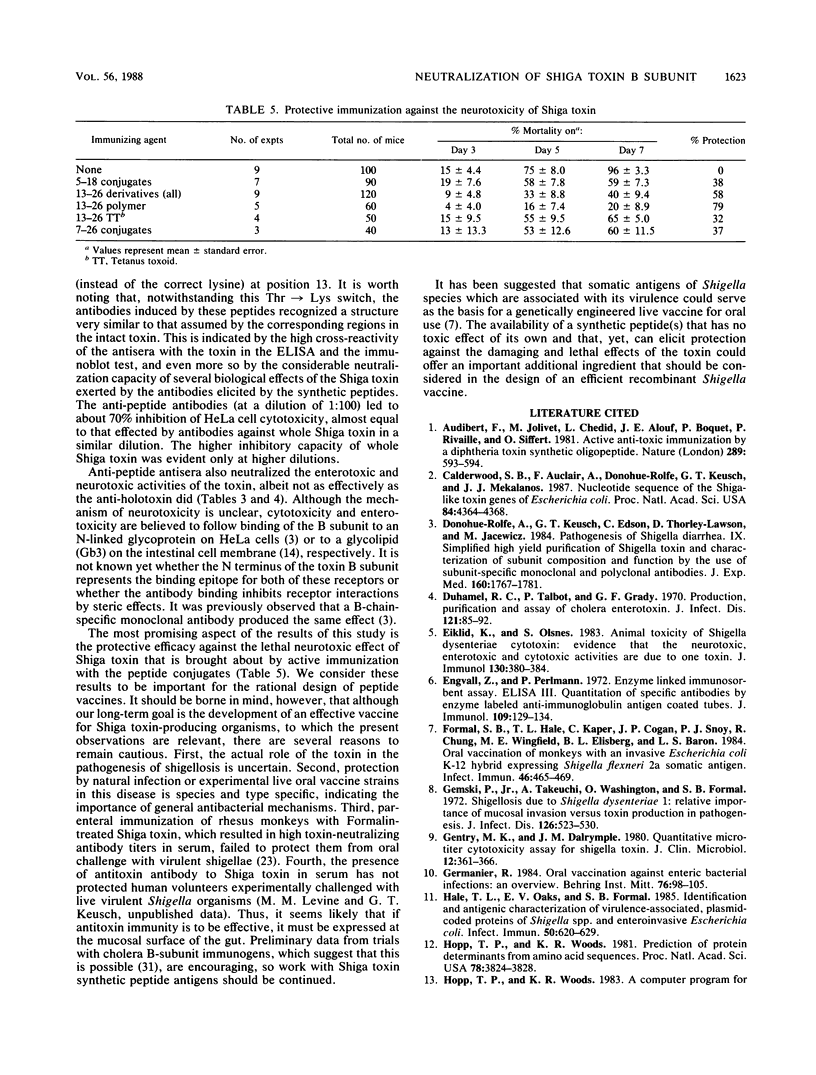
Abstract
Shiga toxin B chain, the binding subunit of Shiga toxin, was recently purified; and the amino acid sequence of this 7,716-dalton polypeptide was determined (N.G. Seidah, A. Donohue-Rolfe, C. Lazure, F. Auclair, G. T. Keusch, and M. Chretien, J. Biol. Chem. 261:13928-13931, 1986). In the present study, synthetic peptides corresponding to three overlapping sequences from the N-terminal region of this subunit were prepared. The peptides synthesized consisted of residues 5 to 18, 13 to 26, and 7 to 26. This region coincides with the major peak of hydrophilicity and surface area residues predicted from a computer analysis. For the purpose of immunization, the peptides either were conjugated with a protein or synthetic carrier or were polymerized with glutaraldehyde. Antisera against these peptide derivatives raised in rabbits reacted not only with the respective homologous peptide but also to a comparable extent with the intact Shiga toxin. The anti-peptide antisera effectively neutralized the various biological activities of the Shiga toxin, namely, cytotoxicity to HeLa cells, enterotoxic activity (the fluid secretion into ligated ileal loops in rats), and neurotoxicity in mice. Furthermore, active immunization with the peptide conjugates was found to protect mice against the lethal effect of Shiga toxin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Audibert F., Jolivet M., Chedid L., Alouf J. E., Boquet P., Rivaille P., Siffert O. Active antitoxic immunization by a diphtheria toxin synthetic oligopeptide. Nature. 1981 Feb 12;289(5798):593–594. doi: 10.1038/289593a0. [DOI] [PubMed] [Google Scholar]
- Calderwood S. B., Auclair F., Donohue-Rolfe A., Keusch G. T., Mekalanos J. J. Nucleotide sequence of the Shiga-like toxin genes of Escherichia coli. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4364–4368. doi: 10.1073/pnas.84.13.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue-Rolfe A., Keusch G. T., Edson C., Thorley-Lawson D., Jacewicz M. Pathogenesis of Shigella diarrhea. IX. Simplified high yield purification of Shigella toxin and characterization of subunit composition and function by the use of subunit-specific monoclonal and polyclonal antibodies. J Exp Med. 1984 Dec 1;160(6):1767–1781. doi: 10.1084/jem.160.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhamel R. C., Talbot P., Grady G. F. Production, purification, and assay of cholera enterotoxin. J Infect Dis. 1970 May;121(Suppl):85+–85+. doi: 10.1093/infdis/121.supplement.s85. [DOI] [PubMed] [Google Scholar]
