Abstract
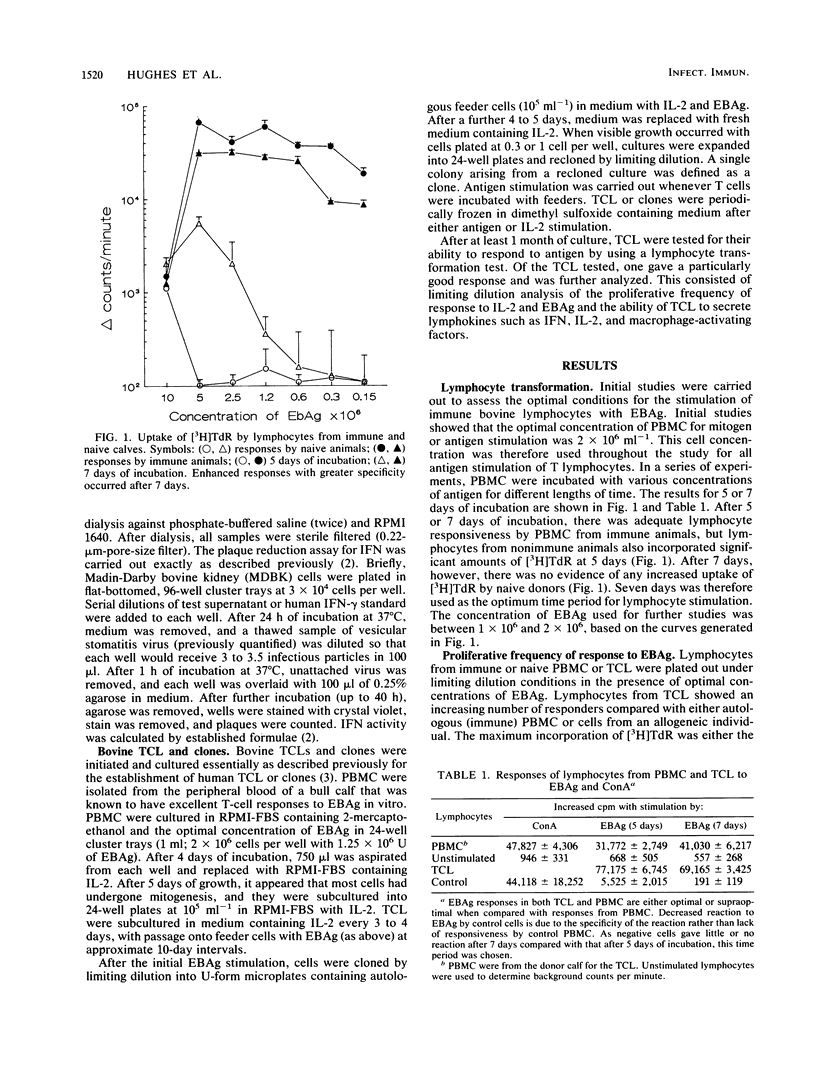
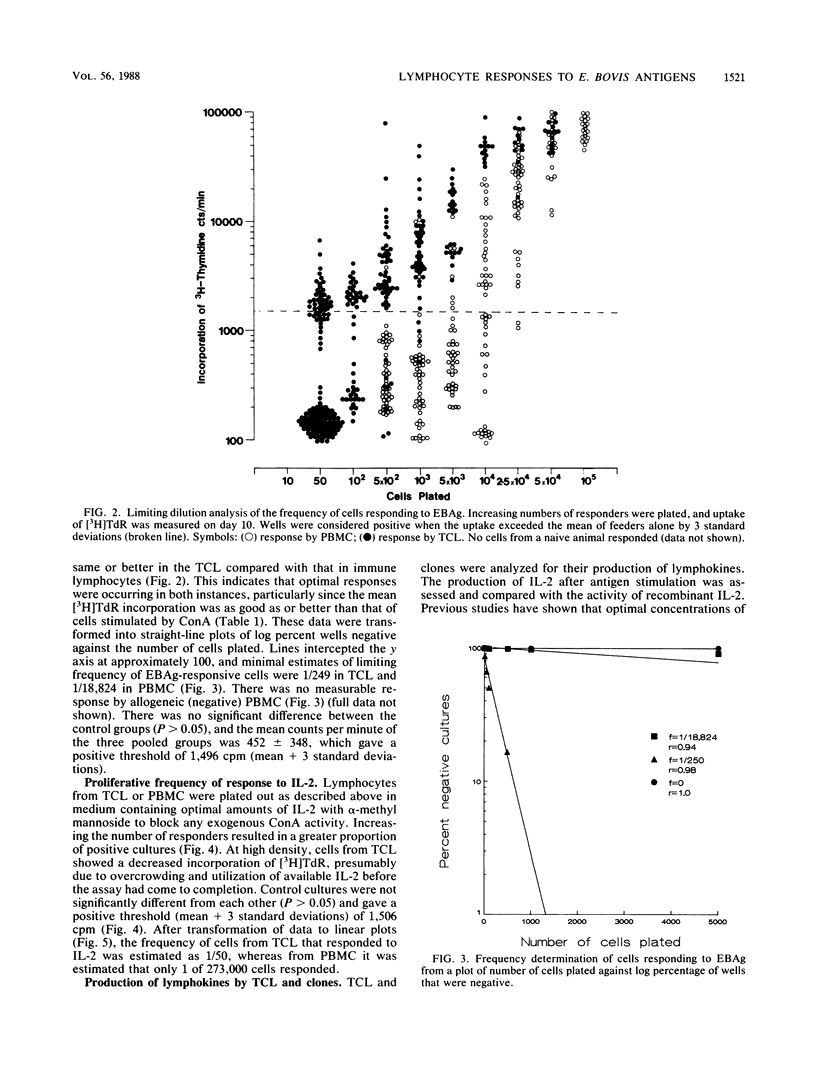
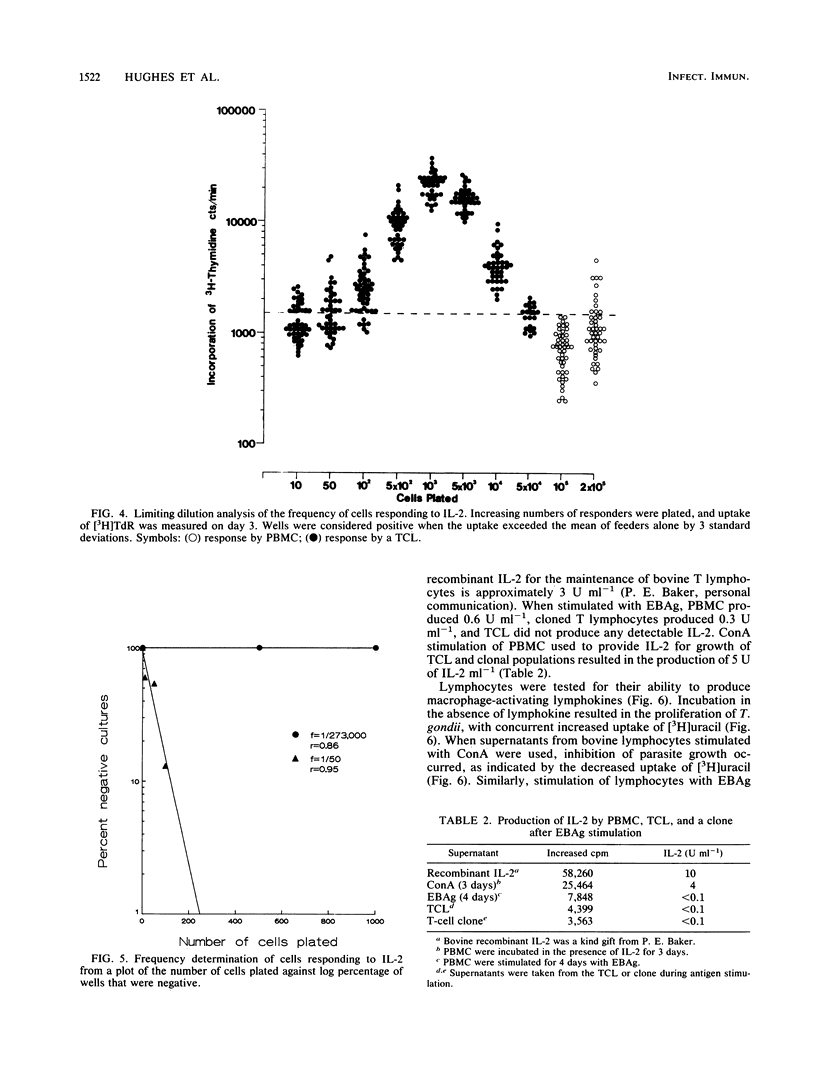
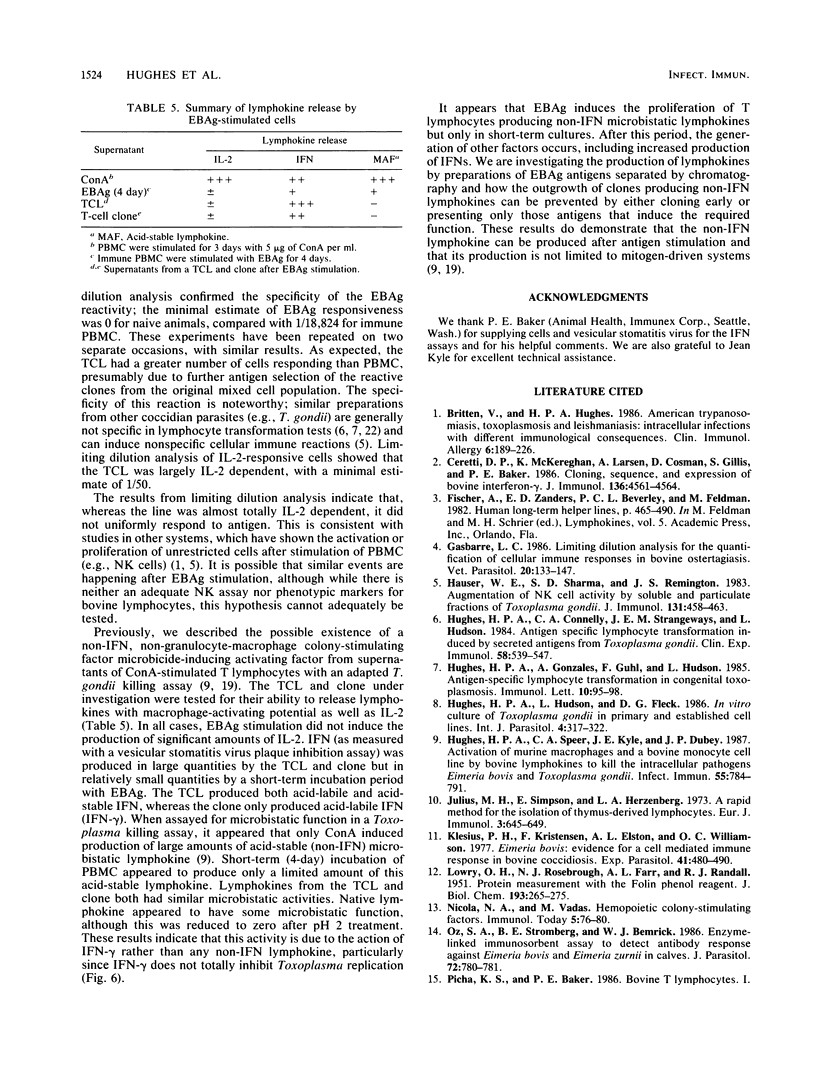
Lymphoproliferative responses against a preparation of Eimeria bovis antigens (EBAg) were measured in E. bovis-immune and naive animals. Optimal lymphocyte responsiveness could be measured after 7 days of culture in the presence of antigen at a cell concentration of 2 X 10(5) cells per well. The specificity of the reaction was confirmed by limiting dilution analysis. Whereas immune peripheral blood mononuclear cells responded to EBAg (f = 1/18,824), naive cells did not (f = 0). The helper function of cells proliferating in response to EBAg was investigated by raising T-cell lines and a clonal population derived from a line. The T-cell line showed an enhanced reactivity to EBAg by limiting dilution analysis (f = 1/256) and was interleukin-2 dependent. Limiting dilution analyses indicated at least two populations of cells: one that was interleukin-2 restricted and antigen dependent and another that was antigen independent. Supernatants from T-cell lines and the clone were analyzed for the production of lymphokines after antigen stimulation. Minimal amounts of interleukin-2 were produced. The T-cell line produced both gamma interferon (IFN-gamma) (750 U) and IFN-alpha (1,250 U), whereas the clone produced IFN-gamma (1,250 U) only. Short-term (4-day) stimulation of immune