Abstract
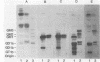
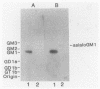
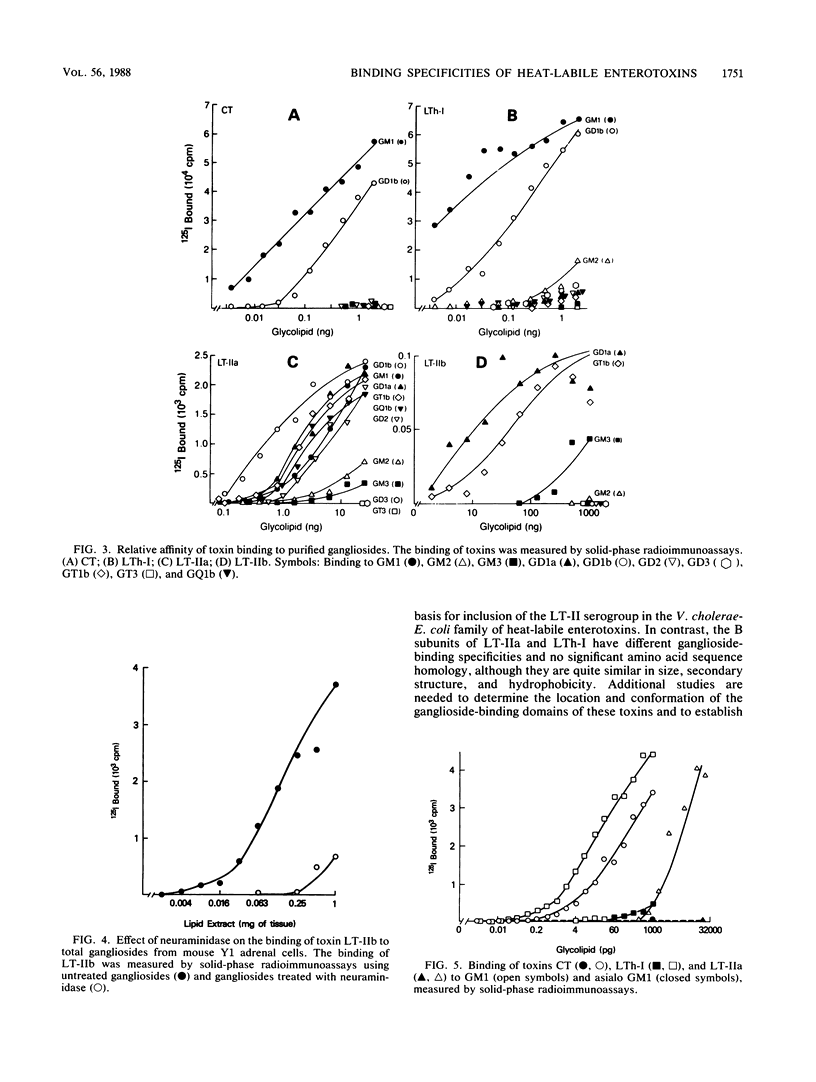
The heat-labile enterotoxins of Vibrio cholerae and Escherichia coli are related in structure and function. They are oligomers consisting of A and B polypeptide subunits. They bind to gangliosides, and they activate adenylate cyclase. The toxins form two antigenically distinct groups; members of each group cross-react but are not necessarily identical. Serogroup I includes cholera toxin (CT) and type I heat-labile enterotoxin (LT-I) of E. coli. LTh-I and LTp-I are antigenic variants of LT-I produced by strains of E. coli from humans and pigs, respectively. Serogroup II contains the type II heat-labile enterotoxin (LT-II) of E. coli. Two antigenic variants designated LT-IIa and LT-IIb have been described. The binding of CT, LTh-I, LT-IIa, and LT-IIb to gangliosides was analyzed by immunostaining thin-layer chromatograms and by solid-phase radioimmunoassay. The four toxins have different glycolipid-binding specificities. LTh-I and CT bind strongly to ganglioside GM1 and less strongly to ganglioside GD1b. However, LTh-I, unlike CT, also binds weakly to GM2 and asialo GM1. LTh-I, like CT, probably binds to the terminal sugar sequence Gal beta 1-3GalNAc beta 1-4(NeuAc alpha 2-3)Gal . . ., where GalNAc is N-acetylgalactosamine and NeuAc is N-acetylneuraminic acid. LT-IIa probably binds to the same sugar sequence to which CT and LTh-I bind, with the additional contribution to binding of a second NeuAc as in GD1b and GD2. Also, LT-IIa must bind the Gal beta 1-3GalNAc . . . sequence in such a way that its binding is relatively unaffected by attachment of NeuAc to the terminal galactose residue as in GD1a, GT1b, and GQ1b. LT-IIb probably binds to the terminal sugar sequence NeuAc alpha 2-3Gal beta 1-4GalNAc . . ., as it binds to gangliosides GD1a and GT1b but not to GM1.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belisle B. W., Twiddy E. M., Holmes R. K. Monoclonal antibodies with an expanded repertoire of specificities and potent neutralizing activity for Escherichia coli heat-labile enterotoxin. Infect Immun. 1984 Dec;46(3):759–764. doi: 10.1128/iai.46.3.759-764.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhaus M., Magnani J. L., Blaszczyk M., Steplewski Z., Koprowski H., Karlsson K. A., Larson G., Ginsburg V. Monoclonal antibodies directed against the human Leb blood group antigen. J Biol Chem. 1981 Dec 25;256(24):13223–13225. [PubMed] [Google Scholar]
- Chang P. P., Moss J., Twiddy E. M., Holmes R. K. Type II heat-labile enterotoxin of Escherichia coli activates adenylate cyclase in human fibroblasts by ADP ribosylation. Infect Immun. 1987 Aug;55(8):1854–1858. doi: 10.1128/iai.55.8.1854-1858.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G. F., Krivan H. C., Wilkins T. D., Smith D. F. Toxin A from Clostridium difficile binds to rabbit erythrocyte glycolipids with terminal Gal alpha 1-3Gal beta 1-4GlcNAc sequences. Arch Biochem Biophys. 1987 Aug 15;257(1):217–229. doi: 10.1016/0003-9861(87)90561-3. [DOI] [PubMed] [Google Scholar]
