Abstract
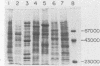
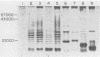
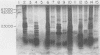
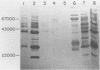
Previous experiments with the carrageenan model for ulcerative colitis demonstrated that the inflammatory response in guinea pigs can be enhanced by immunization with Bacteroides vulgatus and subsequent feeding of this organism to experimental animals. The studies reported here show that antigens extractable from the bacterial outer membrane by EDTA are responsible for this effect. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was used to analyze the outer membrane proteins from various strains as well as the lipopolysaccharides (LPS) extractable by the phenol-water method. Although the observed pattern of outer membrane proteins was complex, the strains could be divided into two electrophoretic types (phenons) on the basis of immunoblotting against a panel of antisera. Cross-absorbed antisera used in immunoblotting experiments identified four outer membrane proteins uniquely associated with the phenon capable of enhancing the colitis inflammatory response. These proteins had molecular weights of 100,000, 57,000, 34,000, and 21,000 when measured in 8% to 12% acrylamide gradient sodium dodecyl sulfate gels. Other antigens identified included at least one type of smooth LPS, three types of rough LPS, and a common antigen of 30,000 molecular weight among the strains of B. vulgatus tested. The outer membrane preparations were used in animal immunization and challenge experiments, and the severity of colitis was correlated with one electrophoresis type. The potential role of membrane proteins in the enhancement of colitis is discussed.
Full text
PDF

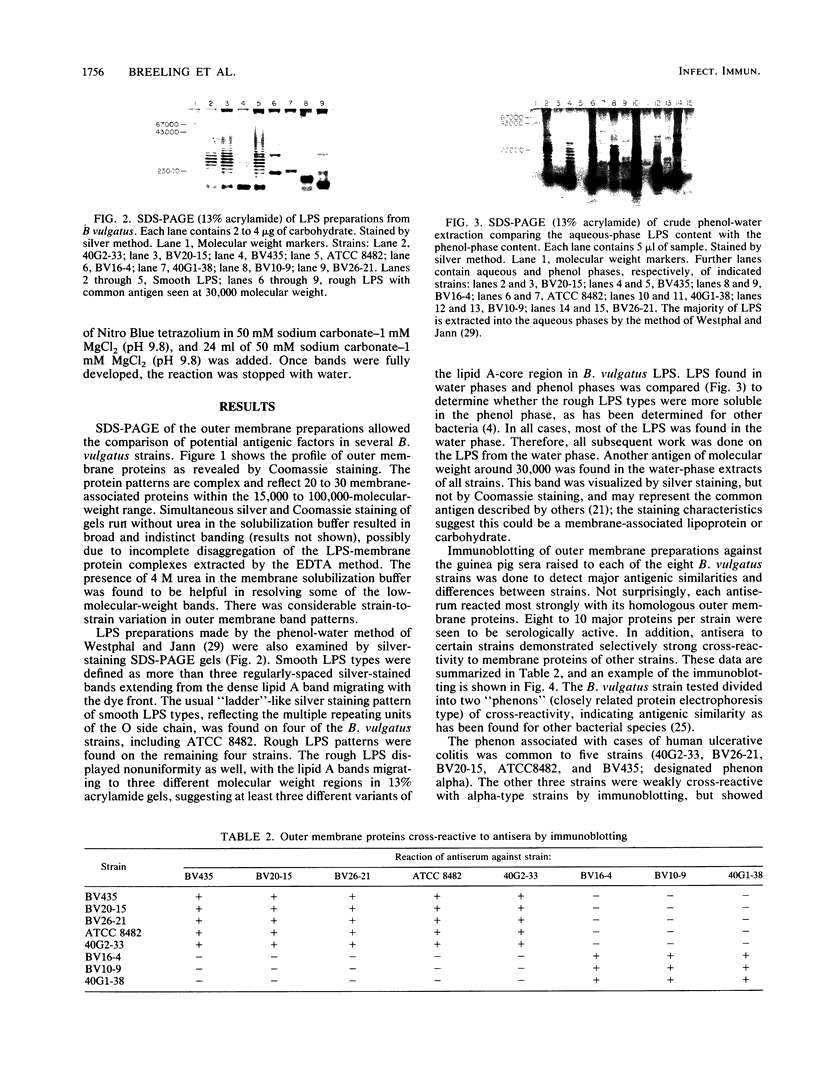


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Suhail A. A., Reid P. E., Culling C. F., Dunn W. L., Clay M. G. Studies of the degraded carrageenan-induced colitis of rabbits. I. Changes in the epithelial glycoprotein O-acylated sialic acids associated with ulceration. Histochem J. 1984 May;16(5):543–553. doi: 10.1007/BF01041354. [DOI] [PubMed] [Google Scholar]
- Chong A. S., Parish C. R. Nonimmune lymphocyte-macrophage interaction. II. Evidence that the interaction involves sulfated polysaccharide recognition. Cell Immunol. 1985 May;92(2):277–289. doi: 10.1016/0008-8749(85)90009-7. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., McAdam K. P., Kasper D. L. Lipopolysaccharides from Bacteroides fragilis are mitogenic for spleen cells from endotoxin responder and nonresponder mice. Infect Immun. 1982 Jun;36(3):1139–1145. doi: 10.1128/iai.36.3.1139-1145.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper D. L., Hayes M. E., Reinap B. G., Craft F. O., Onderdonk A. B., Polk B. F. Isolation and identification of encapsulated strains of Bacteroides fragilis. J Infect Dis. 1977 Jul;136(1):75–81. doi: 10.1093/infdis/136.1.75. [DOI] [PubMed] [Google Scholar]
- Kasper D. L., Seiler M. W. Immunochemical characterization of the outer membrane complex of Bacteroides fragilis subspecies fragilis. J Infect Dis. 1975 Oct;132(4):440–450. doi: 10.1093/infdis/132.4.440. [DOI] [PubMed] [Google Scholar]
- Kotarski S. F., Salyers A. A. Isolation and characterization of outer membranes of Bacteroides thetaiotaomicron grown on different carbohydrates. J Bacteriol. 1984 Apr;158(1):102–109. doi: 10.1128/jb.158.1.102-109.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lederer E. Synthetic immunostimulants derived from the bacterial cell wall. J Med Chem. 1980 Aug;23(8):819–825. doi: 10.1021/jm00182a001. [DOI] [PubMed] [Google Scholar]