cells by EBAg induced the production of IFN-gamma (600 U) and a non-IFN macrophage-activating lymphokine. We conclude that this macrophage-activating lymphokine is only produced after short-term culture and that further culture of T cells results in the proliferation of other clones producing other factors (such as IFN).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cerretti D. P., McKereghan K., Larsen A., Cosman D., Gillis S., Baker P. E. Cloning, sequence, and expression of bovine interferon-gamma. J Immunol. 1986 Jun 15;136(12):4561–4564. [PubMed] [Google Scholar]
- Gasbarre L. C. Limiting dilution analyses for the quantification of cellular immune responses in bovine ostertagiasis. Vet Parasitol. 1986 Mar;20(1-3):133–147. doi: 10.1016/0304-4017(86)90097-x. [DOI] [PubMed] [Google Scholar]
- Hauser W. E., Jr, Sharma S. D., Remington J. S. Augmentation of NK cell activity by soluble and particulate fractions of Toxoplasma gondii. J Immunol. 1983 Jul;131(1):458–463. [PubMed] [Google Scholar]
- Hughes H. P., Connelly C. A., Strangeways J. E., Hudson L. Antigen specific lymphocyte transformation induced by secreted antigens from Toxoplasma gondii. Clin Exp Immunol. 1984 Dec;58(3):539–547. [PMC free article] [PubMed] [Google Scholar]
- Hughes H. P., Gonzalez A., Guhl F., Hudson L. Antigen-specific lymphocyte transformation in congenital toxoplasmosis. Immunol Lett. 1985;10(2):95–98. doi: 10.1016/0165-2478(85)90182-8. [DOI] [PubMed] [Google Scholar]
