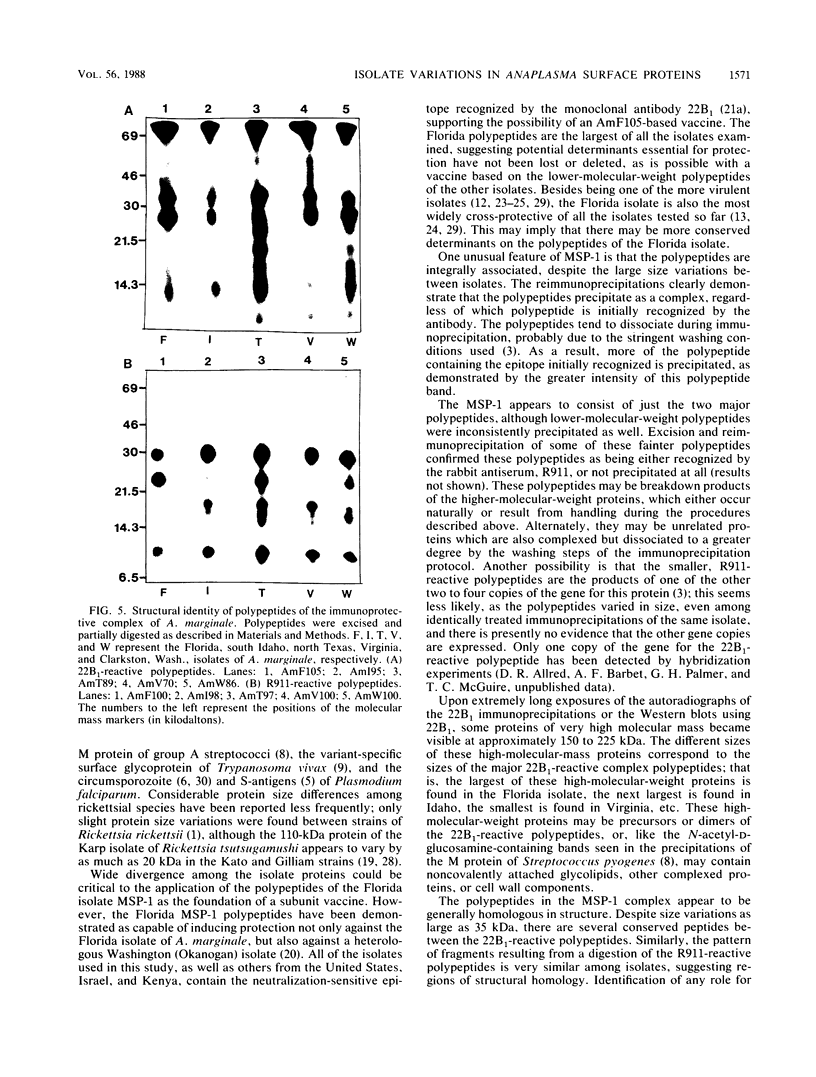
Abstract
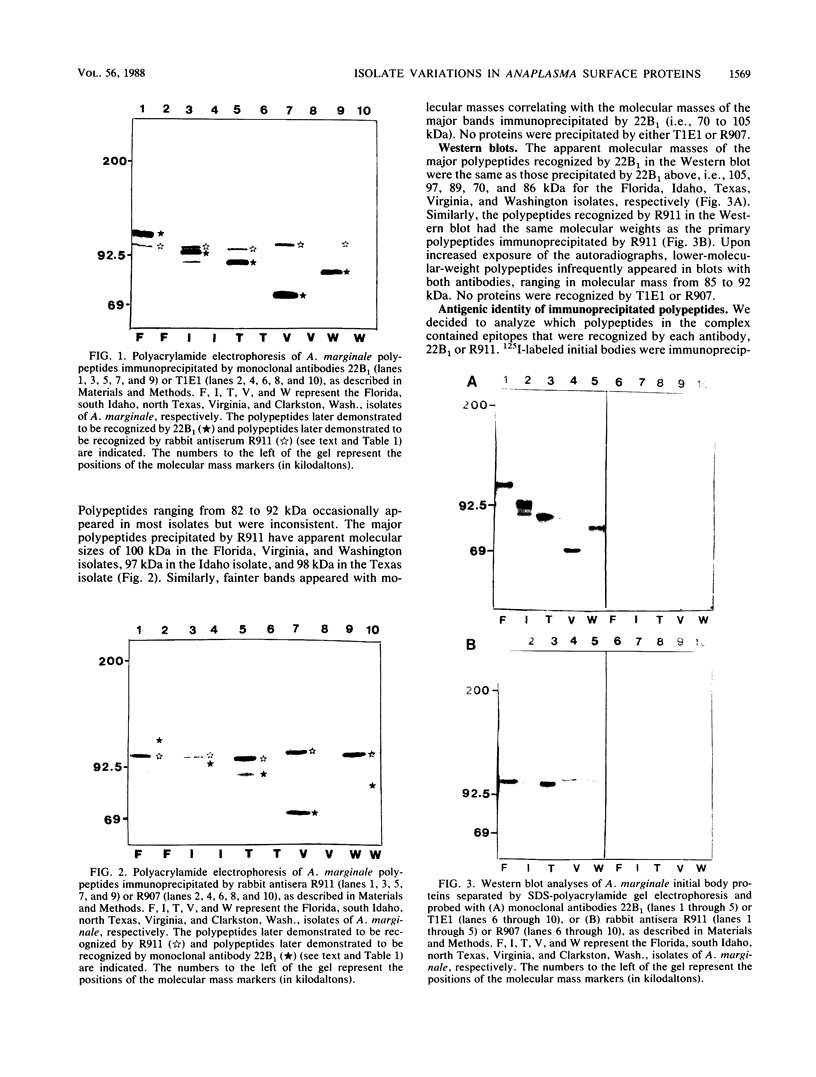
A major surface protein complex from the Florida isolate of Anaplasma marginale has been previously shown to induce protection in immunized cattle and has been proposed as the basis of a subunit vaccine against anaplasmosis. This complex in the Florida isolate is composed of two noncovalently associated polypeptides with molecular masses of 105 and 100 kilodaltons (kDa). The analogous protein complex from four geographically different isolates of A. marginale was immunoprecipitated and compared with the protein complex of the Florida isolate. The polypeptides of the complex varied in apparent molecular mass among the isolates. By using antibodies recognizing epitopes on each polypeptide of the Florida isolate, the antigenic identity of the polypeptides in the analogous complexes was determined. The polypeptides recognized by the neutralizing monoclonal antibody 22B1, which recognizes a 105-kDa polypeptide in the Florida isolate, ranged from 70 to 100 kDa in the other isolates. Those polypeptides recognized by rabbit antiserum R911, which recognizes a 100-kDa polypeptide in the Florida isolate, ranged from 97 to 100 kDa. The surface-exposed peptides in the complexes were compared by limited enzymatic digestion to assess structural homology among isolates. Despite the marked variations in molecular weight, there were conserved peptides between the 22B1-reactive polypeptides and between the R911-reactive peptides. Determination of the role of the conserved peptides in inducing immunity will be critical in the application of these polypeptides as the basis of a subunit vaccine for bovine anaplasmosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anacker R. L., List R. H., Mann R. E., Wiedbrauk D. L. Antigenic heterogeneity in high- and low-virulence strains of Rickettsia rickettsii revealed by monoclonal antibodies. Infect Immun. 1986 Feb;51(2):653–660. doi: 10.1128/iai.51.2.653-660.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbet A. F., Anderson L. W., Palmer G. H., McGuire T. C. Comparison of proteins synthesized by two different isolates of Anaplasma marginale. Infect Immun. 1983 Jun;40(3):1068–1074. doi: 10.1128/iai.40.3.1068-1074.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbet A. F., Palmer G. H., Myler P. J., McGuire T. C. Characterization of an immunoprotective protein complex of Anaplasma marginale by cloning and expression of the gene coding for polypeptide Am105L. Infect Immun. 1987 Oct;55(10):2428–2435. doi: 10.1128/iai.55.10.2428-2435.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cowman A. F., Saint R. B., Coppel R. L., Brown G. V., Anders R. F., Kemp D. J. Conserved sequences flank variable tandem repeats in two S-antigen genes of Plasmodium falciparum. Cell. 1985 Apr;40(4):775–783. doi: 10.1016/0092-8674(85)90337-x. [DOI] [PubMed] [Google Scholar]
