Abstract
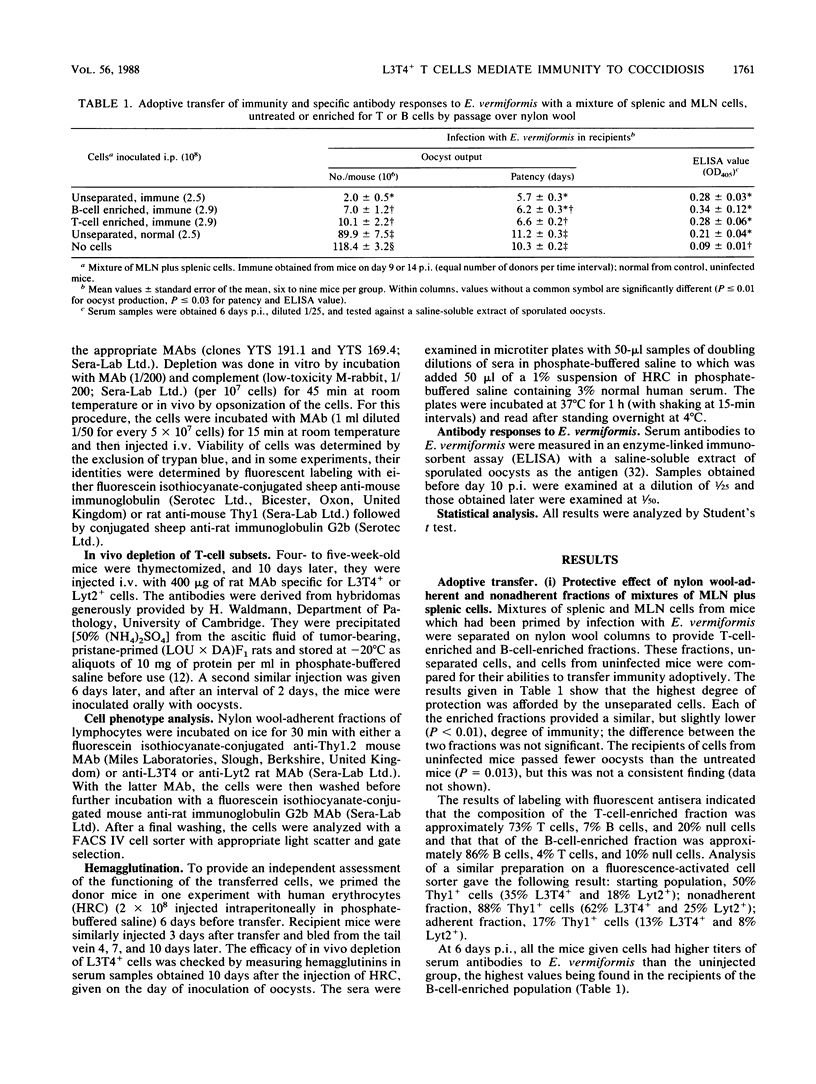
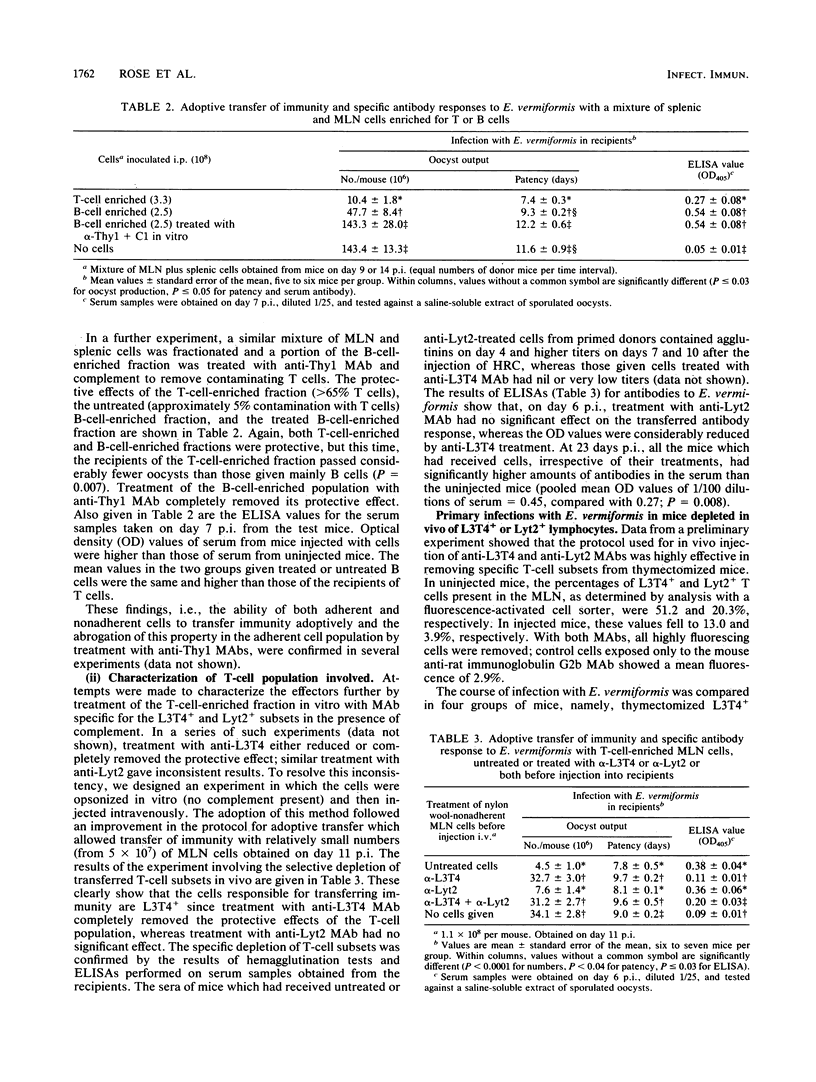
Immunity to infection with Eimeria vermiformis was transferred in NIH mice by both the nylon wool-adherent (B-cell-enriched) and nonadherent (T-cell-enriched) fractions of lymphocytes (spleen and mesenteric lymph node) taken from infected donors. Transfer was more variable with the adherent fraction, and when contaminating T cells were removed by treatment with anti-Thy1 monoclonal antibody (MAb) and complement, this fraction lost all protective activity. The protective effect of T-cell-enriched populations of mesenteric lymphocytes was abrogated by treatment with anti-L3T4 MAb and complement in vitro before transfer or by opsonization with this MAb in vitro before intravenous inoculation into recipients. Similar treatments of cells with anti-Lyt2 MAb did not have this effect, confirming that Thy1+ L3T4+ cells mediate the adoptive transfer of immunity to E. vermiformis. Thy1+ L3T4+ cells were also shown to limit the replication of E. vermiformis in primary infections: mice depleted of this subset (by thymectomy followed by intravenous injection of anti-L3T4 MAb) passed greater numbers of oocysts over a longer period of time than did mice similarly depleted of Lyt2+ cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chinchilla M., Frenkel J. K. Mediation of immunity to intracellular infection (Toxoplasma and Besnoitia) within somatic cells. Infect Immun. 1978 Mar;19(3):999–1012. doi: 10.1128/iai.19.3.999-1012.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P. J., Parry S. H., Porter P. The role of secretory IgA in anti-coccidial immunity in the chicken. Immunology. 1978 May;34(5):879–888. [PMC free article] [PubMed] [Google Scholar]
- Douglass T. G., Speer C. A. Effects of intestinal contents from normal and immunized mice on sporozoites of Eimeria falciformis. J Protozool. 1985 Feb;32(1):156–163. doi: 10.1111/j.1550-7408.1985.tb03031.x. [DOI] [PubMed] [Google Scholar]
- Grencis R. K., Riedlinger J., Wakelin D. L3T4-positive T lymphoblasts are responsible for transfer of immunity to Trichinella spiralis in mice. Immunology. 1985 Oct;56(2):213–218. [PMC free article] [PubMed] [Google Scholar]
- Handwerger B. S., Schwartz R. H. Separation of murine lymphoid cells using nylon wool columns. Recovery of the B cell-enriched population. Transplantation. 1974 Dec;18(6):544–548. doi: 10.1097/00007890-197412000-00013. [DOI] [PubMed] [Google Scholar]
