Abstract
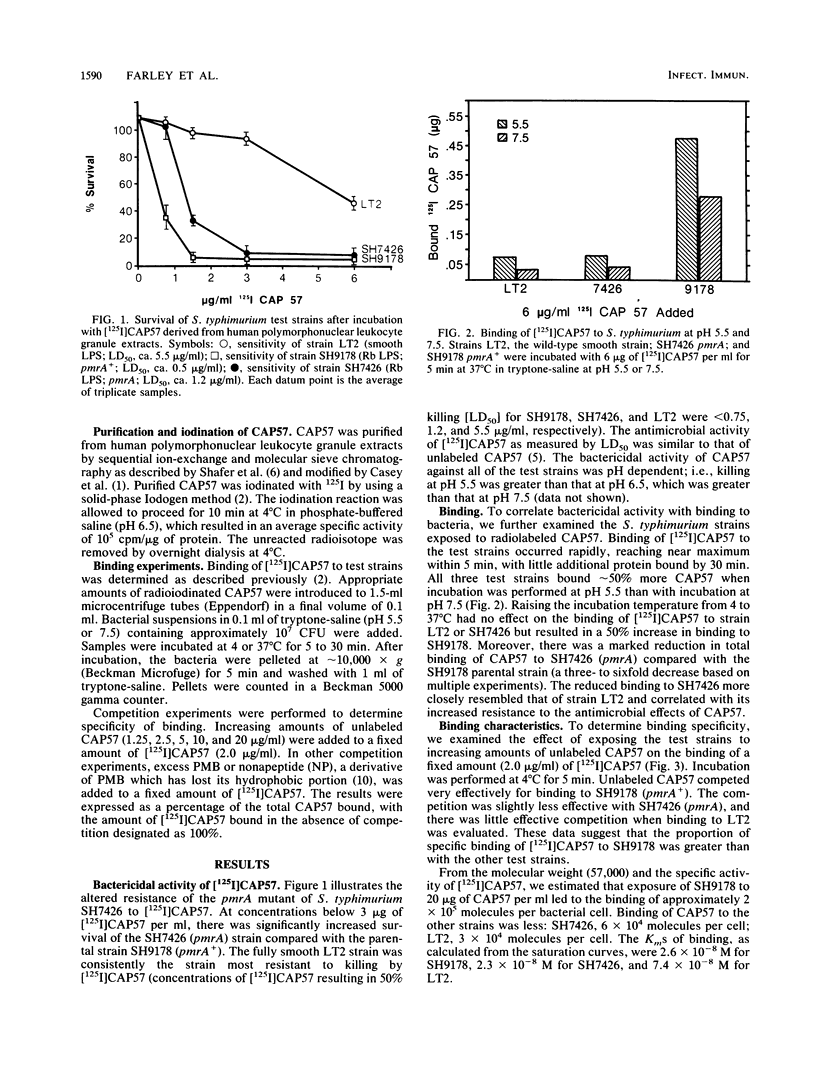
Bactericidal activity and binding of a 57,000-dalton cationic antimicrobial neutrophil granule protein (CAP57) are determined by the presence on bacteria of O-antigen polysaccharide chains and the availability of negatively charged groups in the lipid A region, the inner core region, or both regions of lipopolysaccharide. Polymyxin B (PMB)-resistant mutants with well-defined alterations in lipid A structure and charge (pmrA) are also more resistant to CAP57. We used biologically active radioiodinated CAP57 to study the characteristics and kinetics of binding to a sensitive Rb lipopolysaccharide chemotype, Salmonella typhimurium SH9178, and the relatively resistant pmrA mutant strain SH7426. Binding occurred rapidly and was specific and saturable. Because CAP57 appears to be bound in a manner similar to that of PMB, competition binding studies were performed. Excess PMB did compete with CAP57 for binding to SH9178. Nonapeptide, a polycationic derivative of PMB that has lost its hydrophobic portions, demonstrated a marked decrease in ability to compete for binding with CAP57 compared with PMB. This demonstrated the importance of hydrophobic binding in the interaction of CAP57 with the microbial surface. Thus, we have shown that binding of CAP57 to SH9178 is specific, saturable, and similar to binding of PMB. Both hydrophobic and ionic properties of CAP57 appear to be necessary for binding.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casey S. G., Shafer W. M., Spitznagel J. K. Anaerobiosis increases resistance of Neisseria gonorrhoeae to O2-independent antimicrobial proteins from human polymorphonuclear granulocytes. Infect Immun. 1985 Feb;47(2):401–407. doi: 10.1128/iai.47.2.401-407.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley M. M., Shafer W. M., Spitznagel J. K. Antimicrobial binding of a radiolabeled cationic neutrophil granule protein. Infect Immun. 1987 Jun;55(6):1536–1539. doi: 10.1128/iai.55.6.1536-1539.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Jacobs D. M. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976 Oct;13(10):813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- Rest R. F., Cooney M. H., Spitznagel J. K. Susceptibility of lipopolysaccharide mutants to the bactericidal action of human neutrophil lysosomal fractions. Infect Immun. 1977 Apr;16(1):145–151. doi: 10.1128/iai.16.1.145-151.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Casey S. G., Spitznagel J. K. Lipid A and resistance of Salmonella typhimurium to antimicrobial granule proteins of human neutrophil granulocytes. Infect Immun. 1984 Mar;43(3):834–838. doi: 10.1128/iai.43.3.834-838.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Martin L. E., Spitznagel J. K. Cationic antimicrobial proteins isolated from human neutrophil granulocytes in the presence of diisopropyl fluorophosphate. Infect Immun. 1984 Jul;45(1):29–35. doi: 10.1128/iai.45.1.29-35.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuber M., Bader J. Action of polymyxin B on bacterial membranes. Binding capacities for polymyxin B of inner and outer membranes isolated from Salmonella typhimurium G30. Arch Microbiol. 1976 Aug;109(1-2):51–58. doi: 10.1007/BF00425112. [DOI] [PubMed] [Google Scholar]
- Vaara M. Increased outer membrane resistance to ethylenediaminetetraacetate and cations in novel lipid A mutants. J Bacteriol. 1981 Nov;148(2):426–434. doi: 10.1128/jb.148.2.426-434.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Outer membrane permeability barrier disruption by polymyxin in polymyxin-susceptible and -resistant Salmonella typhimurium. Antimicrob Agents Chemother. 1981 Apr;19(4):578–583. doi: 10.1128/aac.19.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Sensitization of Gram-negative bacteria to antibiotics and complement by a nontoxic oligopeptide. Nature. 1983 Jun 9;303(5917):526–528. doi: 10.1038/303526a0. [DOI] [PubMed] [Google Scholar]
- Vaara M., Viljanen P. Binding of polymyxin B nonapeptide to gram-negative bacteria. Antimicrob Agents Chemother. 1985 Apr;27(4):548–554. doi: 10.1128/aac.27.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Beckerdite-Quagliata S., Elsbach P. Resistance of gram-negative bacteria to purified bactericidal leukocyte proteins: relation to binding and bacterial lipopolysaccharide structure. J Clin Invest. 1980 Mar;65(3):619–628. doi: 10.1172/JCI109707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Victor M., Elsbach P. Role of charge and hydrophobic interactions in the action of the bactericidal/permeability-increasing protein of neutrophils on gram-negative bacteria. J Clin Invest. 1983 Mar;71(3):540–549. doi: 10.1172/JCI110798. [DOI] [PMC free article] [PubMed] [Google Scholar]



