Abstract
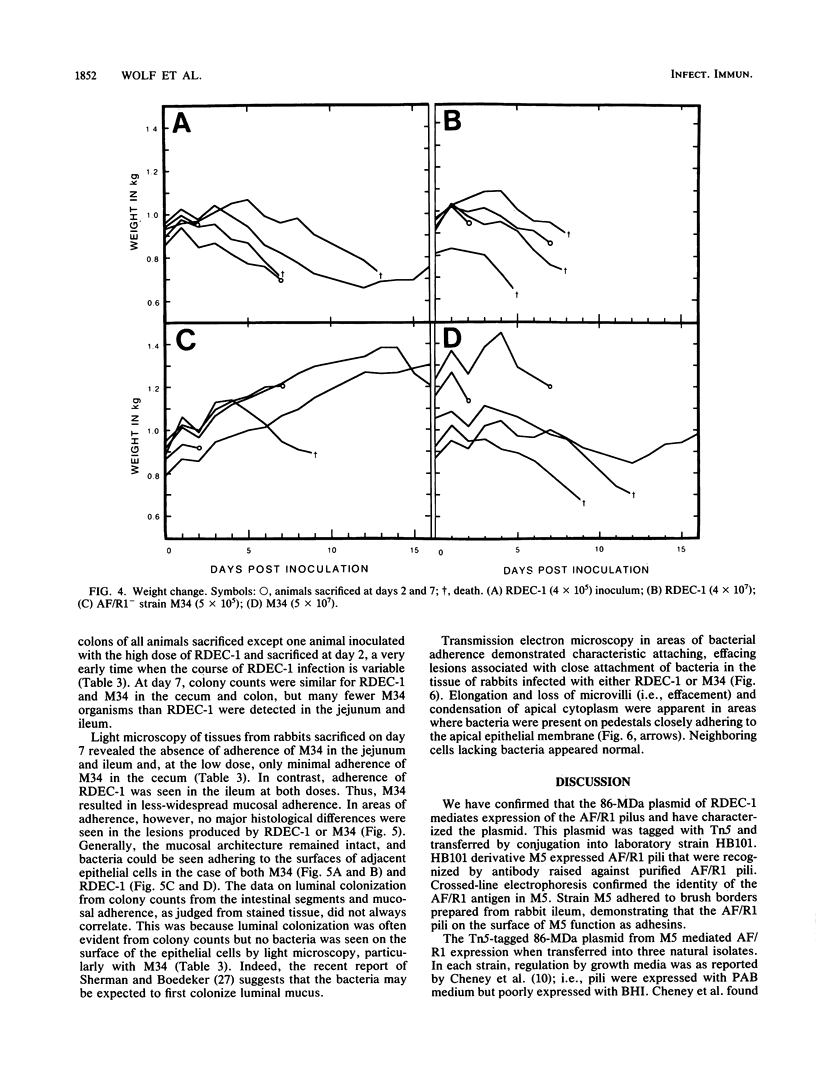
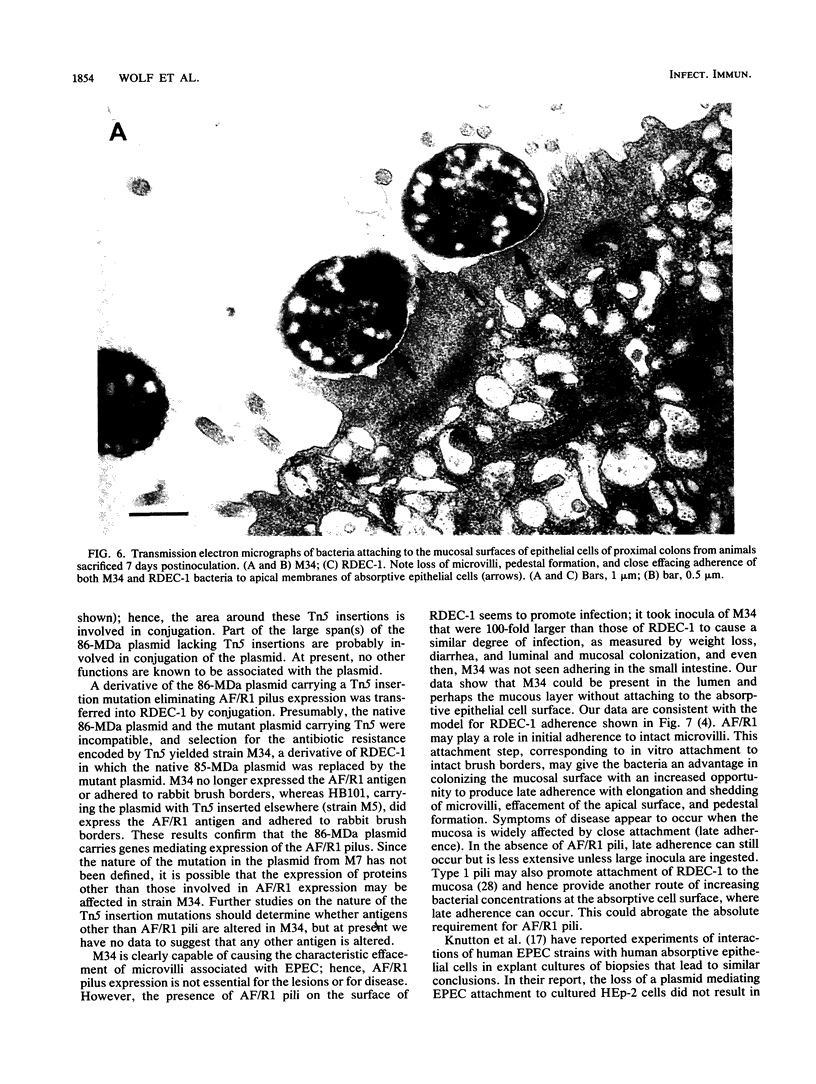
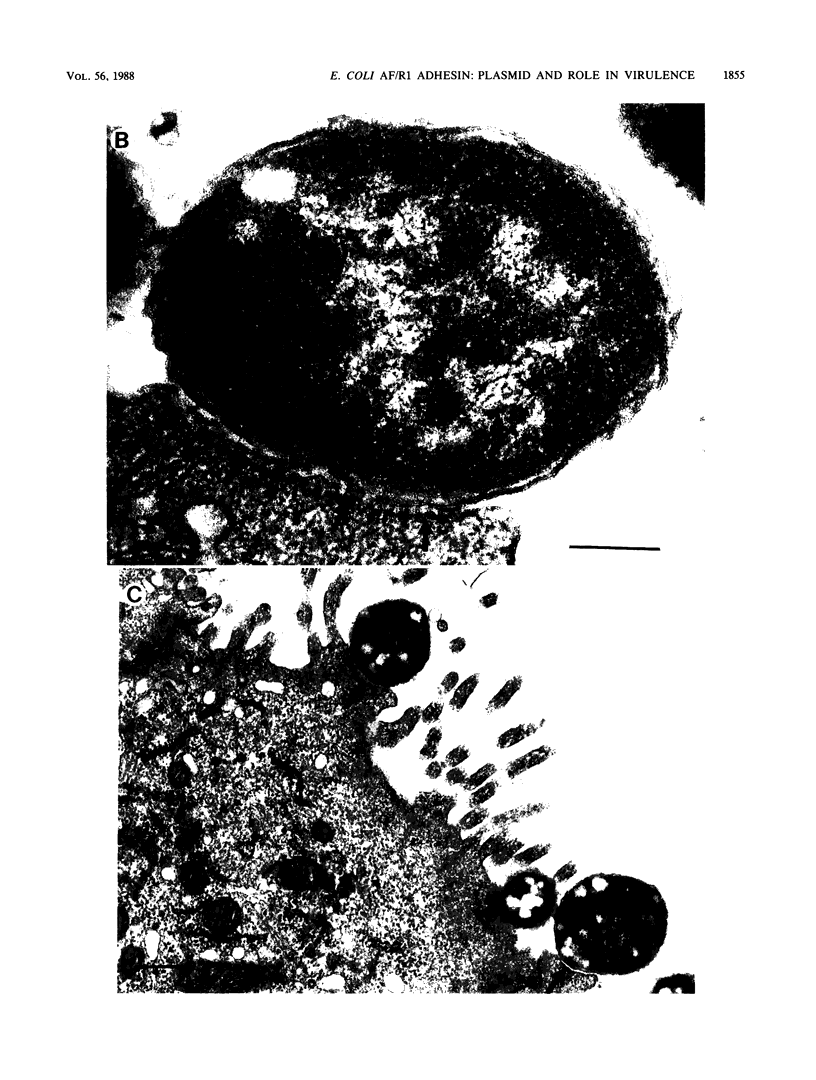
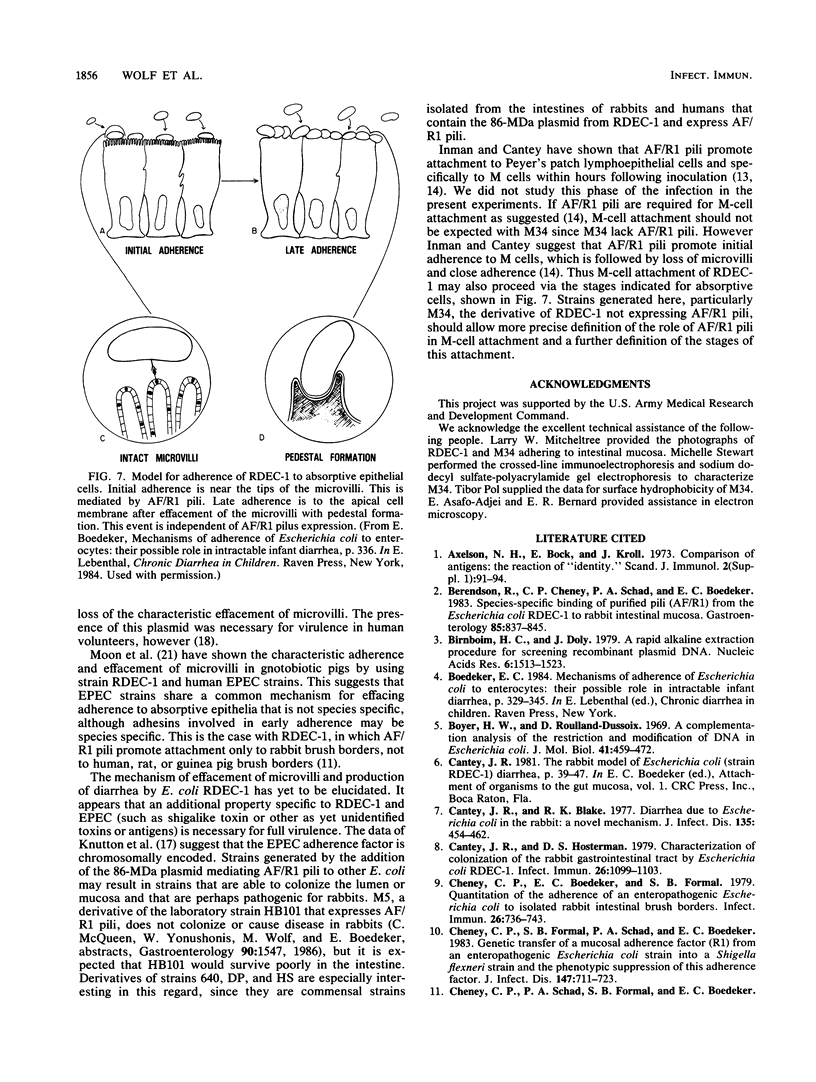
RDEC-1, an Escherichia coli strain that adheres to rabbit mucosa and causes an attaching, effacing lesion, expresses the pilus adhesin AF/R1 which determines in vitro attachment to rabbit intestinal brush borders. In order to determine the role of AF/R1 pili in the pathogenesis of enteropathogenic diarrhea in rabbits, we localized the genes for AF/R1 expression, constructed an AF/R1- strain, and compared the virulence of the AF/R1+ and AF/R1- strains with particular attention to the development of attaching, effacing lesions. We introduced Tn5 into the 86-megadalton (MDa) conjugative plasmid known to mediate expression of AF/R1 pili and transferred the derivative plasmids into laboratory strain HB101. Transconjugant M5 was found to contain the 86-MDa plasmid from RDEC-1 and to express AF/R1 pili. Pilus expression on M5 was confirmed by reaction with antiserum raised against purified AF/R1 pili and allowed the bacteria to adhere to the rabbit ileum in an in vitro assay. Three Tn5 insertions in the 86-MDa plasmid were obtained which resulted in loss of AF/R1 expression. Part of the plasmid was mapped, including a region necessary for AF/R1 pilus expression. AF/R1- mutant strain M34 was constructed, and its pathogenesis was investigated. M34 produced disease in rabbits but was less virulent than the parent. The characteristic effacing lesions of RDEC-1 and enteropathogenic E. coli developed in the intestine of rabbits infected with either M34 or RDEC-1, although with M34 they were much less frequent and did not involve the small bowel. We conclude that AF/R1 pilus expression is not essential for the attaching, effacing lesion but serves as an accessory virulence factor which promotes an initial interaction of RDEC-1 with normal epithelial cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelsen N. H., Bock E., Kroll J. Comparison of antigens: the reaction of 'identity'. Scand J Immunol Suppl. 