- Heyworth M. F., Carlson J. R., Ermak T. H. Clearance of Giardia muris infection requires helper/inducer T lymphocytes. J Exp Med. 1987 Jun 1;165(6):1743–1748. doi: 10.1084/jem.165.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kiyono H., McGhee J. R., Mosteller L. M., Eldridge J. H., Koopman W. J., Kearney J. F., Michalek S. M. Murine Peyer's patch T cell clones. Characterization of antigen-specific helper T cells for immunoglobulin A responses. J Exp Med. 1982 Oct 1;156(4):1115–1130. doi: 10.1084/jem.156.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klesius P. H., Hinds S. E. Strain-dependent differences in murine susceptibility to coccidia. Infect Immun. 1979 Dec;26(3):1111–1115. doi: 10.1128/iai.26.3.1111-1115.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. D., Bogitsh B. J. Schistosoma japonicum: biochemistry and cytochemistry of dipeptidyl aminopeptidase-II-like activity in adults. Exp Parasitol. 1985 Oct;60(2):163–170. doi: 10.1016/0014-4894(85)90019-0. [DOI] [PubMed] [Google Scholar]
- Liburd E. M., Armstrong W. D., Mahrt J. L. Immunity to the protozoan parasite Eimeria nieschulzi in inbred CD-F rats. Cell Immunol. 1973 Jun;7(3):444–452. doi: 10.1016/0008-8749(73)90208-6. [DOI] [PubMed] [Google Scholar]
- Lightman S., Cobbold S., Waldmann H., Askonas B. A. Do L3T4+ T cells act as effector cells in protection against influenza virus infection. Immunology. 1987 Sep;62(1):139–144. [PMC free article] [PubMed] [Google Scholar]
- Lillehoj H. S., Ruff M. D. Comparison of disease susceptibility and subclass-specific antibody response in SC and FP chickens experimentally inoculated with Eimeria tenella, E. acervulina, or E. maxima. Avian Dis. 1987 Jan-Mar;31(1):112–119. [PubMed] [Google Scholar]
- Long P. L., Millard B. J., Joyner L. P., Norton C. C. A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet Lat. 1976 Jul-Sep;6(3):201–217. [PubMed] [Google Scholar]
- MacDonald H. R., Blanc C., Lees R. K., Sordat B. Abnormal distribution of T cell subsets in athymic mice. J Immunol. 1986 Jun 15;136(12):4337–4339. [PubMed] [Google Scholar]
- Mesfin G. M., Bellamy J. E. Thymic dependence of immunity to Eimeria falciformis var. pragensis in mice. Infect Immun. 1979 Feb;23(2):460–464. doi: 10.1128/iai.23.2.460-464.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockett A. P., Rose M. E. Immune responses to eimeria: quantification of antibody isotypes to Eimeria tenella in chicken serum and bile by means of the ELISA. Parasite Immunol. 1986 Sep;8(5):481–489. doi: 10.1111/j.1365-3024.1986.tb00863.x. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Guyre P. M. Inhibition of growth of Toxoplasma gondii in cultured fibroblasts by human recombinant gamma interferon. Infect Immun. 1984 May;44(2):211–216. doi: 10.1128/iai.44.2.211-216.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommel M., Heydorn A. O. Versuche zur Ubertragung der Immunität gegen Eimeria-Infektionen durch Lymphozyten. Z Parasitenkd. 1971;36(3):242–250. [PubMed] [Google Scholar]