- Dennis R. D., Wiegandt H., Haustein D., Knowles B. H., Ellar D. J. Thin layer chromatography overlay technique in the analysis of the binding of the solubilized protoxin of Bacillus thuringiensis var. kurstaki to an insect glycosphingolipid of known structure. Biomed Chromatogr. 1986 Feb;1(1):31–37. doi: 10.1002/bmc.1130010108. [DOI] [PubMed] [Google Scholar]
- Donta S. T., Moon H. W., Whipp S. C. Detection of heat-labile Escherichia coli enterotoxin with the use of adrenal cells in tissue culture. Science. 1974 Jan 25;183(4122):334–336. doi: 10.1126/science.183.4122.334. [DOI] [PubMed] [Google Scholar]
- Eidels L., Proia R. L., Hart D. A. Membrane receptors for bacterial toxins. Microbiol Rev. 1983 Dec;47(4):596–620. doi: 10.1128/mr.47.4.596-620.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Burks M. F., Zupan A., Dallas W. S., Jacob C. O., Ludwig D. S. Epitopes of the cholera family of enterotoxins. Rev Infect Dis. 1987 May-Jun;9(3):544–561. doi: 10.1093/clinids/9.3.544. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Green B. A., Neill R. J., Ruyechan W. T., Holmes R. K. Evidence that a new enterotoxin of Escherichia coli which activates adenylate cyclase in eucaryotic target cells is not plasmid mediated. Infect Immun. 1983 Jul;41(1):383–390. doi: 10.1128/iai.41.1.383-390.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths S. L., Finkelstein R. A., Critchley D. R. Characterization of the receptor for cholera toxin and Escherichia coli heat-labile toxin in rabbit intestinal brush borders. Biochem J. 1986 Sep 1;238(2):313–322. doi: 10.1042/bj2380313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth B. E., Pickett C. L., Twiddy E. M., Holmes R. K., Gomes T. A., Lima A. A., Guerrant R. L., Franco B. D., Trabulsi L. R. Production of type II heat-labile enterotoxin by Escherichia coli isolated from food and human feces. Infect Immun. 1986 Nov;54(2):587–589. doi: 10.1128/iai.54.2.587-589.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth B. E., Twiddy E. M., Trabulsi L. R., Holmes R. K. Variation in chemical properties and antigenic determinants among type II heat-labile enterotoxins of Escherichia coli. Infect Immun. 1986 Nov;54(2):529–536. doi: 10.1128/iai.54.2.529-536.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakomori S., Kannagi R. Glycosphingolipids as tumor-associated and differentiation markers. J Natl Cancer Inst. 1983 Aug;71(2):231–251. [PubMed] [Google Scholar]
- Holmes R. K., Twiddy E. M. Characterization of monoclonal antibodies that react with unique and cross-reacting determinants of cholera enterotoxin and its subunits. Infect Immun. 1983 Dec;42(3):914–923. doi: 10.1128/iai.42.3.914-923.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R. K., Twiddy E. M., Pickett C. L. Purification and characterization of type II heat-labile enterotoxin of Escherichia coli. Infect Immun. 1986 Sep;53(3):464–473. doi: 10.1128/iai.53.3.464-473.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Fredman P., Lindblad M., Svennerholm A. M., Svennerholm L. Rabbit intestinal glycoprotein receptor for Escherichia coli heat-labile enterotoxin lacking affinity for cholera toxin. Infect Immun. 1982 Nov;38(2):424–433. doi: 10.1128/iai.38.2.424-433.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Lindblad M., Fredman P., Svennerholm L., Myrvold H. Comparison of receptors for cholera and Escherichia coli enterotoxins in human intestine. Gastroenterology. 1985 Jul;89(1):27–35. doi: 10.1016/0016-5085(85)90741-3. [DOI] [PubMed] [Google Scholar]