- Hughes H. P., Hudson L., Fleck D. G. In vitro culture of Toxoplasma gondii in primary and established cell lines. Int J Parasitol. 1986 Aug;16(4):317–322. doi: 10.1016/0020-7519(86)90109-8. [DOI] [PubMed] [Google Scholar]
- Hughes H. P., Speer C. A., Kyle J. E., Dubey J. P. Activation of murine macrophages and a bovine monocyte cell line by bovine lymphokines to kill the intracellular pathogens Eimeria bovis and Toxoplasma gondii. Infect Immun. 1987 Mar;55(3):784–791. doi: 10.1128/iai.55.3.784-791.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Klesius P. H., Kristensen F., Elston A. L., Williamson O. C. Eimeria bovis: evidence for a cell-mediated immune response in bovine coccidiosis. Exp Parasitol. 1977 Apr;41(2):480–490. doi: 10.1016/0014-4894(77)90120-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Oz H. S., Stromberg B. E., Bemrick W. J. Enzyme-linked immunosorbent assay to detect antibody response against Eimeria bovis and Eimeria zurnii in calves. J Parasitol. 1986 Oct;72(5):780–781. [PubMed] [Google Scholar]
- Picha K. S., Baker P. E. Bovine T lymphocytes. I. Generation and maintenance of an interleukin-2-dependent, cytotoxic T-lymphocyte cell line. Immunology. 1986 Jan;57(1):131–136. [PMC free article] [PubMed] [Google Scholar]
- Rabin H., Hopkins R. F., 3rd, Ruscetti F. W., Neubauer R. H., Brown R. L., Kawakami T. G. Spontaneous release of a factor with properties of T cell growth factor from a continuous line of primate tumor T cells. J Immunol. 1981 Nov;127(5):1852–1856. [PubMed] [Google Scholar]
