Abstract
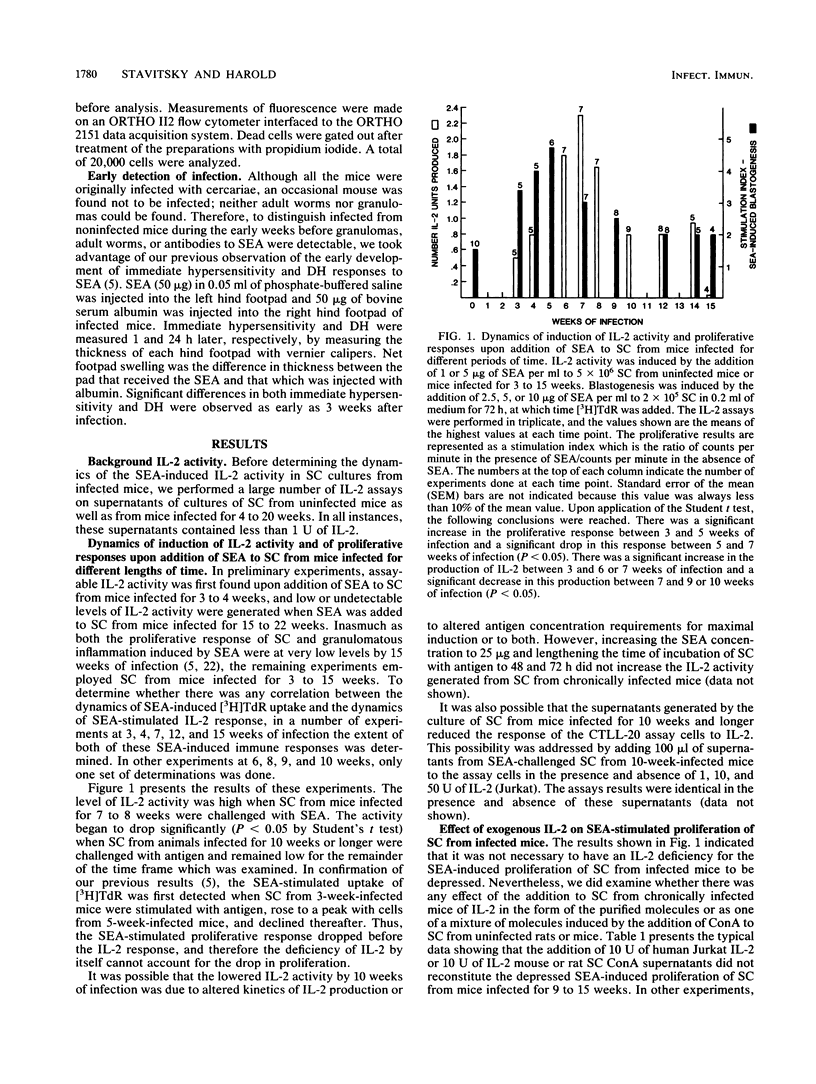
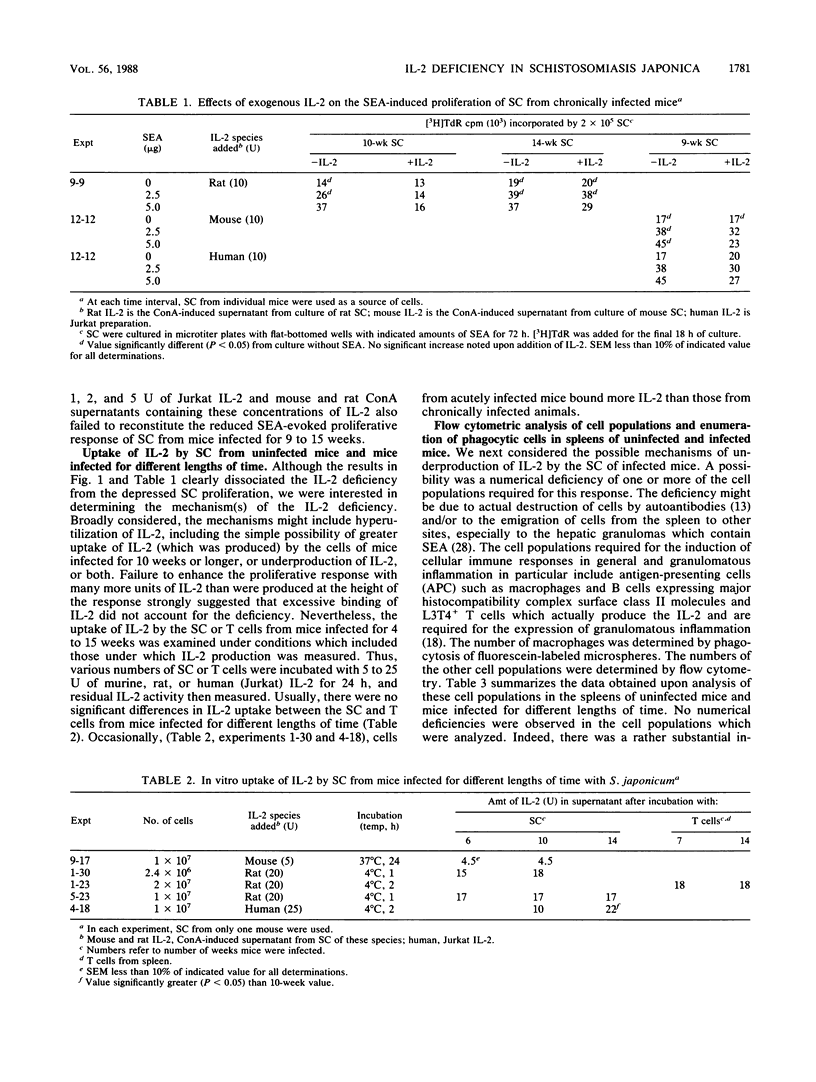
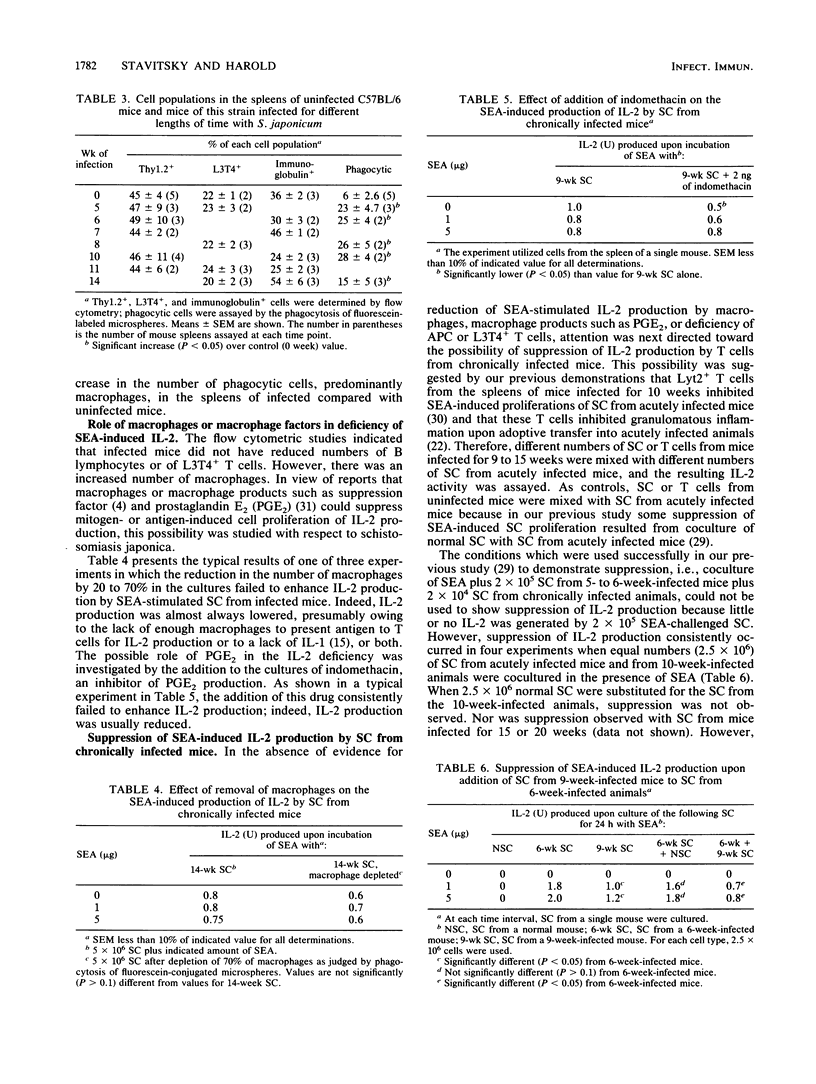
Schistosoma japonicum-infected C57BL/6 mice show similar dynamics of hepatic granulomatous inflammation (HGI) and delayed hypersensitivity (DH) elicited by soluble egg antigens (SEA) which reach peak levels at 9 weeks of infection and then spontaneously regress. The in vitro SEA-induced proliferation of spleen cells (SC) from infected animals attained its high point and then declined when SC from 5-week-infected mice were used. The present study determined the dynamics of interleukin-2 (IL-2) production by SEA-challenged SC from infected mice in an attempt to link the level of IL-2 production to the spontaneous regression of the aforementioned T-cell-mediated immune responses. The production of IL-2 by SEA-stimulated SC reached its peak when cells from 7-week-infected mice were challenged at least 2 weeks after the peak of the proliferative response, but declined at about the same time as the HGI and DH responses. Therefore, the decline in IL-2 activity cannot alone explain the diminished proliferative response but could account for the reduction in HGI and DH in vivo. Some possible mechanisms that might explain the IL-2 deficiency were examined. This deficiency is not due to the in vitro binding of IL-2 by the SC of infected mice and is, therefore, likely to be due to underproduction of IL-2. Nor is the deficiency explained by reduced numbers of antigen-presenting cells (macrophages and B cells) or of L3T4+ T lymphocytes or by suppression of IL-2 production by macrophages or macrophage products such as prostaglandins. However, suppression of IL-2 production was observed consistently upon coculture of SC from acutely infected mice with SC from mice infected for 10 weeks. The cells which suppress appear to be Lyt2+ T cells. The data are consistent with the hypothesis that suppressor T cells inhibit the production of IL-2 and perhaps of other cytokines or lymphokines and that this suppression explains the spontaneous down-regulation of HGI which occurs during schistosomiasis japonica.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell J. D., Robb R. J., Malek T. R. Proliferation of T lymphocytes in response to interleukin 2 varies with their state of activation. J Immunol. 1986 Oct 15;137(8):2572–2578. [PubMed] [Google Scholar]