- Honda T., Tsuji T., Takeda Y., Miwatani T. Immunological nonidentity of heat-labile enterotoxins from human and porcine enterotoxigenic Escherichia coli. Infect Immun. 1981 Nov;34(2):337–340. doi: 10.1128/iai.34.2.337-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamori M., Shimomura J., Nagai Y. Specific binding of cholera toxin to rat erythrocytes revealed by analysis with a fluorescence-activated cell sorter. J Biochem. 1985 Mar;97(3):729–735. doi: 10.1093/oxfordjournals.jbchem.a135112. [DOI] [PubMed] [Google Scholar]
- Kannagi R., Nudelman E., Hakomori S. Possible role of ceramide in defining structure and function of membrane glycolipids. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3470–3474. doi: 10.1073/pnas.79.11.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson K. A., Strömberg N. Overlay and solid-phase analysis of glycolipid receptors for bacteria and viruses. Methods Enzymol. 1987;138:220–232. doi: 10.1016/0076-6879(87)38019-x. [DOI] [PubMed] [Google Scholar]
- Ledeen R. W., Yu R. K. Gangliosides: structure, isolation, and analysis. Methods Enzymol. 1982;83:139–191. doi: 10.1016/0076-6879(82)83012-7. [DOI] [PubMed] [Google Scholar]
- Magnani J. L., Smith D. F., Ginsburg V. Detection of gangliosides that bind cholera toxin: direct binding of 125I-labeled toxin to thin-layer chromatograms. Anal Biochem. 1980 Dec;109(2):399–402. doi: 10.1016/0003-2697(80)90667-3. [DOI] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Bacterial toxins: cellular mechanisms of action. Microbiol Rev. 1984 Sep;48(3):199–221. doi: 10.1128/mr.48.3.199-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill R. J., Twiddy E. M., Holmes R. K. Synthesis of plasmid-coded heat-labile enterotoxin in wild-type and hypertoxinogenic strains of Escherichia coli and in other genera of Enterobacteriaceae. Infect Immun. 1983 Sep;41(3):1056–1061. doi: 10.1128/iai.41.3.1056-1061.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett C. L., Twiddy E. M., Belisle B. W., Holmes R. K. Cloning of genes that encode a new heat-labile enterotoxin of Escherichia coli. J Bacteriol. 1986 Feb;165(2):348–352. doi: 10.1128/jb.165.2.348-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett C. L., Weinstein D. L., Holmes R. K. Genetics of type IIa heat-labile enterotoxin of Escherichia coli: operon fusions, nucleotide sequence, and hybridization studies. J Bacteriol. 1987 Nov;169(11):5180–5187. doi: 10.1128/jb.169.11.5180-5187.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamizawa K., Iwamori M., Kozaki S., Sakaguchi G., Tanaka R., Takayama H., Nagai Y. TLC immunostaining characterization of Clostridium botulinum type A neurotoxin binding to gangliosides and free fatty acids. FEBS Lett. 1986 Jun 9;201(2):229–232. doi: 10.1016/0014-5793(86)80614-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Nakazawa T., Miyata T., Kaji A., Yokota T. Evolution and structure of two ADP-ribosylation enterotoxins, Escherichia coli heat-labile toxin and cholera toxin. FEBS Lett. 1984 Apr 24;169(2):241–246. doi: 10.1016/0014-5793(84)80326-9. [DOI] [PubMed] [Google Scholar]