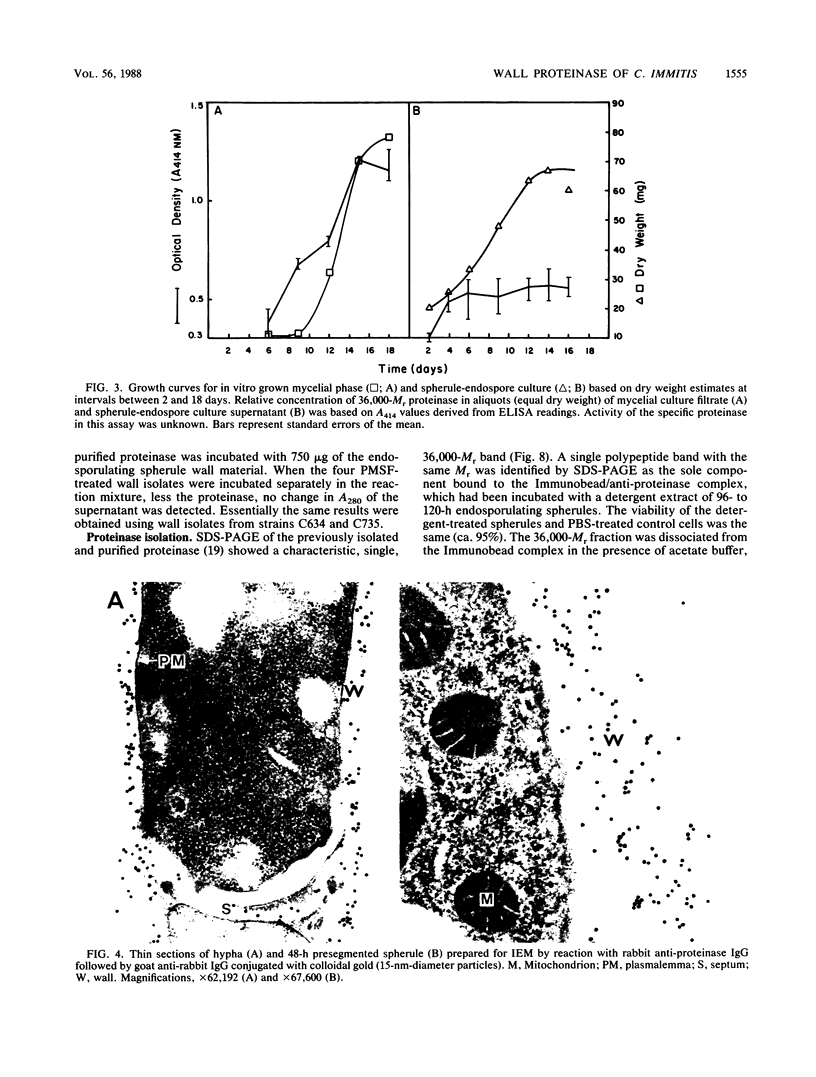
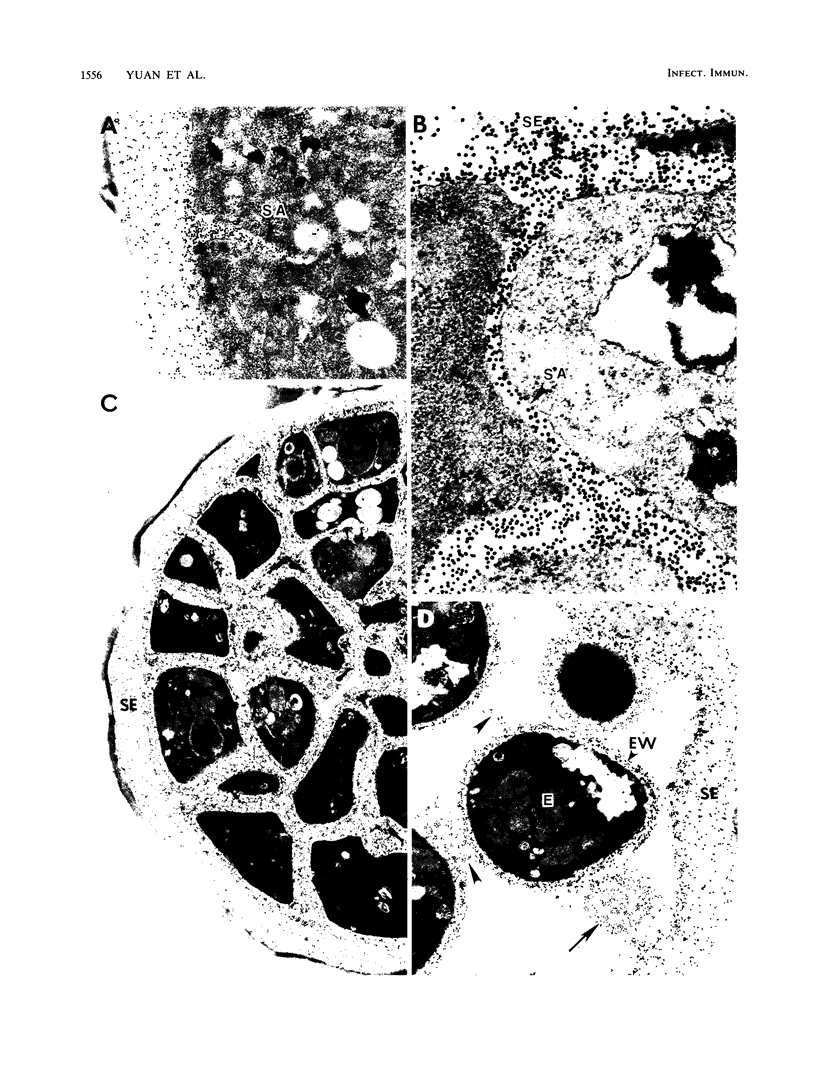
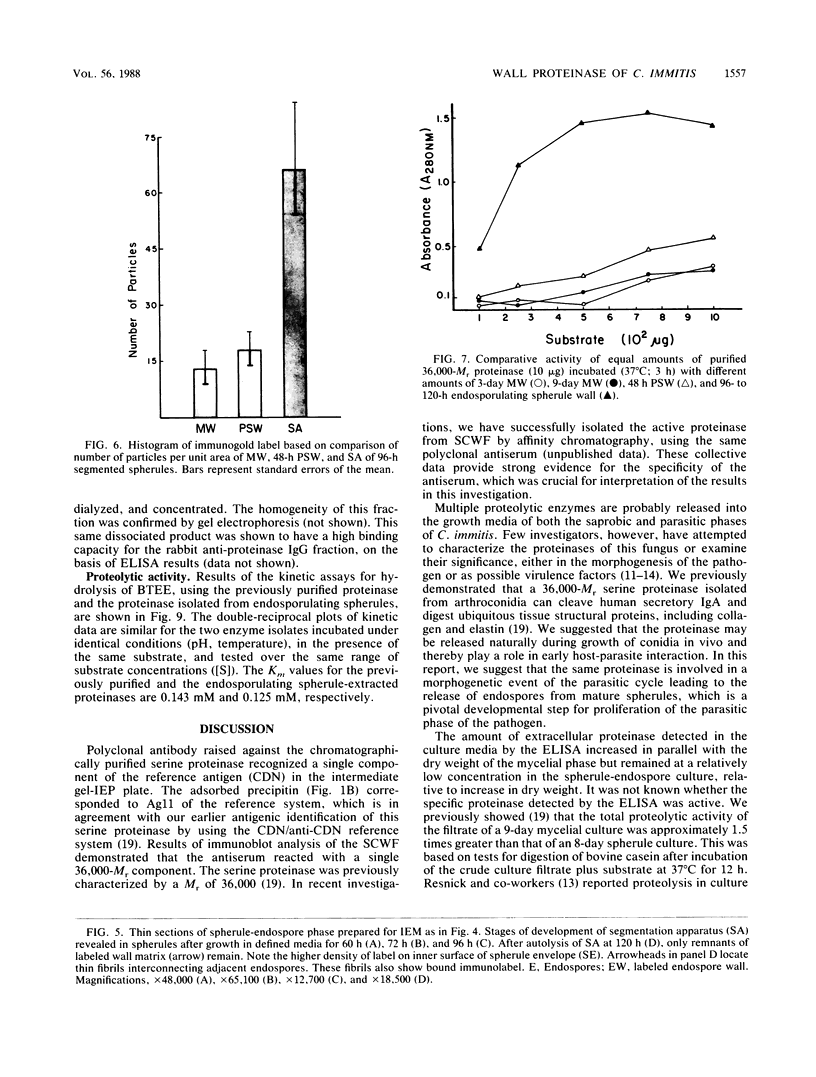
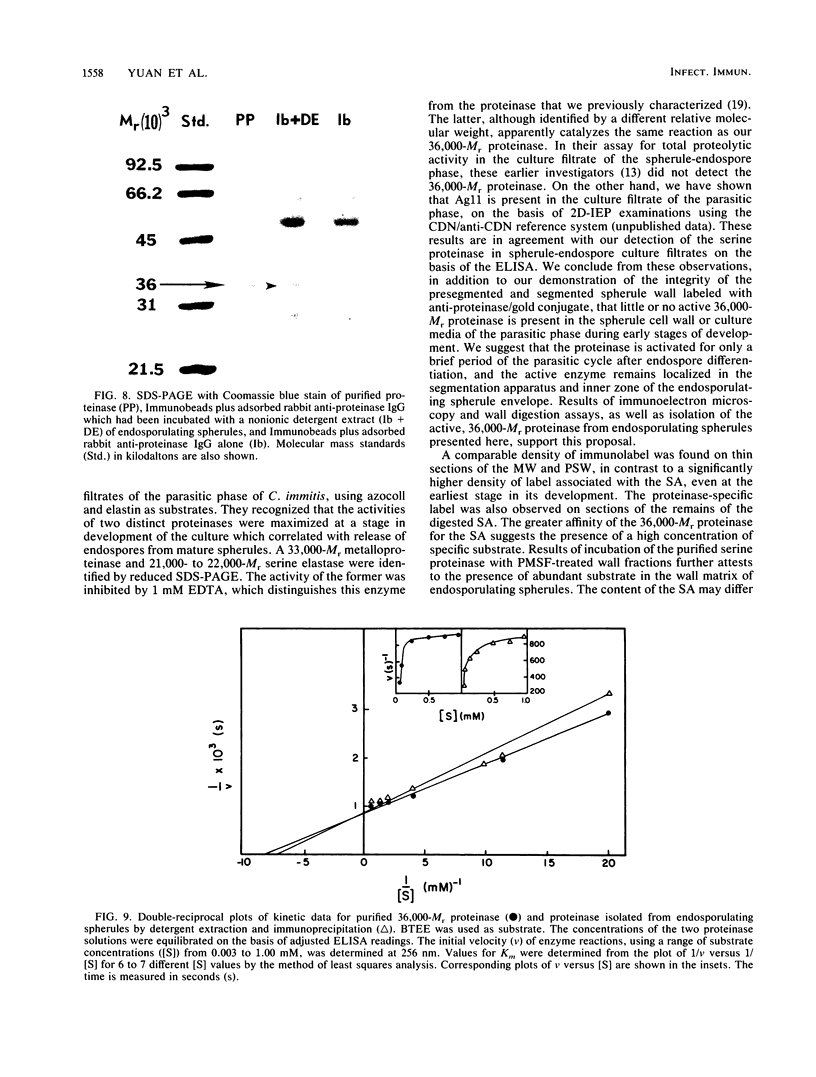
Abstract
We previously reported isolation of a serine proteinase from the soluble conidial wall fraction of Coccidioides immitis. The purified proteinase was identified as a polypeptide band of 36,000 Mr by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. In this study, we raised monospecific antiserum in rabbits against the purified proteinase for use in immunoelectron microscopy. We showed that immunolabel was localized in the cell wall of both the saprobic and parasitic phases but was most concentrated in the wall of the segmentation apparatus of spherules just prior to endospore differentiation. The total wall fractions of the mycelial phase, as well as those of presegmented and endosporulating spherules, were isolated from in vitro grown cells and then treated with a proteinase inhibitor (phenylmethylsulfonyl fluoride [PMSF]) which irreversibly binds to the residual proteolytic enzyme in the wall isolates. Each fraction was dialyzed, lyophilized, and separately incubated with the active, purified 36,000-Mr proteinase. The reaction mixtures were examined spectrophotometrically (A280) for decomposition of the substrates. Only the PMSF-treated wall isolated from endosporulating spherules was significantly digested. Active, 36,000-Mr proteinase was isolated from intact and viable, endosporulating spherules by brief extraction of the cells with 1% octyl-beta-D-thioglucoside, a nonionic detergent. The serine proteinase may be partly responsible for autolysis of the segmentation apparatus of mature spherules, a morphogenetic process which is pivotal for release of endospores and subsequent proliferation of the pathogen.
