Abstract
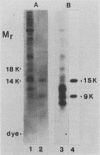
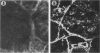
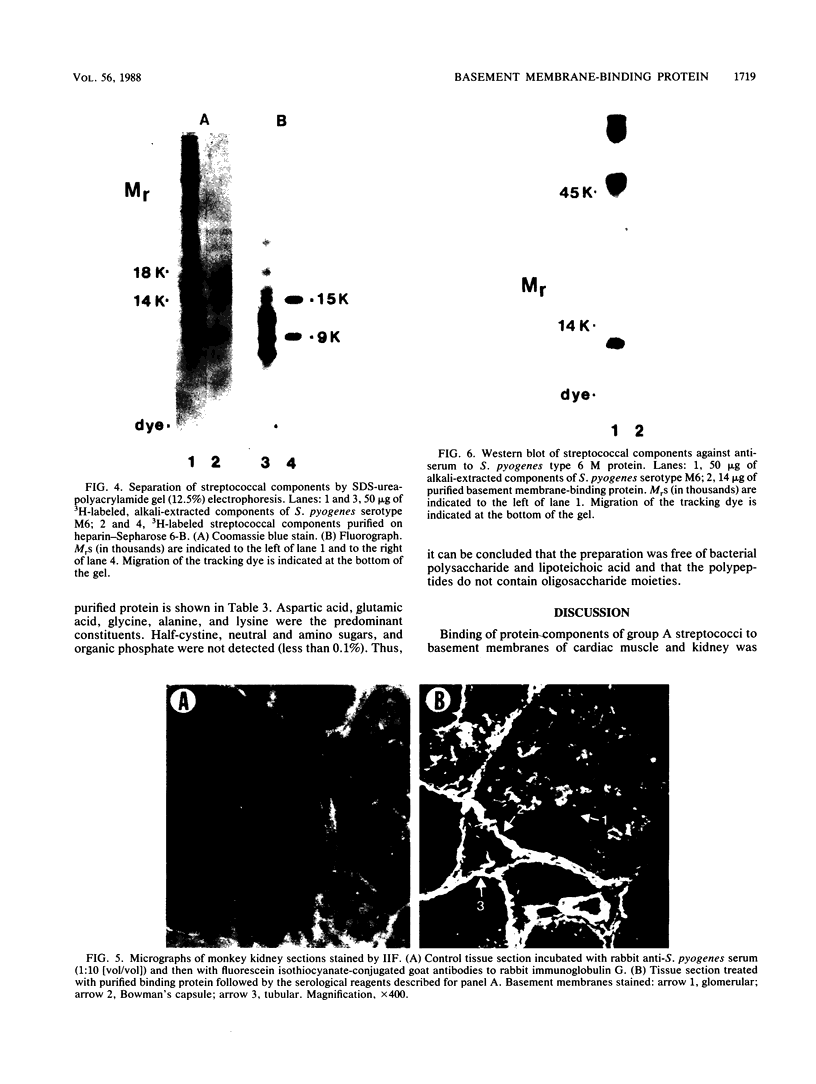
Solubilized surface proteins of Streptococcus pyogenes serotype M6 were found by indirect immunofluorescence assays to bind selectively to proteoglycan-containing regions of basement membranes of kidney and cardiac muscle in vitro. Epithelial, endothelial, and interstitial cells were unstained. Binding of streptococcal protein to basement membranes was competitively inhibited by heparin and, to a lesser extent, by heparan sulfate. Weak inhibition was also observed with other glycosaminoglycans, including dermatan sulfate, chondroitin sulfate, and hyaluronic acid. Type IV collagen, gelatin, serum fibronectin, glucuronic acid, and a selection of monosaccharides had no significant effects on binding. The heparin-inhibitable basement membrane-binding protein was purified by affinity chromatography on heparin-Sepharose 6-B. Polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate and urea dissociated the affinity-purified protein into two polypeptides of 9,000 and 15,000 mrs. Chemical analyses revealed that the purified protein was devoid of cysteine, amino and neutral sugars, and phosphate. Thus, the polypeptides are not glycosylated or complexed with trace amounts of lipoteichoic acid or polysaccharide. Binding of purified protein to tissue was determined by direct radioassay and indirect immunofluorescence and was inhibitable by heparin. Although the in vivo effects of this streptococcal component remain to be determined, its deposition on basement membranes in vitro supports the hypothesis that it contributes to the pathogenesis of poststreptococcal glomerulonephritis or acute rheumatic fever.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini B., Nisengard R. J., Glurich I., Neiders M. E., Stinson M. W. Streptococcus mutans-induced nephritis in rabbits. Am J Pathol. 1985 Mar;118(3):408–418. [PMC free article] [PubMed] [Google Scholar]
- Andres G. A., Accinni L., Hsu K. C., Zabriskie J. B., Seegal B. C. Electron microscopic studies of human glomerulonephritis with ferritin-conjugated antibody. Localization of antigen-antibody complexes in glomerular structures of patients with acute glomerulonephritis. J Exp Med. 1966 Feb 1;123(2):399–412. doi: 10.1084/jem.123.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Ceri H., Kobiler D., Barondes S. H. Heparin-inhibitable lectin. Purification from chicken liver and embryonic chicken muscle. J Biol Chem. 1981 Jan 10;256(1):390–394. [PubMed] [Google Scholar]
- Eibl H., Lands W. E. A new, sensitive determination of phosphate. Anal Biochem. 1969 Jul;30(1):51–57. doi: 10.1016/0003-2697(69)90372-8. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A., Jones K. F., Scott J. R. Size variation of the M protein in group A streptococci. J Exp Med. 1985 Jun 1;161(6):1384–1401. doi: 10.1084/jem.161.6.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornstedt N., Porath J. Characterization studies on a new lectin found in seeds of Vicia ervilia. FEBS Lett. 1975 Sep 15;57(2):187–191. doi: 10.1016/0014-5793(75)80713-7. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Kronvall G. A surface component in group A, C, and G streptococci with non-immune reactivity for immunoglobulin G. J Immunol. 1973 Nov;111(5):1401–1406. [PubMed] [Google Scholar]
