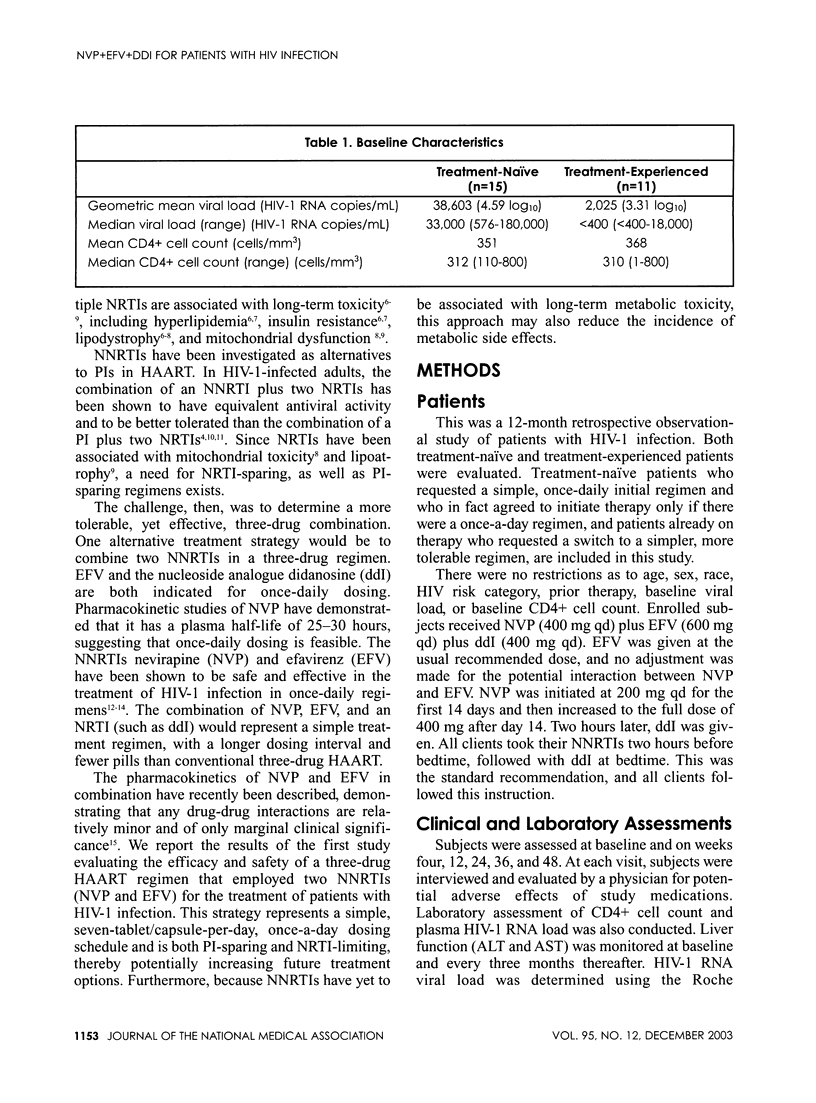
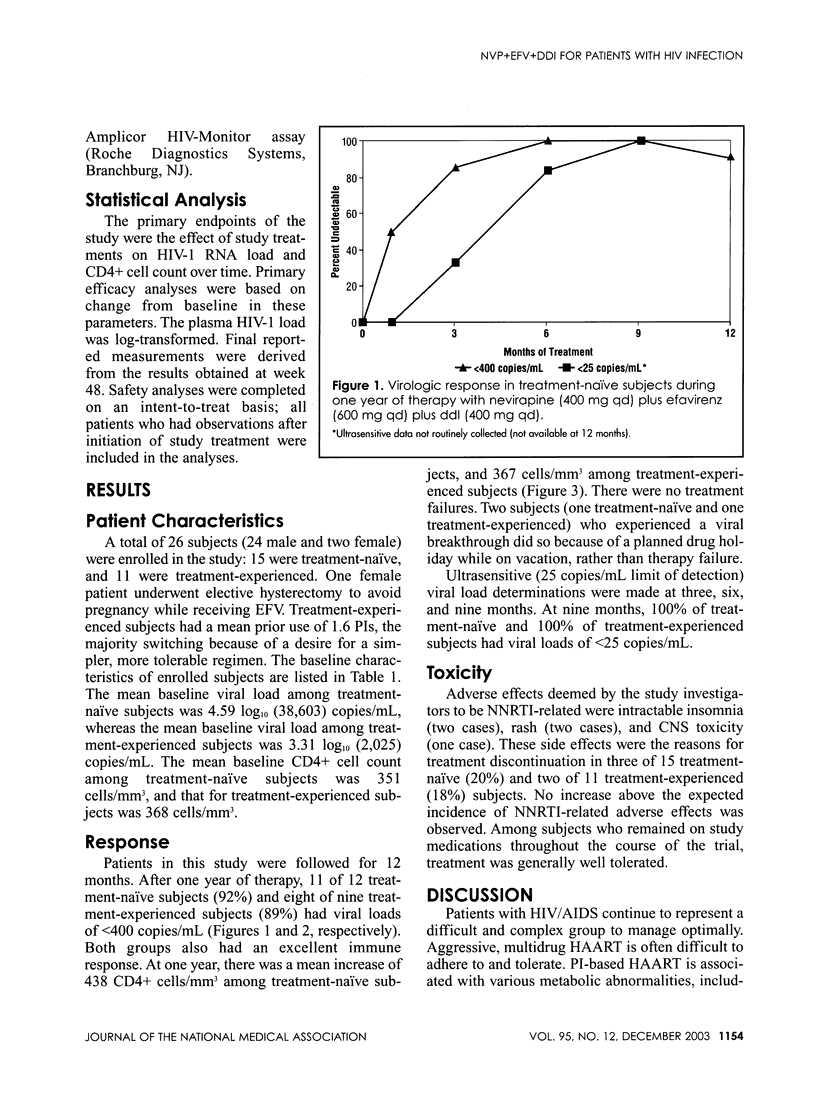
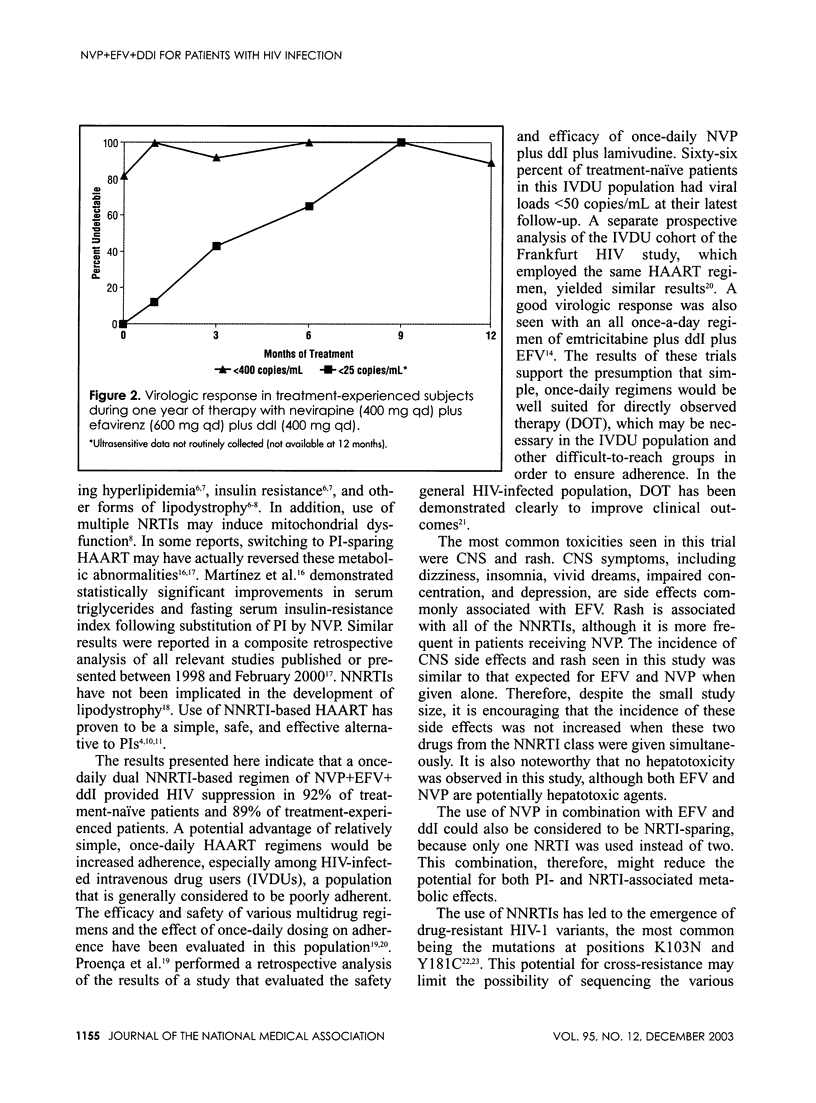
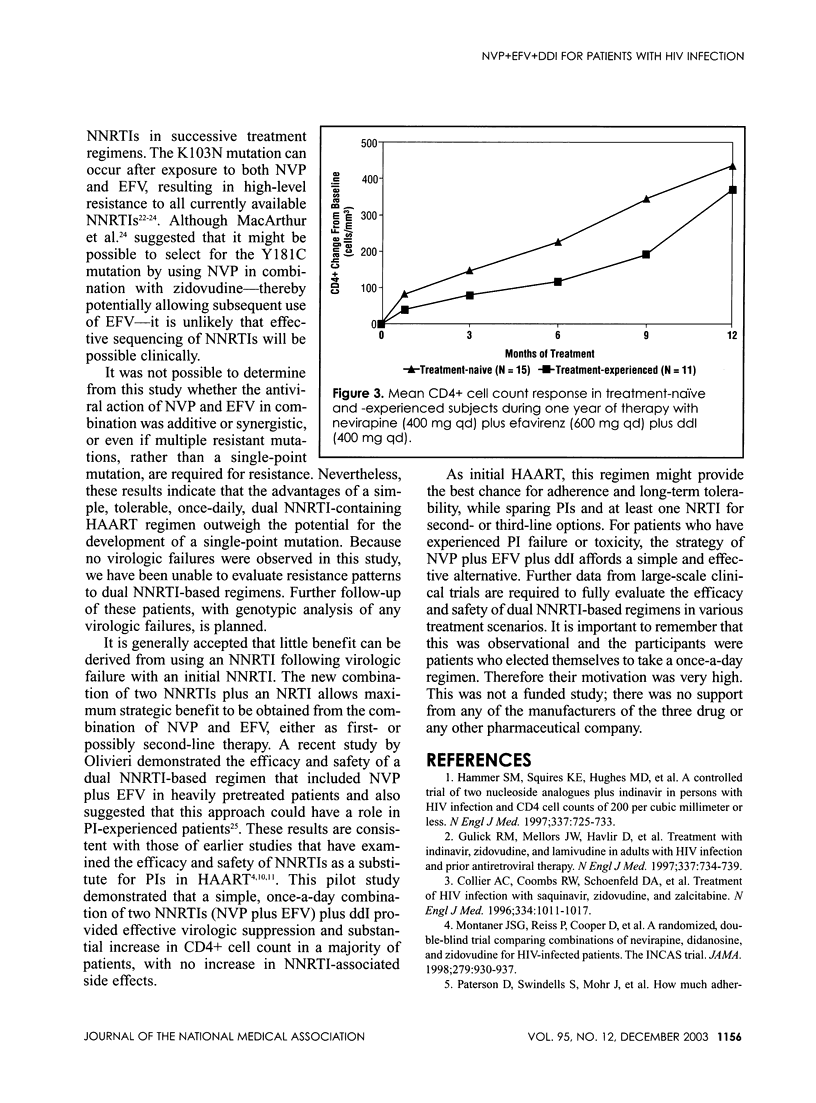
Abstract
Conventional highly active antiretroviral therapy (HAART) regimens used to treat human immunodeficiency virus (HIV) infection typically use nucleoside reverse transcriptase inhibitors (NRTIs) and either a protease inhibitor (PI) or a non-nucleoside reverse transcriptase inhibitor (NNRTI). Because PI-based regimens are associated with significant long-term toxicity and adherence difficulty, there is a need for novel regimens that maximize combination treatment options. This 12-month, observational, cohort study evaluated the efficacy, safety, and tolerability of a novel three-drug HAART regimen. Drug treatment consisted of nevirapine (NVP), efavirenz (EFV), and didanosine (ddl). Twenty-six treatment-naive and -experienced HIV-1+ men and women were included in the study. Assessment consisted of CD4+ cell count, plasma HIV-1 RNA load, and adverse effects of study medications. After one year of therapy, 11/12 treatment-naive subjects (92%) and 8/9 treatment-experienced subjects (89%) had viral loads < 400 copies/mL. Both groups also had