Abstract
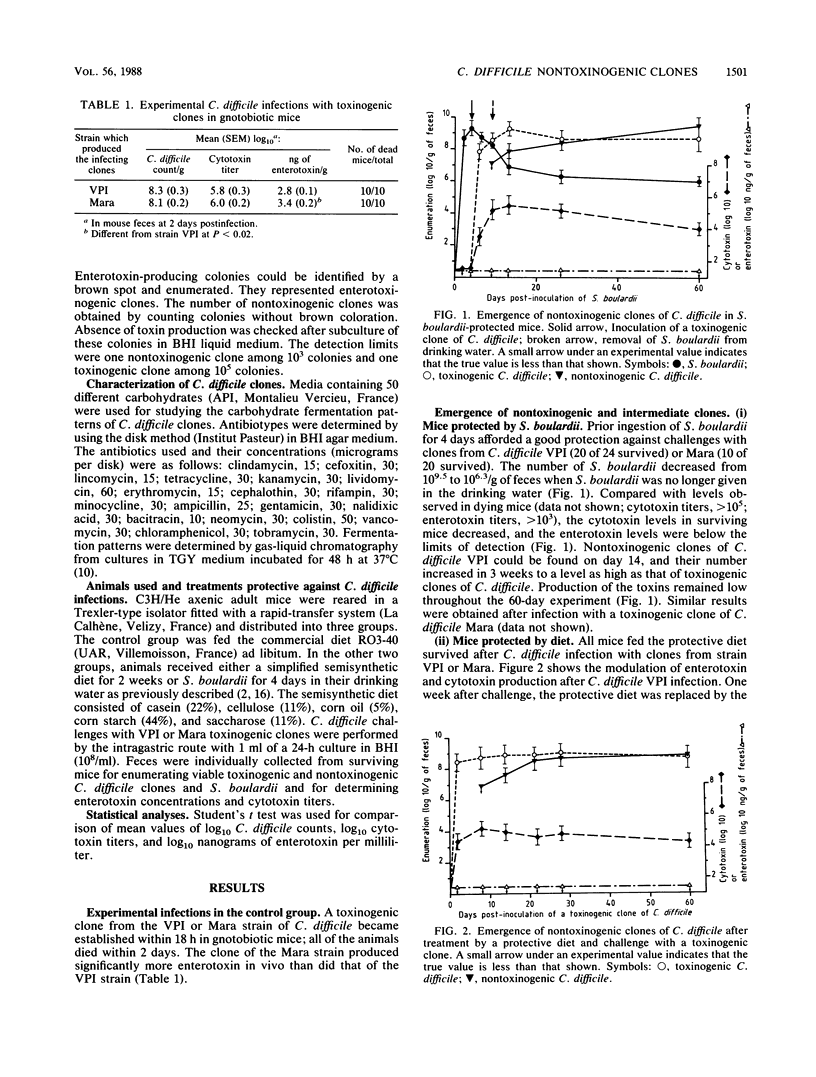
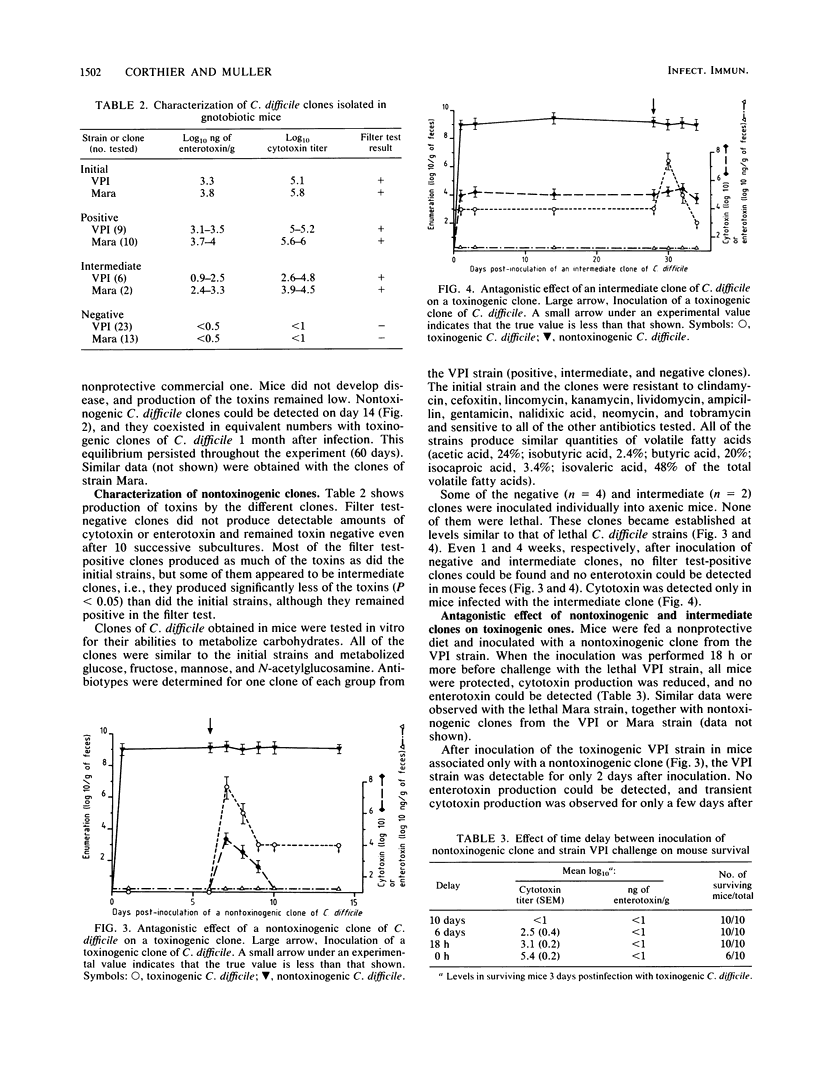
In previous studies, we showed that diet composition or Saccharomyces boulardii ingestion could protect gnotobiotic mice against lethal Clostridium difficile infection. Using an original method, we detected nontoxinogenic clones from feces of protected mice challenged with a toxinogenic clone of C. difficile. These clones became established at the same level as the toxinogenic one after about 30 days. In these protected mice bearing nontoxinogenic clones, no enterotoxin production could be detected and cytotoxin titers were highly reduced. These nontoxinogenic clones were genetically stable because nontoxinogenic clones and clones that produce intermediate levels of toxins in vivo did not revert to toxin production, even after repeated culture in vitro. Furthermore, the nontoxinogenic clones were shown to arise from a single toxinogenic clone and were identical to that clone in metabolic patterns and antibiotic sensitivity tests. When mice fed a nonprotective diet were challenged with a nontoxinogenic or intermediate clone, they remained healthy and no toxin production could be detected in their feces. Moreover, these mice were protected against further infections with toxinogenic strains of C. difficile, and a strong antagonism between nontoxinogenic and toxinogenic clones was observed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett J. G., Chang T. W., Gurwith M., Gorbach S. L., Onderdonk A. B. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978 Mar 9;298(10):531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- Corthier G., Dubos F., Ducluzeau R. Prevention of Clostridium difficile induced mortality in gnotobiotic mice by Saccharomyces boulardii. Can J Microbiol. 1986 Nov;32(11):894–896. doi: 10.1139/m86-164. [DOI] [PubMed] [Google Scholar]
- Corthier G., Dubos F., Raibaud P. Ability of two Clostridium difficile strains from man and hare to produce cytotoxin in vitro and in gnotobiotic rodent intestines. Ann Inst Pasteur Microbiol. 1986 Jul-Aug;137B(1):113–121. doi: 10.1016/s0769-2609(86)80098-9. [DOI] [PubMed] [Google Scholar]
