Abstract
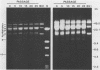
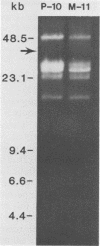
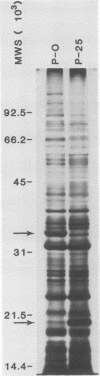
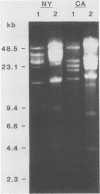
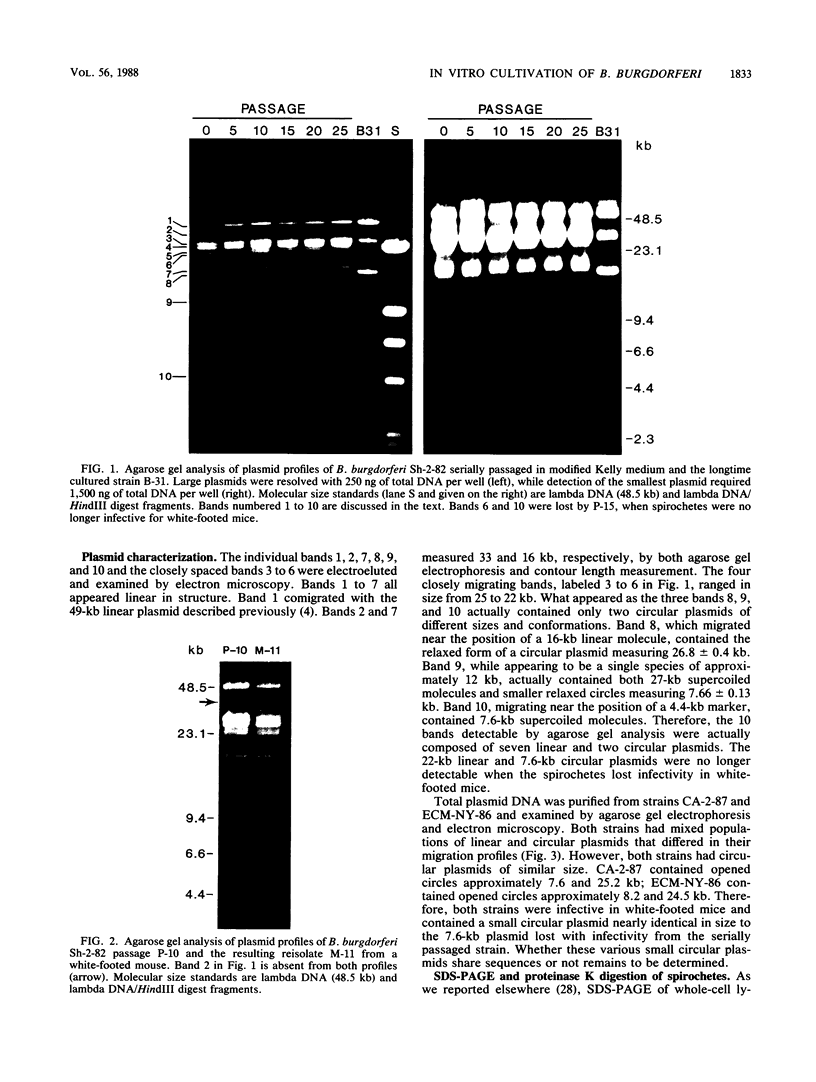
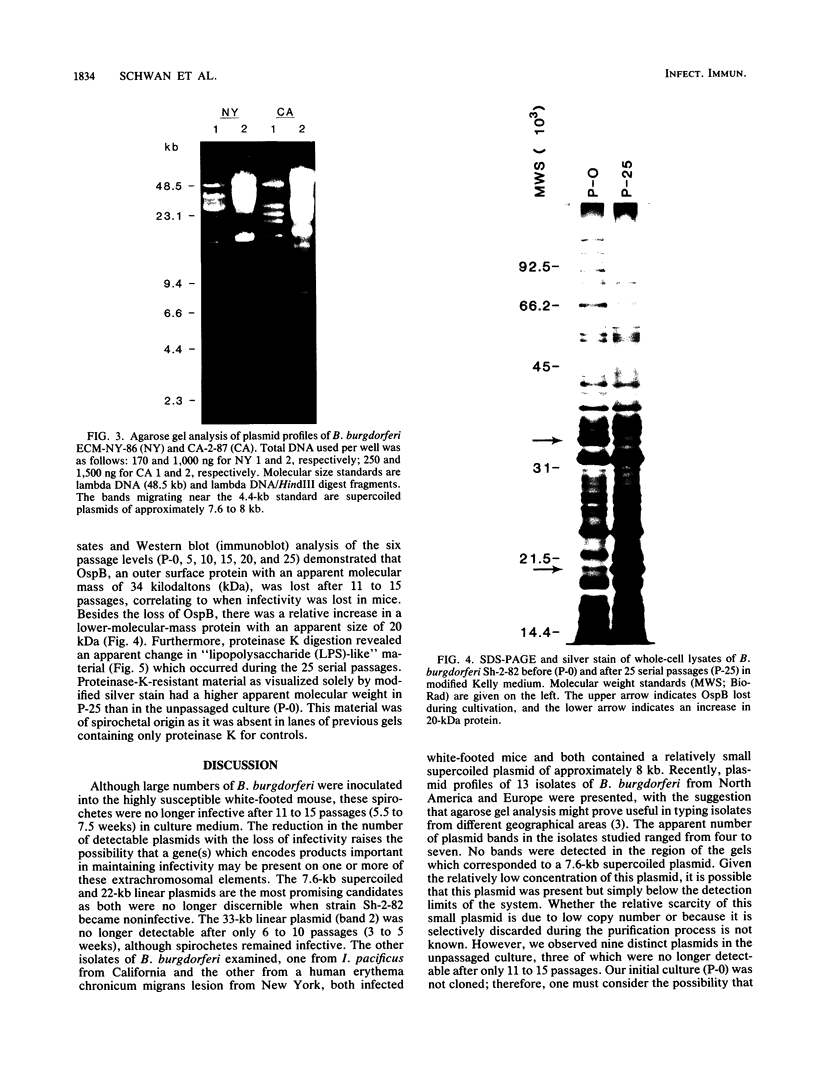
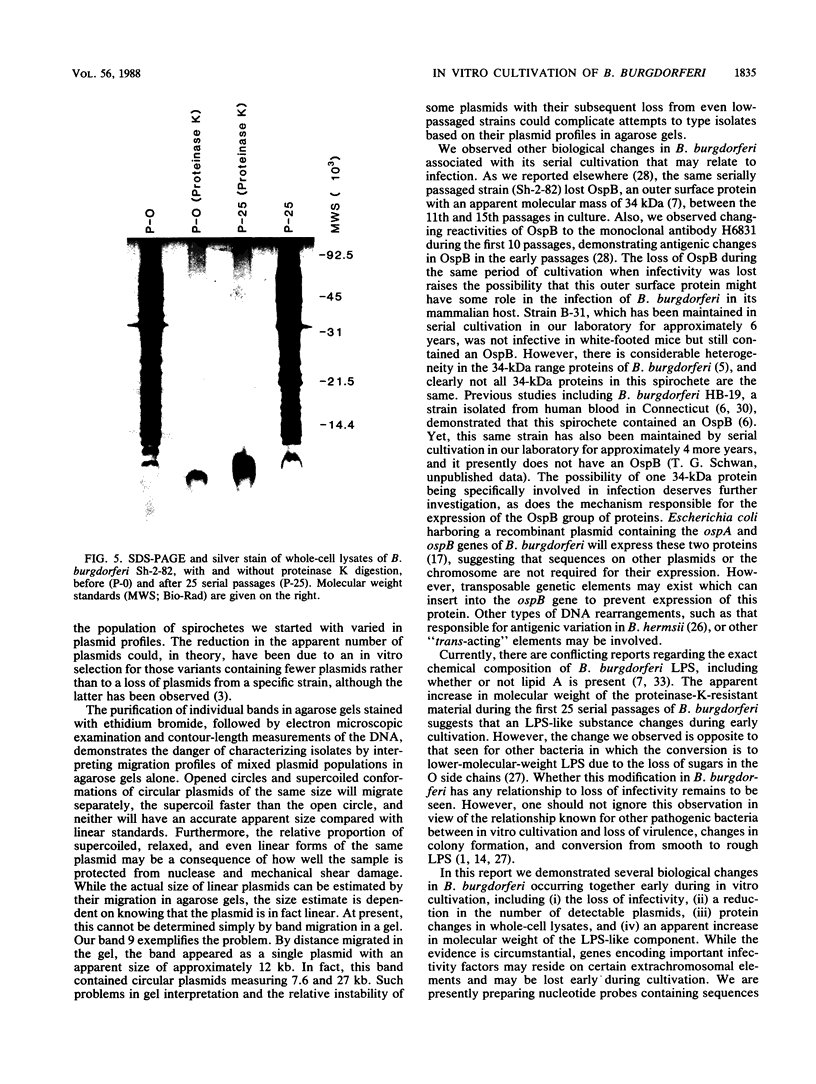
In vitro cultivation of Borrelia burgdorferi, the etiologic agent of Lyme spirochetosis, allows for the isolation and growth of this bacterium from infected tissues. However, continuous cultivation in modified Kelly medium causes a reduction in the number of detectable plasmids and the loss of infectivity in the white-footed mouse, Peromyscus leucopus. In an unpassaged culture of B. burgdorferi, nine plasmids were present, including seven linear plasmids ranging in size from 49 to 16 kilobases (kb) and two circular plasmids of 27 and 7.6 kb. The 7.6-kb circular and 22-kb linear plasmids were no longer detectable in spirochetes noninfective in white-footed mice, suggesting that a gene(s) encoding for factors responsible for infection may be present on one or more of these extrachromosomal elements. Furthermore, changes in spirochetal proteins and lipopolysaccharide-like material were observed also during early cultivation and may be related to loss of infectivity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G., Garon C. F. Linear plasmids of the bacterium Borrelia burgdorferi have covalently closed ends. Science. 1987 Jul 24;237(4813):409–411. doi: 10.1126/science.3603026. [DOI] [PubMed] [Google Scholar]
