Abstract
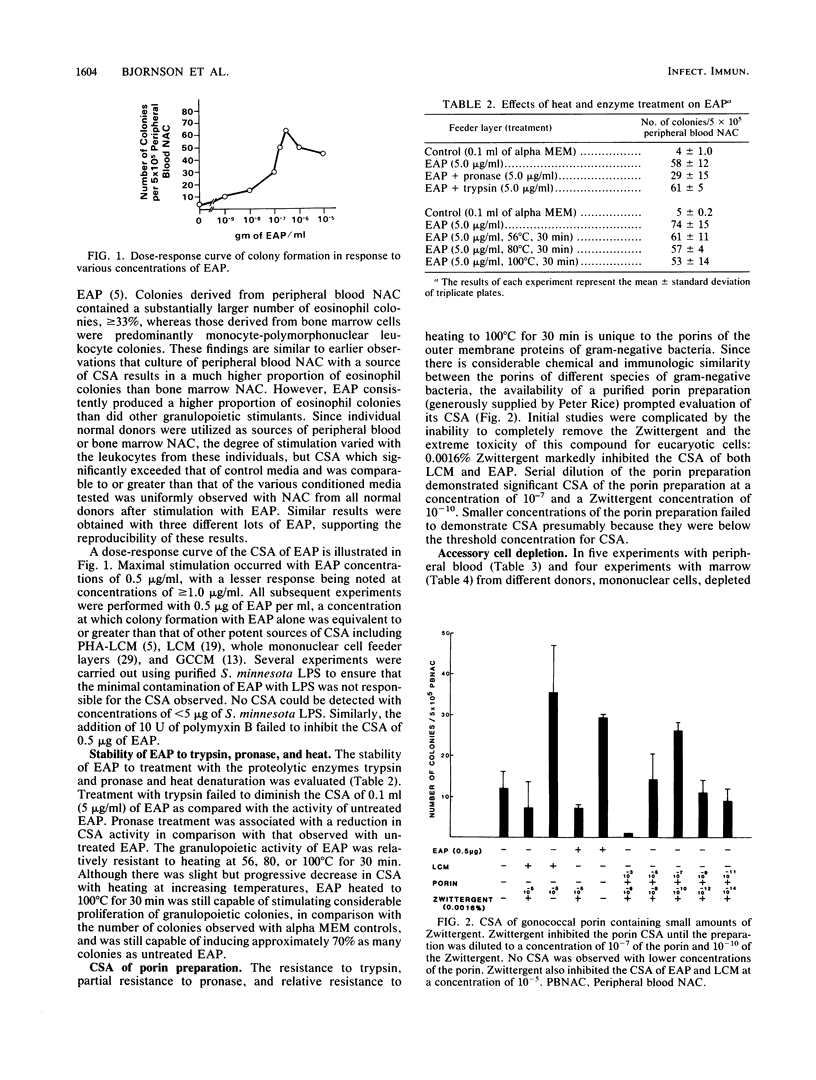
Proteins coextracted with endotoxin, termed endotoxin-associated protein (EAP), have been shown to exert interleukin 1-like activities. The present studies demonstrate that EAP also exerts potent granulopoietic colony-stimulating activity (CSA) on human peripheral blood and bone marrow progenitor cells, comparable to that seen with various types of conditioned media. The CSA observed with EAP appeared to be heat (100 degrees C, 30 min) and trypsin resistant and partially pronase resistant. Similar resistance was observed with the porin proteins of the outer membrane of gram-negative bacteria, and similar CSA activity was observed with a purified porin preparation of Neisseria gonorrhoeae. The CSA of EAP could be demonstrated in human peripheral blood and bone marrow leukocytes rigorously depleted of monocytes, T lymphocytes, and B lymphocytes by treatment with specific monoclonal antibodies and complement.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews R. G., Torok-Storb B., Bernstein I. D. Myeloid-associated differentiation antigens on stem cells and their progeny identified by monoclonal antibodies. Blood. 1983 Jul;62(1):124–132. [PubMed] [Google Scholar]
- Apte R. N., Galanos C., Pluznik D. H. Lipid A, the active part of bacterial endotoxins in inducing serum colony stimulating activity and proliferation of splenic granulocyte/macrophage progenitor cells. J Cell Physiol. 1976 Jan;87(1):71–78. doi: 10.1002/jcp.1040870110. [DOI] [PubMed] [Google Scholar]
- Bagby G. C., Jr, Dinarello C. A., Wallace P., Wagner C., Hefeneider S., McCall E. Interleukin 1 stimulates granulocyte macrophage colony-stimulating activity release by vascular endothelial cells. J Clin Invest. 1986 Nov;78(5):1316–1323. doi: 10.1172/JCI112717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessler W. G., Henning U. Protein I and protein II from the outer membrane of Escherichia coli are mouse B-lymphocyte mitogens. Z Immunitatsforsch Immunobiol. 1979 Jun;155(5):387–398. [PubMed] [Google Scholar]
- Bjornson B. H., André-Schwartz J., Desforges J. F. In vitro culture of circulating CFUEOS from normal donors. Exp Hematol. 1982 Mar;10(3):271–276. [PubMed] [Google Scholar]
- Blake M. S., Gotschlich E. C. Purification and partial characterization of the major outer membrane protein of Neisseria gonorrhoeae. Infect Immun. 1982 Apr;36(1):277–283. doi: 10.1128/iai.36.1.277-283.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Chervenick P. A., LoBuglio A. F. Human blood monocytes: stimulators of granulocyte and mononuclear colony formation in vitro. Science. 1972 Oct 13;178(4057):164–166. doi: 10.1126/science.178.4057.164. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Golde D. W. Production of colony-stimulating activity by human lymphocytes. Nature. 1974 Apr 19;248(5450):703–704. doi: 10.1038/248703a0. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Rothman B., Golde D. W. Effect of endotoxin on the production of colony-stimulating factor by human monocytes and macrophages. J Cell Physiol. 1974 Oct;84(2):193–196. doi: 10.1002/jcp.1040840205. [DOI] [PubMed] [Google Scholar]
- Di Persio J. F., Brennan J. K., Lichtman M. A., Speiser B. L. Human cell lines that elaborate colon-stimulating activity for the marrow cells of man and other species. Blood. 1978 Mar;51(3):507–519. [PubMed] [Google Scholar]