- Corthier G., Dubos F., Raibaud P. Modulation of cytotoxin production by Clostridium difficile in the intestinal tracts of gnotobiotic mice inoculated with various human intestinal bacteria. Appl Environ Microbiol. 1985 Jan;49(1):250–252. doi: 10.1128/aem.49.1.250-252.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Johnson W. J., Balish E., Wilkins T. Pseudomembranous colitis in Clostridium difficile-monoassociated rats. Infect Immun. 1983 Mar;39(3):1368–1376. doi: 10.1128/iai.39.3.1368-1376.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval-Iflah Y., Raibaud P., Rousseau M. Antagonisms among isogenic strains of Escherichia coli in the digestive tracts of gnotobiotic mice. Infect Immun. 1981 Dec;34(3):957–969. doi: 10.1128/iai.34.3.957-969.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine O., Ducluzeau R., Raibaud P., Chabanet C., Popoff M. R., Badoual J., Gabilan J. C., Andremont A. Comparaison entre le nombre et la nature des Clostridium fécaux et d'autres facteurs de risque impliqués dans la pathologie intestinale des nouveau-nés. Ann Inst Pasteur Microbiol. 1986 Jul-Aug;137B(1):61–75. [PubMed] [Google Scholar]
- Freter R., Stauffer E., Cleven D., Holdeman L. V., Moore W. E. Continuous-flow cultures as in vitro models of the ecology of large intestinal flora. Infect Immun. 1983 Feb;39(2):666–675. doi: 10.1128/iai.39.2.666-675.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George R. H., Symonds J. M., Dimock F., Brown J. D., Arabi Y., Shinagawa N., Keighley M. R., Alexander-Williams J., Burdon D. W. Identification of Clostridium difficile as a cause of pseudomembranous colitis. Br Med J. 1978 Mar 18;1(6114):695–695. doi: 10.1136/bmj.1.6114.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson H. E., Barclay F. E., Honour P., Hill I. D. Epidemiology of Clostridium difficile in infants. J Infect Dis. 1982 Dec;146(6):727–733. doi: 10.1093/infdis/146.6.727. [DOI] [PubMed] [Google Scholar]
- Larson H. E., Price A. B., Honour P., Borriello S. P. Clostridium difficile and the aetiology of pseudomembranous colitis. Lancet. 1978 May 20;1(8073):1063–1066. doi: 10.1016/s0140-6736(78)90912-1. [DOI] [PubMed] [Google Scholar]