- Dame J. B., Williams J. L., McCutchan T. F., Weber J. L., Wirtz R. A., Hockmeyer W. T., Maloy W. L., Haynes J. D., Schneider I., Roberts D. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984 Aug 10;225(4662):593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A., Jones K. F., Scott J. R. Size variation of the M protein in group A streptococci. J Exp Med. 1985 Jun 1;161(6):1384–1401. doi: 10.1084/jem.161.6.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner P. R., Pearson T. W., Clarke M. W., Mutharia L. M. Identification and isolation of a variant surface glycoprotein from Trypanosoma vivax. Science. 1987 Feb 13;235(4790):774–777. doi: 10.1126/science.3810164. [DOI] [PubMed] [Google Scholar]
- KREIER J. P., RISTIC M. Anaplasmosis. X. Morphologic characteristics of the parasites present in the blood of calves infected with the Oregon strain of Anaplasma marginale. Am J Vet Res. 1963 Jul;24:676–687. [PubMed] [Google Scholar]
- KREIER J. P., RISTIC M. Anaplasmosis. XI. Immunoserologic characteristics of the parasites present in the blood of calves infected with the Oregon strain of Anaplasma marginale. Am J Vet Res. 1963 Jul;24:688–696. [PubMed] [Google Scholar]
- Kuttler K. L., Zaugg J. L., Johnson L. W. Serologic and clinical responses of premunized, vaccinated, and previously infected cattle to challenge exposure by two different Anaplasma marginale isolates. Am J Vet Res. 1984 Nov;45(11):2223–2226. [PubMed] [Google Scholar]
- Langford C. J., Edwards S. J., Smith G. L., Mitchell G. F., Moss B., Kemp D. J., Anders R. F. Anchoring a secreted plasmodium antigen on the surface of recombinant vaccinia virus-infected cells increases its immunogenicity. Mol Cell Biol. 1986 Sep;6(9):3191–3199. doi: 10.1128/mcb.6.9.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love J. N. Cryogenic preservation of Anaplasma marginale with Dimethyl sulfoxide. Am J Vet Res. 1972 Dec;33(12):2557–2560. [PubMed] [Google Scholar]
- McGuire T. C., Palmer G. H., Goff W. L., Johnson M. I., Davis W. C. Common and isolate-restricted antigens of Anaplasma marginale detected with monoclonal antibodies. Infect Immun. 1984 Sep;45(3):697–700. doi: 10.1128/iai.45.3.697-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks E. V., Stover C. K., Rice R. M. Molecular cloning and expression of Rickettsia tsutsugamushi genes for two major protein antigens in Escherichia coli. Infect Immun. 