1973;1:91–94. doi: 10.1111/j.1365-3083.1973.tb03785.x. [DOI] [PubMed] [Google Scholar]
- Berendson R., Cheney C. P., Schad P. A., Boedeker E. C. Species-specific binding of purified pili (AF/R1) from the Escherichia coli RDEC-1 to rabbit intestinal mucosa. Gastroenterology. 1983 Oct;85(4):837–845. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Cantey J. R., Blake R. K. Diarrhea due to Escherichia coli in the rabbit: a novel mechanism. J Infect Dis. 1977 Mar;135(3):454–462. doi: 10.1093/infdis/135.3.454. [DOI] [PubMed] [Google Scholar]
- Cantey J. R., Hosterman D. S. Characterization of colonization of the rabbit gastrointestinal tract by Escherichia coli RDEC-1. Infect Immun. 1979 Dec;26(3):1099–1103. doi: 10.1128/iai.26.3.1099-1103.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney C. P., Boedeker E. C., Formal S. B. Quantitation of the adherence of an enteropathogenic Escherichia coli to isolated rabbit intestinal brush borders. Infect Immun. 1979 Nov;26(2):736–743. doi: 10.1128/iai.26.2.736-743.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney C. P., Formal S. B., Schad P. A., Boedeker E. C. Genetic transfer of a mucosal adherence factor (R1) from an enteropathogenic Escherichia coli strain into a Shigella flexneri strain and the phenotypic suppression of this adherence factor. J Infect Dis. 1983 Apr;147(4):711–723. doi: 10.1093/infdis/147.4.711. [DOI] [PubMed] [Google Scholar]