- Butler L. D., DeRiso P. E., Marder P., Scheetz M. E. In vivo expression and regulation of murine IL 2 receptors after antigen sensitization. J Immunol. 1987 Jan 15;138(2):470–477. [PubMed] [Google Scholar]
- Farrar W. L., Birchenall-Sparks M. C., Young H. B. Interleukin 2 induction of interferon-gamma mRNA synthesis. J Immunol. 1986 Dec 15;137(12):3836–3840. [PubMed] [Google Scholar]
- Fujiwara H., Toossi Z., Ohnishi K., Edmonds K., Ellner J. J. Spontaneous production of a suppressor factor by a human macrophage-like cell line U937. II. Suppression of antigen- and mitogen-induced blastogenesis, IL 2 production and IL 2 receptor expression in T lymphocytes. J Immunol. 1987 Jan 1;138(1):197–203. [PubMed] [Google Scholar]
- Garb K. S., Stavitsky A. B., Mahmoud A. A. Dynamics of antigen and mitogen-induced responses in murine schistosomiasis japonica: in vitro comparison between hepatic granulomas and splenic cells. J Immunol. 1981 Jul;127(1):115–120. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Gillis S., Smith K. A., Watson J. Biochemical characterization of lymphocyte regulatory molecules. II. Purification of a class of rat and human lymphokines. J Immunol. 1980 Apr;124(4):1954–1962. [PubMed] [Google Scholar]
- Heckford S. E., Gelmann E. P., Agnor C. L., Jacobson S., Zinn S., Matis L. A. Distinct signals are required for proliferation and lymphokine gene expression in murine T cell clones. J Immunol. 1986 Dec 1;137(11):3652–3663. [PubMed] [Google Scholar]
- Hoffenbach A., Lagrange P. H., Bach M. A. Deficit of interleukin 2 production associated with impaired T-cell proliferative responses in Mycobacterium lepraemurium infection. Infect Immun. 1983 Jan;39(1):109–116. doi: 10.1128/iai.39.1.109-116.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M. K., Pardoll D. M., Mizuguchi J., Chused T. M., Schwartz R. H. Molecular events in the induction of a nonresponsive state in interleukin 2-producing helper T-lymphocyte clones. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5409–5413. doi: 10.1073/pnas.84.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan G. W. Basis for the probit analysis of an interferon plaque reduction assay. J Gen Virol. 1972 Jan;14(1):49–61. doi: 10.1099/0022-1317-14-1-49. [DOI] [PubMed] [Google Scholar]