- Libby J. M., Jortner B. S., Wilkins T. D. Effects of the two toxins of Clostridium difficile in antibiotic-associated cecitis in hamsters. Infect Immun. 1982 May;36(2):822–829. doi: 10.1128/iai.36.2.822-829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Lockwood D. E., Richardson S. H., Wilkins T. D. Biological activities of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1147–1150. doi: 10.1128/iai.35.3.1147-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahe S., Corthier G., Dubos F. Effect of various diets on toxin production by two strains of Clostridium difficile in gnotobiotic mice. Infect Immun. 1987 Aug;55(8):1801–1805. doi: 10.1128/iai.55.8.1801-1805.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massot J., Sanchez O., Couchy R., Astoin J., Parodi A. L. Bacterio-pharmacological activity of Saccharomyces boulardii in clindamycin-induced colitis in the hamster. Arzneimittelforschung. 1984;34(7):794–797. [PubMed] [Google Scholar]
- Stark P. L., Lee A., Parsonage B. D. Colonization of the large bowel by Clostridium difficile in healthy infants: quantitative study. Infect Immun. 1982 Mar;35(3):895–899. doi: 10.1128/iai.35.3.895-899.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan N. M., Pellett S., Wilkins T. D. Purification and characterization of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1032–1040. doi: 10.1128/iai.35.3.1032-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toothaker R. D., Elmer G. W. Prevention of clindamycin-induced mortality in hamsters by Saccharomyces boulardii. Antimicrob Agents Chemother. 1984 Oct;26(4):552–556. doi: 10.1128/aac.26.4.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytgat F. Fréquence d'isolement de Clostridium difficile dans les selles de malades hospitalisés: sensibilité aux antibiotiques des souches isolées. Ann Microbiol (Paris) 1980 Jul-Aug;131B(1):11–20. [PubMed] [Google Scholar]
- Wilson K. H., Sheagren J. N. Antagonism of toxigenic Clostridium difficile by nontoxigenic C. difficile. J Infect Dis. 1983 Apr;147(4):733–736. doi: 10.1093/infdis/147.4.733. [DOI] [PubMed] [Google Scholar]
- Wilson K. H., Sheagren J. N., Freter R., Weatherbee L., Lyerly D. Gnotobiotic models for study of the microbial ecology of Clostridium difficile and Escherichia coli. J Infect Dis. 1986 Mar;153(3):547–551. doi: 10.1093/infdis/153.3.547. [DOI] [PubMed] [Google Scholar]