- Inman L. R., Cantey J. R. Peyer's patch lymphoid follicle epithelial adherence of a rabbit enteropathogenic Escherichia coli (strain RDEC-1). Role of plasmid-mediated pili in initial adherence. J Clin Invest. 1984 Jul;74(1):90–95. doi: 10.1172/JCI111423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman L. R., Cantey J. R. Specific adherence of Escherichia coli (strain RDEC-1) to membranous (M) cells of the Peyer's patch in Escherichia coli diarrhea in the rabbit. J Clin Invest. 1983 Jan;71(1):1–8. doi: 10.1172/JCI110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P. Fimbrial adhesions of Escherichia coli. Rev Infect Dis. 1985 May-Jun;7(3):321–340. doi: 10.1093/clinids/7.3.321. [DOI] [PubMed] [Google Scholar]
- Knutton S., Baldini M. M., Kaper J. B., McNeish A. S. Role of plasmid-encoded adherence factors in adhesion of enteropathogenic Escherichia coli to HEp-2 cells. Infect Immun. 1987 Jan;55(1):78–85. doi: 10.1128/iai.55.1.78-85.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Lloyd D. R., McNeish A. S. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect Immun. 1987 Jan;55(1):69–77. doi: 10.1128/iai.55.1.69-77.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Nataro J. P., Karch H., Baldini M. M., Kaper J. B., Black R. E., Clements M. L., O'Brien A. D. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J Infect Dis. 1985 Sep;152(3):550–559. doi: 10.1093/infdis/152.3.550. [DOI] [PubMed] [Google Scholar]
- McDowell E. M., Trump B. F. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med. 1976 Aug;100(8):405–414. [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C., Argenzio R. A., Levine M. M., Giannella R. A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983 Sep;41(3):1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataro J. P., Maher K. O., Mackie P., Kaper J. B. Characterization of plasmids encoding the adherence factor of enteropathogenic Escherichia coli. Infect Immun. 1987 Oct;55(10):2370–2377. doi: 10.1128/iai.55.10.2370-2377.