. Tubercle. 1956 Oct;37(5):327–332. doi: 10.1016/s0041-3879(56)80078-0. [DOI] [PubMed] [Google Scholar]
- De Libero G., Kaufmann S. H. Antigen-specific Lyt-2+ cytolytic T lymphocytes from mice infected with the intracellular bacterium Listeria monocytogenes. J Immunol. 1986 Oct 15;137(8):2688–2694. [PubMed] [Google Scholar]
- Flesch I., Ferber E. Growth requirements of murine bone marrow macrophages in serum-free cell culture. Immunobiology. 1986 Mar;171(1-2):14–26. doi: 10.1016/S0171-2985(86)80014-6. [DOI] [PubMed] [Google Scholar]
- Flesch I., Früh A., Ferber E. Functional comparison of bone marrow-derived macrophages obtained by cultivation in serum-free or serum-supplemented medium. Immunobiology. 1986 Oct;173(1):72–81. doi: 10.1016/S0171-2985(86)80091-2. [DOI] [PubMed] [Google Scholar]
- Flesch I., Kaufmann S. H. Mycobacterial growth inhibition by interferon-gamma-activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J Immunol. 1987 Jun 15;138(12):4408–4413. [PubMed] [Google Scholar]
- Goren M. B., Vatter A. E., Fiscus J. Polyanionic agents as inhibitors of phagosome-lysosome fusion in cultured macrophages: evolution of an alternative interpretation. J Leukoc Biol. 1987 Feb;41(2):111–121. doi: 10.1002/jlb.41.2.111. [DOI] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Young M. R. Interference with normal phagosome-lysosome fusion in macrophages, using ingested yeast cells and suramin. Nature. 1975 Jul 3;256(5512):47–49. doi: 10.1038/256047a0. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Young M. R. Manipulations of the phagosome-lysosome fusion response in cultured macrophages. Enhancement of fusion by chloroquine and other amines. Exp Cell Res. 1978 Jul;114(2):486–490. doi: 10.1016/0014-4827(78)90516-5. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Young M. R. The effect of inhibitors and enhancers of phagosome--lysosome fusion in cultured macrophages on the phagosome membranes of ingested yeasts. Exp Cell Res. 1979 Feb;118(2):365–375. doi: 10.1016/0014-4827(79)90160-5. [DOI] [PubMed] [Google Scholar]
- Hawking F. Suramin: with special reference to onchocerciasis. Adv Pharmacol Chemother. 1978;15:289–322. doi: 10.1016/s1054-3589(08)60486-x. [DOI] [PubMed] [Google Scholar]
- Jackett P. S., Aber V. R., Mitchison D. A., Lowrie D. B. The contribution of hydrogen peroxide resistance to virulence of Mycobacterium tuberculosis during the first six days after intravenous infection of normal and BCG-vaccinated guinea-pigs. Br J Exp Pathol. 1981 Feb;62(1):34–40. [PMC free article] [PubMed] [Google Scholar]
- Jackett P. S., Andrew P. W., Aber V. R., Lowrie D. B. Guinea pig alveolar macrophages probably kill M. tuberculosis H37Rv and H37Ra in vivo by producing hydrogen peroxide. Adv Exp Med Biol. 1983;162:99–104. doi: 10.1007/978-1-4684-4481-0_10. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Flesch I. Function and antigen recognition pattern of L3T4+ T-cell clones from Mycobacterium tuberculosis-immune mice. Infect Immun. 1986 Nov;54(2):291–296. doi: 10.1128/iai.54.2.291-296.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner H., Keyssner M., Stünkel K. G. Activation of murine B lymphocytes by suramin. Cell Immunol. 1986 Apr 1;98(2):560–563. doi: 10.1016/0008-8749(86)90316-3. [DOI] [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. I. Susceptibility of Toxoplasma gondii to oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):938–949. doi: 10.1084/jem.150.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Juangbhanich C. W., Nathan C. F., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. II. The role of oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):950–964. doi: 10.1084/jem.150.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Carriero S. M., Harris A. M., Jaffee E. A. Human mononuclear phagocyte antiprotozoal mechanisms: oxygen-dependent vs oxygen-independent activity against intracellular Toxoplasma gondii. J Immunol. 1985 Mar;134(3):1982–1988. [PubMed] [Google Scholar]
- Parish C. R., Müllbacher A. Automated colorimetric assay for T cell cytotoxicity. J Immunol Methods. 1983 Mar 11;58(1-2):225–237. doi: 10.1016/0022-1759(83)90277-6. [DOI] [PubMed] [Google Scholar]
- Pesanti E. L. Suramin effects on macrophage phagolysosome formation and antimicrobial activity. Infect Immun. 1978 May;20(2):503–511. doi: 10.1128/iai.20.2.503-511.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci U S A. 1984 Feb;81(3):908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermel C. D., Rubin B. Y., Jaffe E. A., Murray H. W. Oxygen-independent inhibition of intracellular Chlamydia psittaci growth by human monocytes and interferon-gamma-activated macrophages. J Immunol. 1986 Jul 15;137(2):689–692. [PubMed] [Google Scholar]
- Walker L., Lowrie D. B. Killing of Mycobacterium microti by immunologically activated macrophages. Nature. 1981 Sep 3;293(5827):69–71. doi: 10.1038/293069a0. [DOI] [PubMed] [Google Scholar]