1987 May;55(5):1156–1162. doi: 10.1128/iai.55.5.1156-1162.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Davis W. C., McGuire T. C. Immunization with an isolate-common surface protein protects cattle against anaplasmosis. Science. 1986 Mar 14;231(4743):1299–1302. doi: 10.1126/science.3945825. [DOI] [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Kuttler K. L., McGuire T. C. Detection of an Anaplasma marginale common surface protein present in all stages of infection. J Clin Microbiol. 1986 Jun;23(6):1078–1083. doi: 10.1128/jcm.23.6.1078-1083.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Musoke A. J., Katende J. M., Rurangirwa F., Shkap V., Pipano E., Davis W. C., McGuire T. C. Recognition of conserved surface protein epitopes on Anaplasma centrale and Anaplasma marginale isolates from Israel, Kenya and the United States. Int J Parasitol. 1988 Feb;18(1):33–38. doi: 10.1016/0020-7519(88)90033-1. [DOI] [PubMed] [Google Scholar]
- Palmer G. H., McGuire T. C. Immune serum against Anaplasma marginale initial bodies neutralizes infectivity for cattle. J Immunol. 1984 Aug;133(2):1010–1015. [PubMed] [Google Scholar]
- Ristic M., Carson C. A. Methods of immunoprophylaxis against bovine anaplasmosis with emphasis on use of the attenuated Anaplasma marginale vaccine. Adv Exp Med Biol. 1977;93:151–188. doi: 10.1007/978-1-4615-8855-9_10. [DOI] [PubMed] [Google Scholar]
- Rovis L., Barbet A. F., Williams R. O. Characterisation of the surface coat of Trypanosoma congolense. Nature. 1978 Feb 16;271(5646):654–656. doi: 10.1038/271654a0. [DOI] [PubMed] [Google Scholar]
- Smith R. D., Levy M. G., Kuhlenschmidt M. S., Adams J. H., Rzechula D. L., Hardt T. A., Kocan K. M. Isolate of Anaplasma marginale not transmitted by ticks. Am J Vet Res. 1986 Jan;47(1):127–129. [PubMed] [Google Scholar]
- Tamura A., Ohashi N., Urakami H., Takahashi K., Oyanagi M. Analysis of polypeptide composition and antigenic components of Rickettsia tsutsugamushi by polyacrylamide gel electrophoresis and immunoblotting. Infect Immun. 1985 Jun;48(3):671–675. doi: 10.1128/iai.48.3.671-675.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizcaino O., Corrier D. E., Terry M. K., Carson C. A., Lee A. J., Kuttler K. L., Ristic M., Treviño G. S. Comparison of three methods of immunization against bovine anaplasmosis: evaluation of protection afforded against field challenge exposure. Am J Vet Res. 1980 Jul;41(7):1066–1068. [PubMed] [Google Scholar]
- Zavala F., Masuda A., Graves P. M., Nussenzweig V., Nussenzweig R. S. Ubiquity of the repetitive epitope of the CS protein in different isolates of human malaria parasites. J Immunol. 1985 Oct;135(4):2790–2793. [PubMed] [Google Scholar]