- Kawabata M., Hosaka Y., Kumada M., Matsui N., Kobayakawa T. Thymocytotoxic autoantibodies found in mice infected with Schistosoma japonicum. Infect Immun. 1981 May;32(2):438–442. doi: 10.1128/iai.32.2.438-442.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Allred C., Cohen S., Yoshida T. Role of interleukin 1 in experimental pulmonary granuloma in mice. J Immunol. 1985 Jan;134(1):358–364. [PubMed] [Google Scholar]
- Kobayashi K., Allred C., Yoshida T. Mechanisms of suppressed cell-mediated immunity and impaired antigen-induced interleukin 2 production in granuloma-bearing mice. J Immunol. 1985 Nov;135(5):2996–3003. [PubMed] [Google Scholar]
- Kresina T. F., Olds G. R. Concomitant cellular and humoral expression of a regulatory cross-reactive idiotype in acute Schistosoma japonicum infection. Infect Immun. 1986 Jul;53(1):90–94. doi: 10.1128/iai.53.1.90-94.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Mathew R. C., Boros D. L. Anti-L3T4 antibody treatment suppresses hepatic granuloma formation and abrogates antigen-induced interleukin-2 production in Schistosoma mansoni infection. Infect Immun. 1986 Dec;54(3):820–826. doi: 10.1128/iai.54.3.820-826.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlin R. L., Hofman F. M., Horwitz D. A., Husmann L. A., Gillis S., Taylor C. R., Rea T. H. In situ identification of cells in human leprosy granulomas with monoclonal antibodies to interleukin 2 and its receptor. J Immunol. 1984 Jun;132(6):3085–3090. [PubMed] [Google Scholar]
- Olds G. R., Olveda R., Tracy J. W., Mahmoud A. A. Adoptive transfer of modulation of granuloma formation and hepatosplenic disease in murine schistosomiasis japonica by serum from chronically infected animals. J Immunol. 1982 Mar;128(3):1391–1393. [PubMed] [Google Scholar]