- Ralph P., Nacy C. A., Meltzer M. S., Williams N., Nakoinz I., Leonard E. J. Colony-stimulating factors and regulation of macrophage tumoricidal and microbicidal activities. Cell Immunol. 1983 Feb 15;76(1):10–21. doi: 10.1016/0008-8749(83)90343-x. [DOI] [PubMed] [Google Scholar]
- Reduker D. W., Speer C. A. Proteins and antigens of merozoites and sporozoites of Eimeria bovis (Apicomplexa). J Parasitol. 1986 Dec;72(6):901–907. [PubMed] [Google Scholar]
- Speer C. A., Reduker D. W., Burgess D. E., Whitmire W. M., Splitter G. A. Lymphokine-induced inhibition of growth of Eimeria bovis and Eimeria papillata (Apicomplexa) in cultured bovine monocytes. Infect Immun. 1985 Nov;50(2):566–571. doi: 10.1128/iai.50.2.566-571.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981 Apr;126(4):1614–1619. [PubMed] [Google Scholar]
- Vose B. M. Quantitation of proliferative and cytotoxic precursor cells directed against human tumours: limiting dilution analysis in peripheral blood and at the tumour site. Int J Cancer. 1982 Aug 15;30(2):135–142. doi: 10.1002/ijc.2910300202. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Desmonts G., Couvreur J., Remington J. S. Lymphocyte transformation in the diagnosis of congenital toxoplasma infection. N Engl J Med. 1980 Apr 3;302(14):785–788. doi: 10.1056/NEJM198004033021406. [DOI] [PubMed] [Google Scholar]