- Mansheim B. J., Onderdonk A. B., Kasper D. L. Immunochemical characterization of surface antigens of Bacteroides melaninogenicus. Rev Infect Dis. 1979 Mar-Apr;1(2):263–277. doi: 10.1093/clinids/1.2.263. [DOI] [PubMed] [Google Scholar]
- Melchers F., Braun V., Galanos C. The lipoprotein of the outer membrane of Escherichia coli: a B-lymphocyte mitogen. J Exp Med. 1975 Aug 1;142(2):473–482. doi: 10.1084/jem.142.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah I., Glick B. Effect of carrageenan on the histology of the bursa of fabricius and the humoral immune response to Salmonella O antigen. Anat Rec. 1986 Apr;214(4):398–404. doi: 10.1002/ar.1092140410. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Bronson R., Cisneros R. Comparison of Bacteroides vulgatus strains in the enhancement of experimental ulcerative colitis. Infect Immun. 1987 Mar;55(3):835–836. doi: 10.1128/iai.55.3.835-836.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderdonk A. B., Cisneros R. L., Bronson R. T. Enhancement of experimental ulcerative colitis by immunization with Bacteroides vulgatus. Infect Immun. 1983 Nov;42(2):783–788. doi: 10.1128/iai.42.2.783-788.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderdonk A. B., Hermos J. A., Dzink J. L., Bartlett J. G. Protective effect of metronidazole in experimental ulcerative colitis. Gastroenterology. 1978 Mar;74(3):521–526. [PubMed] [Google Scholar]
- Onderdonk A. B., Steeves R. M., Cisneros R. L., Bronson R. T. Adoptive transfer of immune enhancement of experimental ulcerative colitis. Infect Immun. 1984 Oct;46(1):64–67. doi: 10.1128/iai.46.1.64-67.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poxton I. R., Brown R. Immunochemistry of the surface carbohydrate antigens of Bacteroides fragilis and definition of a common antigen. J Gen Microbiol. 1986 Sep;132(9):2475–2481. doi: 10.1099/00221287-132-9-2475. [DOI] [PubMed] [Google Scholar]
- Poxton I. R., Ip M. K. The cell-surface antigens of Bacteroides vulgatus. J Gen Microbiol. 1981 Sep;126(1):103–109. doi: 10.1099/00221287-126-1-103. [DOI] [PubMed] [Google Scholar]
- Takahashi F., Das K. M. Isolation and characterization of a colonic autoantigen specifically recognized by colon tissue-bound immunoglobulin G from idiopathic ulcerative colitis. J Clin Invest. 1985 Jul;76(1):311–318. doi: 10.1172/JCI111963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targan S. R., Kagnoff M. F., Brogan M. D., Shanahan F. Immunologic mechanisms in intestinal diseases. Ann Intern Med. 1987 Jun;106(6):853–870. doi: 10.7326/0003-4819-106-6-853. [DOI] [PubMed] [Google Scholar]
- Taylor A. J., Costas M., Owen R. J. Numerical analysis of electrophoretic protein patterns of Bacteroides ureolyticus clinical isolates. J Clin Microbiol. 1987 Apr;25(4):660–666. doi: 10.1128/jcm.25.4.660-666.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. J., Dawson C. A., Owen R. J. The identification of Bacteroides ureolyticus from patients with non-gonococcal urethritis by conventional biochemical tests and by DNA and protein analyses. J Med Microbiol. 1986 Mar;21(2):109–116. doi: 10.1099/00222615-21-2-109. [DOI] [PubMed] [Google Scholar]
- Weintraub A., Larsson B. E., Lindberg A. A. Chemical and immunochemical analyses of Bacteroides fragilis lipopolysaccharides. Infect Immun. 1985 Jul;49(1):197–201. doi: 10.1128/iai.49.1.197-201.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]