- Rose M. E., Hesketh P. Coccidiosis: T-lymphocyte-dependent effects of infection with Eimeria nieschulzi in rats. Vet Immunol Immunopathol. 1982 Sep;3(5):499–508. doi: 10.1016/0165-2427(82)90015-0. [DOI] [PubMed] [Google Scholar]
- Rose M. E., Hesketh P. Eimeria tenella: effects of immunity on sporozoites within the lumen of the small intestine. Exp Parasitol. 1987 Jun;63(3):337–344. doi: 10.1016/0014-4894(87)90181-0. [DOI] [PubMed] [Google Scholar]
- Rose M. E., Hesketh P. Eimerian life cycles: the patency of eimeria vermiformis, but not Eimeria pragensis, is subject to host (Mus musculus) influence. J Parasitol. 1986 Dec;72(6):949–954. [PubMed] [Google Scholar]
- Rose M. E., Hesketh P. Immunity to coccidia in chickens: adoptive transfer with peripheral blood lymphocytes and spleen cells. Parasite Immunol. 1982 May;4(3):171–185. doi: 10.1111/j.1365-3024.1982.tb00429.x. [DOI] [PubMed] [Google Scholar]
- Rose M. E., Hesketh P. Immunity to coccidiosis: T-lymphocyte- or B-lymphocyte-deficient animals. Infect Immun. 1979 Nov;26(2):630–637. doi: 10.1128/iai.26.2.630-637.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. E., Lawn A. M., Millard B. J. The effect of immunity on the early events in the life-cycle of Eimeria tenella in the caecal mucosa of the chicken. Parasitology. 1984 Apr;88(Pt 2):199–210. doi: 10.1017/s0031182000054470. [DOI] [PubMed] [Google Scholar]
- Rose M. E., Ogilvie B. M., Hesketh P., Festing M. F. Failure of nude (athymic) rats to become resistant to reinfection with the intestinal coccidian parasite Eimeria nieschulzi or the nematode Nippostrongylus brasiliensis. Parasite Immunol. 1979 Summer;1(2):125–132. doi: 10.1111/j.1365-3024.1979.tb00700.x. [DOI] [PubMed] [Google Scholar]
- Rose M. E., Owen D. G., Hesketh P. Susceptibility to coccidiosis: effect of strain of mouse on reproduction of Eimeria vermiformis. Parasitology. 1984 Feb;88(Pt 1):45–54. doi: 10.1017/s0031182000054330. [DOI] [PubMed] [Google Scholar]
- Rose M. E., Peppard J. V., Hobbs S. M. Coccidiosis: characterization of antibody responses to infection with Eimeria nieschulzi. Parasite Immunol. 1984 Jan;6(1):1–12. doi: 10.1111/j.1365-3024.1984.tb00777.x. [DOI] [PubMed] [Google Scholar]
- Rose M. E., Wakelin D., Joysey H. S., Hesketh P. Immunity to coccidiosis: adoptive transfer in NIH mice challenged with Eimeria vermiformis. Parasite Immunol. 1988 Jan;10(1):59–69. doi: 10.1111/j.1365-3024.1988.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Speer C. A., Reduker D. W., Burgess D. E., Whitmire W. M., Splitter G. A. Lymphokine-induced inhibition of growth of Eimeria bovis and Eimeria papillata (Apicomplexa) in cultured bovine monocytes. Infect Immun. 1985 Nov;50(2):566–571. doi: 10.1128/iai.50.2.566-571.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