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Birch-Andersen A. Comparison of Escherichia coli fimbrial antigen F7 with type 1 fimbriae. Infect Immun. 1980 Feb;27(2):657–666. doi: 10.1128/iai.27.2.657-666.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters J. E., Charlier G. J., Halen P. H. Pathogenicity of attaching effacing enteropathogenic Escherichia coli isolated from diarrheic suckling and weanling rabbits for newborn rabbits. Infect Immun. 1984 Dec;46(3):690–696. doi: 10.1128/iai.46.3.690-696.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein S. J., Reznikoff W. S. The functional differences in the inverted repeats of Tn5 are caused by a single base pair nonhomology. Cell. 1981 Jan;23(1):191–199. doi: 10.1016/0092-8674(81)90284-1. [DOI] [PubMed] [Google Scholar]
- Sansonetti P. J., Kopecko D. J., Formal S. B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982 Mar;35(3):852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P. M., Boedeker E. C. Pilus-mediated interactions of the Escherichia coli strain RDEC-1 with mucosal glycoproteins in the small intestine of rabbits. Gastroenterology. 1987 Oct;93(4):734–743. doi: 10.1016/0016-5085(87)90435-5. [DOI] [PubMed] [Google Scholar]
- Sherman P. M., Houston W. L., Boedeker E. C. Functional heterogeneity of intestinal Escherichia coli strains expressing type 1 somatic pili (fimbriae): assessment of bacterial adherence to intestinal membranes and surface hydrophobicity. Infect Immun. 1985 Sep;49(3):797–804. doi: 10.1128/iai.49.3.797-804.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. R., Willshaw G. A., Rowe B. Mapping of a plasmid, coding for colonization, factor antigen I and heat-stable enterotoxin production, isolated from an enterotoxigenic strain of Escherichia coli. J Bacteriol. 1982 Jan;149(1):264–275. doi: 10.1128/jb.149.1.264-275.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]