an excellent immune response. At one year, there was a mean increase of 438 CD4+ cells/mm3 among treatment-naive subjects and 367 cells/mm3 among treatment-experienced subjects. Treatment-limiting adverse effects occurred in 3/15 treatment-naive (20%) and 2/11 treatment-experienced (18%) subjects. These preliminary data suggest that the combination of NVP, EFV, and ddl is simple, safe, and effective.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinkman K., Smeitink J. A., Romijn J. A., Reiss P. Mitochondrial toxicity induced by nucleoside-analogue reverse-transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapy-related lipodystrophy. Lancet. 1999 Sep 25;354(9184):1112–1115. doi: 10.1016/S0140-6736(99)06102-4. [DOI] [PubMed] [Google Scholar]
- Carr A., Samaras K., Chisholm D. J., Cooper D. A. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 1998 Jun 20;351(9119):1881–1883. doi: 10.1016/S0140-6736(98)03391-1. [DOI] [PubMed] [Google Scholar]
- Collier A. C., Coombs R. W., Schoenfeld D. A., Bassett R. L., Timpone J., Baruch A., Jones M., Facey K., Whitacre C., McAuliffe V. J. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine, and zalcitabine. AIDS Clinical Trials Group. N Engl J Med. 1996 Apr 18;334(16):1011–1017. doi: 10.1056/NEJM199604183341602. [DOI] [PubMed] [Google Scholar]