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G. Plasmid analysis of Borrelia burgdorferi, the Lyme disease agent. J Clin Microbiol. 1988 Mar;26(3):475–478. doi: 10.1128/jcm.26.3.475-478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Hayes S. F. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984 Jul;45(1):94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Todd W. J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983 Aug;41(2):795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck G., Habicht G. S., Benach J. L., Coleman J. L. Chemical and biologic characterization of a lipopolysaccharide extracted from the Lyme disease spirochete (Borrelia burgdorferi). J Infect Dis. 1985 Jul;152(1):108–117. doi: 10.1093/infdis/152.1.108. [DOI] [PubMed] [Google Scholar]
- Bissett M. L., Hill W. Characterization of Borrelia burgdorferi strains isolated from Ixodes pacificus ticks in California. J Clin Microbiol. 1987 Dec;25(12):2296–2301. doi: 10.1128/jcm.25.12.2296-2301.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Burgess E. C., Amundson T. E., Davis J. P., Kaslow R. A., Edelman R. Experimental inoculation of Peromyscus spp. with Borrelia burgdorferi: evidence of contact transmission. Am J Trop Med Hyg. 1986 Mar;35(2):355–359. doi: 10.4269/ajtmh.1986.35.355. [DOI] [PubMed] [Google Scholar]
- Elwell L. P., Shipley P. L. Plasmid-mediated factors associated with virulence of bacteria to animals. Annu Rev Microbiol. 1980;34:465–496. doi: 10.1146/annurev.mi.34.100180.002341. [DOI] [PubMed] [Google Scholar]
- Garon C. F. Electron microscopy of nucleic acids. Gene Amplif Anal. 1981;2:573–589. [PubMed] [Google Scholar]