- Eaves A. C., Bruce W. R. In vitro production of colony-stimulating activity. I. Exposure of mouse peritoneal cells to endotoxin. Cell Tissue Kinet. 1974 Jan;7(1):19–30. doi: 10.1111/j.1365-2184.1974.tb00395.x. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., White D., Leive L. Identification of outer membrane proteins, including known lymphocyte mitogens, as the endotoxin protein of Escherichia coli 0111. J Immunol. 1981 Oct;127(4):1290–1294. [PubMed] [Google Scholar]
- Goodman G. W., Sultzer B. M. Characterization of the chemical and physical properties of a novel B-lymphocyte activator, endotoxin protein. Infect Immun. 1979 Jun;24(3):685–696. doi: 10.1128/iai.24.3.685-696.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman G. W., Sultzer B. M. Endotoxin protein is a mitogen and polyclonal activator of human B lymphocytes. J Exp Med. 1979 Mar 1;149(3):713–723. doi: 10.1084/jem.149.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscove N. N., Senn J. S., Till J. E., McCulloch E. A. Colony formation by normal and leukemic human marrow cells in culture: effect of conditioned medium from human leukocytes. Blood. 1971 Jan;37(1):1–5. [PubMed] [Google Scholar]
- Kaplan M. E., Clark C. An improved rosetting assay for detection of human T lymphocytes. J Immunol Methods. 1974 Jul;5(2):131–135. doi: 10.1016/0022-1759(74)90003-9. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Staub A. M., Westphal O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol Rev. 1966 Mar;30(1):192–255. doi: 10.1128/br.30.1.192-255.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. J., Hansen J. A., Nowinski R. C., Brown M. A. A new human T-cell differentiation antigen: unexpected expression on chronic lymphocytic leukemia cells. Immunogenetics. 1980;11(5):429–439. doi: 10.1007/BF01567812. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Betz S. J., Jacobs D. M. Isolation of a lipid A bound polypeptide responsible for "LPS-initiated" mitogenesis of C3H/HeJ spleen cells. J Exp Med. 1976 Sep 1;144(3):840–846. doi: 10.1084/jem.144.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- Pieroni R. E., Broderick E. J., Bundeally A., Levine L. A simple method for the quantitation of submicrogram amounts of bacterial endotoxin. Proc Soc Exp Biol Med. 1970 Mar;133(3):790–794. doi: 10.3181/00379727-133-34565. [DOI] [PubMed] [Google Scholar]
- Pike B. L., Robinson W. A. Human bone marrow colony growth in agar-gel. J Cell Physiol. 1970 Aug;76(1):77–84. doi: 10.1002/jcp.1040760111. [DOI] [PubMed] [Google Scholar]
- Pluznik D. H., Sachs L. The cloning of normal "mast" cells in tissue culture. J Cell Physiol. 1965 Dec;66(3):319–324. doi: 10.1002/jcp.1030660309. [DOI] [PubMed] [Google Scholar]
- Ruscetti F. W., Chervenick P. A. Regulation of the release of colony-stimulating activity from mitogen-stimulated lymphocytes. J Immunol. 1975 May;114(5):1513–1517. [PubMed] [Google Scholar]
- Ruscetti F. W., Chervenick P. A. Release of colony-stimulating factor from monocytes by endotoxin and polyinosinic-polycytidylic acid. J Lab Clin Med. 1974 Jan;83(1):64–72. [PubMed] [Google Scholar]
- Skidmore B. J., Morrison D. C., Chiller J. M., Weigle W. O. Immunologic properties of bacterial lipopolysaccharide (LPS). II. The unresponsiveness of C3H/HeJ Mouse spleen cells to LPS-induced mitogenesis is dependent on the method used to extract LPS. J Exp Med. 1975 Dec 1;142(6):1488–1508. doi: 10.1084/jem.142.6.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M., Goodman G. W. Endotoxin protein: a B-cell mitogen and polyclonal activator of C3H/HeJ lymphocytes. J Exp Med. 1976 Sep 1;144(3):821–827. doi: 10.1084/jem.144.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]