- De Clercq E. The role of non-nucleoside reverse transcriptase inhibitors (NNRTIs) in the therapy of HIV-1 infection. Antiviral Res. 1998 Jun;38(3):153–179. doi: 10.1016/s0166-3542(98)00025-4. [DOI] [PubMed] [Google Scholar]
- Gulick R. M., Mellors J. W., Havlir D., Eron J. J., Gonzalez C., McMahon D., Richman D. D., Valentine F. T., Jonas L., Meibohm A. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997 Sep 11;337(11):734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- Hammer S. M., Squires K. E., Hughes M. D., Grimes J. M., Demeter L. M., Currier J. S., Eron J. J., Jr, Feinberg J. E., Balfour H. H., Jr, Deyton L. R. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997 Sep 11;337(11):725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- Leitz G., Robinson P. The development of lipodystrophy on a protease inhibitor-sparing highly active antiretroviral therapy regimen. AIDS. 2000 Mar 10;14(4):468–469. doi: 10.1097/00002030-200003100-00026. [DOI] [PubMed] [Google Scholar]
- Martínez E., Conget I., Lozano L., Casamitjana R., Gatell J. M. Reversion of metabolic abnormalities after switching from HIV-1 protease inhibitors to nevirapine. AIDS. 1999 May 7;13(7):805–810. doi: 10.1097/00002030-199905070-00009. [DOI] [PubMed] [Google Scholar]
- Molina J. M., Ferchal F., Rancinan C., Raffi F., Rozenbaum W., Sereni D., Morlat P., Journot V., Decazes J. M., Chêne G. Once-daily combination therapy with emtricitabine, didanosine, and efavirenz in human immunodeficiency virus-infected patients. J Infect Dis. 2000 Jul 13;182(2):599–602. doi: 10.1086/315711. [DOI] [PubMed] [Google Scholar]
- Montaner J. S., Reiss P., Cooper D., Vella S., Harris M., Conway B., Wainberg M. A., Smith D., Robinson P., Hall D. A randomized, double-blind trial comparing combinations of nevirapine, didanosine, and zidovudine for HIV-infected patients: the INCAS Trial. Italy, The Netherlands, Canada and Australia Study. JAMA. 1998 Mar 25;279(12):930–937. doi: 10.1001/jama.279.12.930. [DOI] [PubMed] [Google Scholar]
- Staszewski S., Morales-Ramirez J., Tashima K. T., Rachlis A., Skiest D., Stanford J., Stryker R., Johnson P., Labriola D. F., Farina D. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. Study 006 Team. N Engl J Med. 1999 Dec 16;341(25):1865–1873. doi: 10.1056/NEJM199912163412501. [DOI] [PubMed] [Google Scholar]