- Hackstadt T., Peacock M. G., Hitchcock P. J., Cole R. L. Lipopolysaccharide variation in Coxiella burnetti: intrastrain heterogeneity in structure and antigenicity. Infect Immun. 1985 May;48(2):359–365. doi: 10.1128/iai.48.2.359-365.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe T. R., Mayer L. W., Barbour A. G. A single recombinant plasmid expressing two major outer surface proteins of the Lyme disease spirochete. Science. 1985 Feb 8;227(4687):645–646. doi: 10.1126/science.3969554. [DOI] [PubMed] [Google Scholar]
- Hyde F. W., Johnson R. C. Genetic analysis of Borrelia. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):119–122. doi: 10.1016/s0176-6724(86)80111-0. [DOI] [PubMed] [Google Scholar]
- Hyde F. W., Johnson R. C. Genetic relationship of lyme disease spirochetes to Borrelia, Treponema, and Leptospira spp. J Clin Microbiol. 1984 Aug;20(2):151–154. doi: 10.1128/jcm.20.2.151-154.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Marek N., Kodner C. Infection of Syrian hamsters with Lyme disease spirochetes. J Clin Microbiol. 1984 Dec;20(6):1099–1101. doi: 10.1128/jcm.20.6.1099-1101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. Cultivation of Borrelia hermsi. Science. 1971 Jul 30;173(3995):443–444. doi: 10.1126/science.173.3995.443. [DOI] [PubMed] [Google Scholar]
- Plasterk R. H., Simon M. I., Barbour A. G. Transposition of structural genes to an expression sequence on a linear plasmid causes antigenic variation in the bacterium Borrelia hermsii. Nature. 1985 Nov 21;318(6043):257–263. doi: 10.1038/318257a0. [DOI] [PubMed] [Google Scholar]