- Eiklid K., Olsnes S. Animal toxicity of Shigella dysenteriae cytotoxin: evidence that the neurotoxic, enterotoxic, and cytotoxic activities are due to one toxin. J Immunol. 1983 Jan;130(1):380–384. [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Formal S. B., Hale T. L., Kapfer C., Cogan J. P., Snoy P. J., Chung R., Wingfield M. E., Elisberg B. L., Baron L. S. Oral vaccination of monkeys with an invasive Escherichia coli K-12 hybrid expressing Shigella flexneri 2a somatic antigen. Infect Immun. 1984 Nov;46(2):465–469. doi: 10.1128/iai.46.2.465-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Jr, Takeuchi A., Washington O., Formal S. B. Shigellosis due to Shigella dysenteriae. 1. Relative importance of mucosal invasion versus toxin production in pathogenesis. J Infect Dis. 1972 Nov;126(5):523–530. doi: 10.1093/infdis/126.5.523. [DOI] [PubMed] [Google Scholar]
- Gentry M. K., Dalrymple J. M. Quantitative microtiter cytotoxicity assay for Shigella toxin. J Clin Microbiol. 1980 Sep;12(3):361–366. doi: 10.1128/jcm.12.3.361-366.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germanier R. Oral vaccination against enteric bacterial infections: an overview. Behring Inst Mitt. 1984 Nov;(76):98–105. [PubMed] [Google Scholar]
- Hale T. L., Oaks E. V., Formal S. B. Identification and antigenic characterization of virulence-associated, plasmid-coded proteins of Shigella spp. and enteroinvasive Escherichia coli. Infect Immun. 1985 Dec;50(3):620–629. doi: 10.1128/iai.50.3.620-629.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacewicz M., Clausen H., Nudelman E., Donohue-Rolfe A., Keusch G. T. Pathogenesis of shigella diarrhea. XI. Isolation of a shigella toxin-binding glycolipid from rabbit jejunum and HeLa cells and its identification as globotriaosylceramide. J Exp Med. 1986 Jun 1;163(6):1391–1404. doi: 10.1084/jem.163.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob C. O., Pines M., Arnon R. Neutralization of heat-labile toxin of E. coli by antibodies to synthetic peptides derived from the B subunit of cholera toxin. EMBO J. 1984 Dec 1;3(12):2889–2893. doi: 10.1002/j.1460-2075.1984.tb02226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob C. O., Sela M., Arnon R. Antibodies against synthetic peptides of the B subunit of cholera toxin: crossreaction and neutralization of the toxin. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7611–7615. doi: 10.1073/pnas.80.24.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keusch G. T., Donohue-Rolfe A., Jacewicz M. Shigella toxin(s): description and role in diarrhea and dysentery. Pharmacol Ther. 1981;15(3):403–438. doi: 10.1016/0163-7258(81)90052-8. [DOI] [PubMed] [Google Scholar]
- Keusch G. T., Jacewicz M. Serum enterotoxin-neutralizing antibody in human shigellosis. Nat New Biol. 1973 Jan 3;241(105):31–32. doi: 10.1038/newbio241031a0. [DOI] [PubMed] [Google Scholar]