Full text
PDF



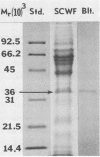
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cole G. T., Kirkland T. N., Sun S. H. An immunoreactive, water-soluble conidial wall fraction of Coccidioides immitis. Infect Immun. 1987 Mar;55(3):657–667. doi: 10.1128/iai.55.3.657-667.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott B. W., Jr, Cohen C. Isolation and characterization of a lysine-specific protease from Pseudomonas aeruginosa. J Biol Chem. 1986 Aug 25;261(24):11259–11265. [PubMed] [Google Scholar]
- Huppert M., Adler J. P., Rice E. H., Sun S. H. Common antigens among systemic disease fungi analyzed by two-dimensional immunoelectrophoresis. Infect Immun. 1979 Feb;23(2):479–485. doi: 10.1128/iai.23.2.479-485.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert M., Spratt N. S., Vukovich K. R., Sun S. H., Rice E. H. Antigenic analysis of coccidioidin and spherulin determined by two-dimensional immunoelectrophoresis. Infect Immun. 1978 May;20(2):541–551. doi: 10.1128/iai.20.2.541-551.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert M., Sun S. H., Harrison J. L. Morphogenesis throughout saprobic and parasitic cycles of Coccidioides immitis. Mycopathologia. 1982 May 22;78(2):107–122. doi: 10.1007/BF00442634. [DOI] [PubMed] [Google Scholar]
- LEVINE H. B. Purification of the spherule-endospore phase of Coccidioides immitis. Sabouraudia. 1961 Jun;1:112–115. doi: 10.1080/00362176285190231. [DOI] [PubMed] [Google Scholar]
- Lupan D. M., Nziramasanga P. Collagenolytic activity of Coccidioides immitis. Infect Immun. 1986 Jan;51(1):360–361. doi: 10.1128/iai.51.1.360-361.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nziramasanga P., Lupan D. M. Elastase activity of Coccidioides immitis. J Med Microbiol. 1985 Feb;19(1):109–114. doi: 10.1099/00222615-19-1-109. [DOI] [PubMed] [Google Scholar]
- Resnick S., Pappagianis D., McKerrow J. H. Proteinase production by the parasitic cycle of the pathogenic fungus Coccidioides immitis. Infect Immun. 1987 Nov;55(11):2807–2815. doi: 10.1128/iai.55.11.2807-2815.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippon J. W., Varadi D. P. The elastases of pathogenic fungi and actinomycetes. J Invest Dermatol. 1968 Jan;50(1):54–58. doi: 10.1038/jid.1968.8. [DOI] [PubMed] [Google Scholar]
- Sun S. H., Cole G. T., Drutz D. J., Harrison J. L. Electron-microscopic observations of the Coccidioides immitis parasitic cycle in vivo. J Med Vet Mycol. 1986 Jun;24(3):183–192. [PubMed] [Google Scholar]
- Sun S. H., Sekhon S. S., Huppert M. Electron microscopic studies of saprobic and parasitic forms of Coccidioides immitis. Sabouraudia. 1979 Sep;17(3):265–273. doi: 10.1080/00362177985380391. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turini P., Kurooka S., Steer M., Corbascio A. N., Singer T. P. The action of phenylmethylsulfonyl fluoride on human acetylcholinesterase, chymotyrpsin and trypsin. J Pharmacol Exp Ther. 1969 May;167(1):98–104. [PubMed] [Google Scholar]
- Yuan L., Cole G. T. Isolation and characterization of an extracellular proteinase of Coccidioides immitis. Infect Immun. 1987 Sep;55(9):1970–1978. doi: 10.1128/iai.55.9.1970-1978.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]