- Roantree R. J. Salmonella O antigens and virulence. Annu Rev Microbiol. 1967;21:443–466. doi: 10.1146/annurev.mi.21.100167.002303. [DOI] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W. Antigenic changes of Borrelia burgdorferi as a result of in vitro cultivation. J Infect Dis. 1987 Nov;156(5):852–853. doi: 10.1093/infdis/156.5.852-a. [DOI] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W., Schrumpf M. E., Karstens R. H. The urinary bladder, a consistent source of Borrelia burgdorferi in experimentally infected white-footed mice (Peromyscus leucopus). J Clin Microbiol. 1988 May;26(5):893–895. doi: 10.1128/jcm.26.5.893-895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere A. C., Grodzicki R. L., Kornblatt A. N., Craft J. E., Barbour A. G., Burgdorfer W., Schmid G. P., Johnson E., Malawista S. E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983 Mar 31;308(13):733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Stoenner H. G. Biology of Borrelia hermsii in Kelly medium. Appl Microbiol. 1974 Oct;28(4):540–543. doi: 10.1128/am.28.4.540-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoenner H. G., Dodd T., Larsen C. Antigenic variation of Borrelia hermsii. J Exp Med. 1982 Nov 1;156(5):1297–1311. doi: 10.1084/jem.156.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Rothenberg R. J., Barbour A. G. Absence of lipopolysaccharide in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1987 Sep;55(9):2311–2313. doi: 10.1128/iai.55.9.2311-2313.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Schierz G., Busch K. V. Immunochemical and immunological analysis of European Borrelia burgdorferi strains. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):92–102. doi: 10.1016/s0176-6724(86)80108-0. [DOI] [PubMed] [Google Scholar]