- Olds G. R., Stavitsky A. B. Mechanisms of in vivo modulation of granulomatous inflammation in murine schistosomiasis japonicum. Infect Immun. 1986 May;52(2):513–518. doi: 10.1128/iai.52.2.513-518.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owhashi M., Maruyama H., Nawa Y. Eosinophil chemotactic lymphokine produced by egg-associated granulomas in murine schistosomiasis japonicum. Infect Immun. 1986 Dec;54(3):723–727. doi: 10.1128/iai.54.3.723-727.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner N. E., Finke J. H. Interleukin 2 deficiency in murine Leishmaniasis donovani and its relationship to depressed spleen cell responses to phytohemagglutinin. J Immunol. 1983 Sep;131(3):1487–1491. [PubMed] [Google Scholar]
- Sileghem M., Hamers R., De Baetselier P. Active suppression of interleukin 2 secretion in mice infected with Trypanosoma brucei AnTat 1.1.E. Parasite Immunol. 1986 Nov;8(6):641–649. doi: 10.1111/j.1365-3024.1986.tb00877.x. [DOI] [PubMed] [Google Scholar]
- Stavitsky A. B., Garb K. S. Spontaneous and egg antigen-induced syntheses of immunoglobulin and antibody by spleen cells and hepatic granulomas of mice infected with Schistosoma japonicum. Infect Immun. 1984 Oct;46(1):276–278. doi: 10.1128/iai.46.1.276-278.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavitsky A. B., Olds G. R., Peterson L. B. Regulation of egg antigen-induced in vitro proliferative response by splenic suppressor T cells in murine Schistosoma japonicum infection. Infect Immun. 1985 Sep;49(3):635–640. doi: 10.1128/iai.49.3.635-640.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarleton R. L., Kuhn R. E. Restoration of in vitro immune responses of spleen cells from mice infected with Trypanosoma cruzi by supernatants containing interleukin 2. J Immunol. 1984 Sep;133(3):1570–1575. [PubMed] [Google Scholar]
- Walker C., Kristensen F., Bettens F., deWeck A. L. Lymphokine regulation of activated (G1) lymphocytes. I. Prostaglandin E2-induced inhibition of interleukin 2 production. J Immunol. 1983 Apr;130(4):1770–1773. [PubMed] [Google Scholar]
- Yamashita T., Watanabe T., Sendo F. Studies on the immunological disturbance in murine schistosomiasis japonica from the viewpoint of the interleukin cascade reaction. Immunology. 1987 Oct;62(2):215–221. [PMC free article] [PubMed] [Google Scholar]