- Levine M. M., DuPont H. L., Formal S. B., Hornick R. B., Takeuchi A., Gangarosa E. J., Snyder M. J., Libonati J. P. Pathogenesis of Shigella dysenteriae 1 (Shiga) dysentery. J Infect Dis. 1973 Mar;127(3):261–270. doi: 10.1093/infdis/127.3.261. [DOI] [PubMed] [Google Scholar]
- McIver J., Grady G. F., Formal S. B. Immunization with Shigella dysenteriae type 1: evaluation of antitoxic immunity in prevention of experimental disease in rhesus monkeys (Macaca mulatta). J Infect Dis. 1977 Sep;136(3):416–421. doi: 10.1093/infdis/136.3.416. [DOI] [PubMed] [Google Scholar]
- Müller G. M., Shapira M., Arnon R. Anti-influenza response achieved by immunization with a synthetic conjugate. Proc Natl Acad Sci U S A. 1982 Jan;79(2):569–573. doi: 10.1073/pnas.79.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkind D., Frey J. A., Petersen E. A., Dinowitz M. Delayed hypersensitivity to fungal antigens in mice. II. Molecular classes in immunogenic RNA extracts that transfer delayed hypersensitivity. J Infect Dis. 1976 May;133(5):523–532. doi: 10.1093/infdis/133.5.523. [DOI] [PubMed] [Google Scholar]
- Rose G. D. Prediction of chain turns in globular proteins on a hydrophobic basis. Nature. 1978 Apr 13;272(5654):586–590. doi: 10.1038/272586a0. [DOI] [PubMed] [Google Scholar]
- Seid R. C., Jr, Kopecko D. J., Sadoff J. C., Schneider H., Baron L. S., Formal S. B. Unusual lipopolysaccharide antigens of a Salmonella typhi oral vaccine strain expressing the Shigella sonnei form I antigen. J Biol Chem. 1984 Jul 25;259(14):9028–9034. [PubMed] [Google Scholar]
- Seidah N. G., Donohue-Rolfe A., Lazure C., Auclair F., Keusch G. T., Chrétien M. Complete amino acid sequence of Shigella toxin B-chain. A novel polypeptide containing 69 amino acids and one disulfide bridge. J Biol Chem. 1986 Oct 25;261(30):13928–13931. [PubMed] [Google Scholar]
- Svennerholm A. M., Jertborn M., Gothefors L., Karim A. M., Sack D. A., Holmgren J. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B subunit-whole cell vaccine. J Infect Dis. 1984 Jun;149(6):884–893. doi: 10.1093/infdis/149.6.884. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Timmis K. N. A small plasmid in Shigella dysenteriae 1 specifies one or more functions essential for O antigen production and bacterial virulence. Infect Immun. 1984 Jan;43(1):391–396. doi: 10.1128/iai.43.1.391-396.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhof E., Altschuh D., Moras D., Bloomer A. C., Mondragon A., Klug A., Van Regenmortel M. H. Correlation between segmental mobility and the location of antigenic determinants in proteins. Nature. 1984 Sep 13;311(5982):123–126. doi: 10.1038/311123a0. [DOI] [PubMed] [Google Scholar]