- Kronvall G., Myhre E. B., Björck L., Berggård I. Binding of aggregated human beta2-microglobulin to surface protein structure in group A, C, and G streptococci. Infect Immun. 1978 Oct;22(1):136–142. doi: 10.1128/iai.22.1.136-142.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronvall G., Schönbeck C., Myhre E. Fibrinogen binding structures in beta-hemolytic streptococci group A, C, and G. Comparisons with receptors for IgG and aggregated beta 2-microglobulin. Acta Pathol Microbiol Scand B. 1979 Oct;87(5):303–310. [PubMed] [Google Scholar]
- Kronvall G., Simmons A., Myhre E. B., Jonsson S. Specific absorption of human serum albumin, immunoglobulin A, and immunoglobulin G with selected strains of group A and G streptococci. Infect Immun. 1979 Jul;25(1):1–10. doi: 10.1128/iai.25.1.1-10.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. Procedures for the chromatographic determination of amino acids on four per cent cross-linked sulfonated polystyrene resins. J Biol Chem. 1954 Dec;211(2):893–906. [PubMed] [Google Scholar]
- Michael A. F., Jr, Drummond K. N., Good R. A., Vernier R. L. Acute poststreptococcal glomerulonephritis: immune deposit disease. J Clin Invest. 1966 Feb;45(2):237–248. doi: 10.1172/JCI105336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H. P., Blobel H. Absorption of human alpha 2-macroglobulin with selected strains of streptococci. Med Microbiol Immunol. 1983;172(1):33–39. doi: 10.1007/BF02123675. [DOI] [PubMed] [Google Scholar]
- Ofek I., Simpson W. A., Beachey E. H. Formation of molecular complexes between a structurally defined M protein and acylated or deacylated lipoteichoic acid of Streptococcus pyogenes. J Bacteriol. 1982 Feb;149(2):426–433. doi: 10.1128/jb.149.2.426-433.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W. A., Hasty D. L., Mason J. M., Beachey E. H. Fibronectin-mediated binding of group A streptococci to human polymorphonuclear leukocytes. Infect Immun. 1982 Aug;37(2):805–810. doi: 10.1128/iai.37.2.805-810.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislawski L., Simpson W. A., Hasty D., Sharon N., Beachey E. H., Ofek I. Role of fibronectin in attachment of Streptococcus pyogenes and Escherichia coli to human cell lines and isolated oral epithelial cells. Infect Immun. 1985 Apr;48(1):257–259. doi: 10.1128/iai.48.1.257-259.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson M. W., Barua P. K., Bergey E. J., Nisengard R. J., Neiders M. E., Albini B. Binding of Streptococcus mutans antigens to heart and kidney basement membranes. Infect Immun. 1984 Oct;46(1):145–151. doi: 10.1128/iai.46.1.145-151.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson M. W., Bergey E. J. Isolation of heart- and kidney-binding protein from group A streptococci. Infect Immun. 1982 Jan;35(1):335–342. doi: 10.1128/iai.35.1.335-342.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson M. W., Nisengard R. J., Bergey E. J. Binding of streptococcal antigens to muscle tissue in vitro. Infect Immun. 1980 Feb;27(2):604–613. doi: 10.1128/iai.27.2.604-613.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzwelder F. J., Barua P. K., Albini B., Stinson M. W. Heart-reactive antibodies in rabbit anti-Streptococcus mutans sera fail to cross-react with Streptococcus mutans. J Immunol. 1988 Feb 1;140(3):954–961. [PubMed] [Google Scholar]
- Switalski L. M., Speziale P., Hök M., Wadström T., Timpl R. Binding of Streptococcus pyogenes to laminin. J Biol Chem. 1984 Mar 25;259(6):3734–3738. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Whitnack E., Dale J. B., Beachey E. H. Common protective antigens of group A streptococcal M proteins masked by fibrinogen. J Exp Med. 1984 Apr 1;159(4):1201–1212. doi: 10.1084/jem.159.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight T. N. Vessel proteoglycans and thrombogenesis. Prog Hemost Thromb. 1980;5:1–39. [PubMed] [Google Scholar]