Abstract
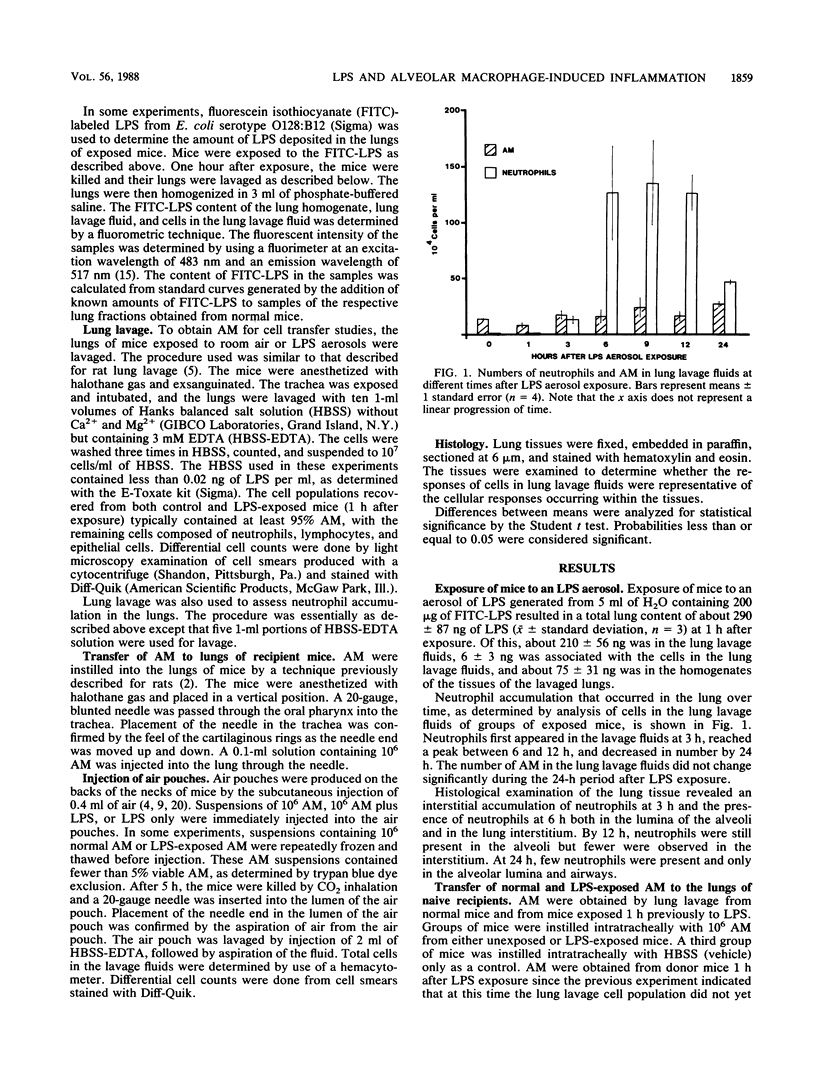
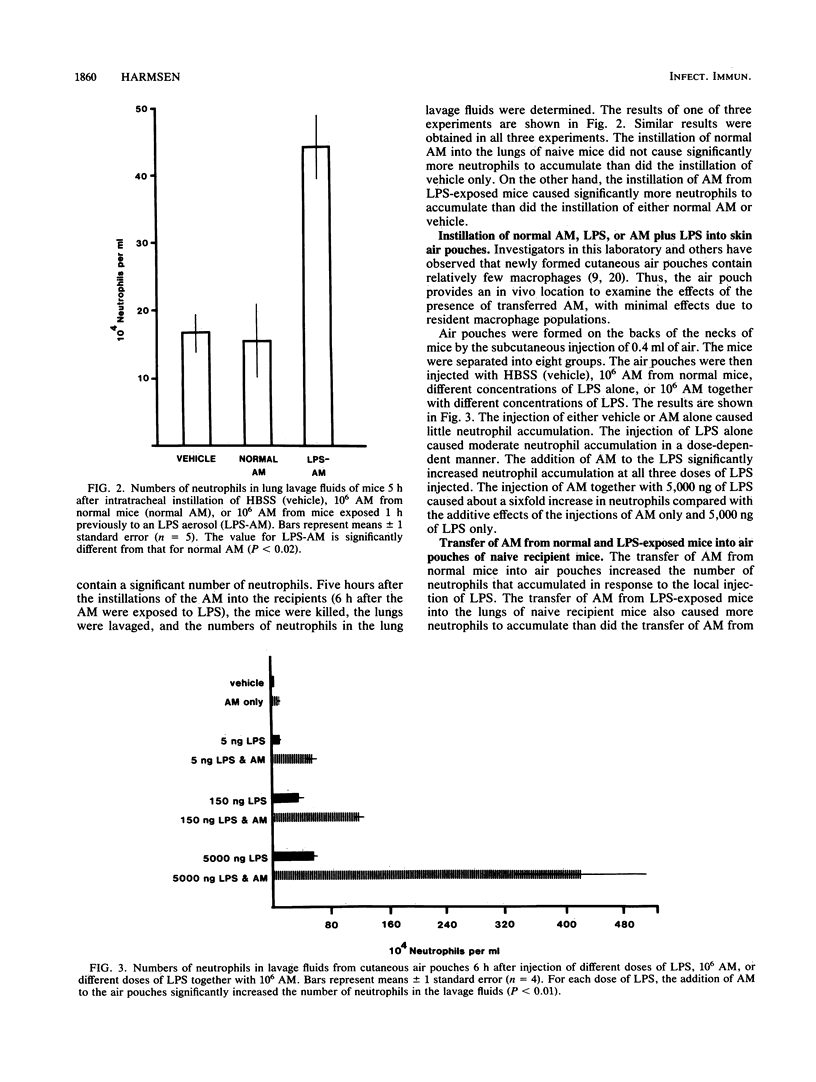
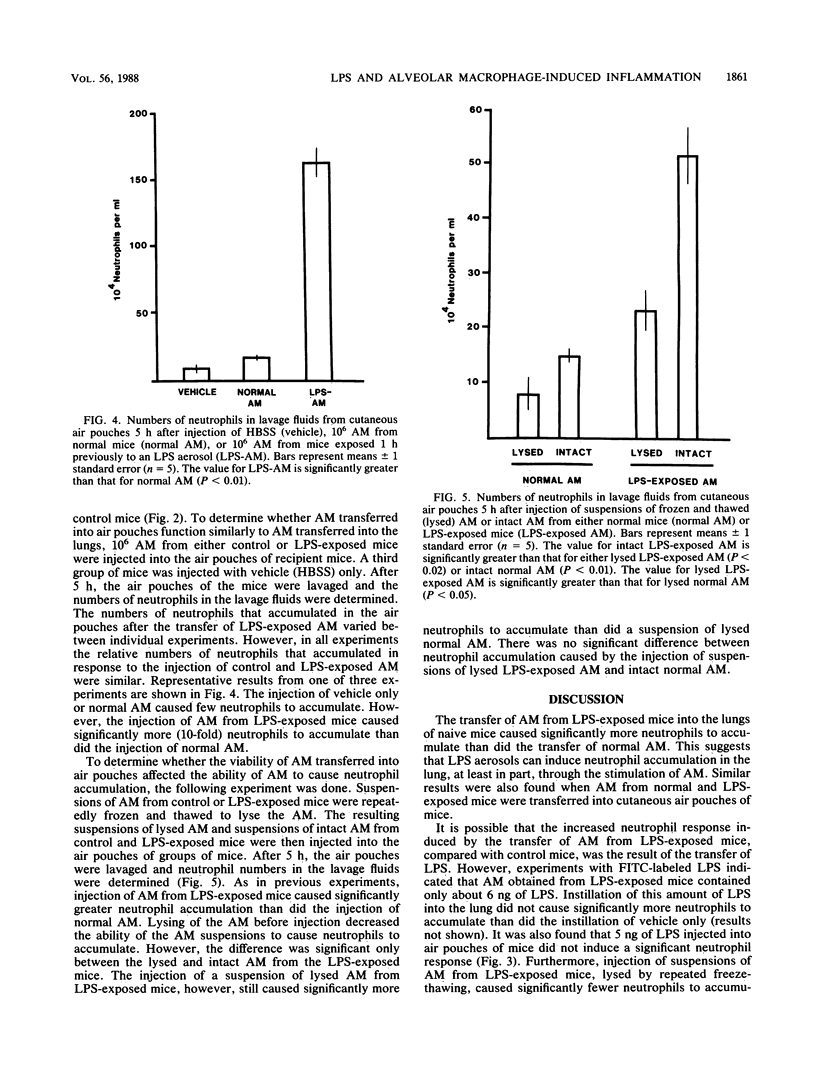
B6D2F1/TRU mice were exposed to a lipopolysaccharide (LPS) aerosol that resulted in a 1-h-postexposure lung burden of about 290 ng of LPS. This exposure caused an accumulation of neutrophils in the lung that peaked between 6 and 12 h after exposure. To determine the potential role of alveolar macrophages (AM) in the induction of neutrophil accumulation by LPS, 10(6) AM from normal or LPS-exposed mice were transferred to the lungs of groups of naive recipient mice. A third group of mice was instilled intratracheally with vehicle only. After 5 h, the lungs of the mice were lavaged and the numbers of neutrophils in the lavage fluids were determined. The instillation of AM from unexposed mice did not cause significantly more neutrophils to accumulate than did the instillation of vehicle only, whereas the instillation of AM from LPS-exposed mice caused nearly a threefold increase in the numbers of neutrophils in lavage fluids. Transfer of AM from LPS-exposed mice into cutaneous air pouches of naive mice also caused greater local neutrophil accumulation (10-fold) than did the transfer of AM from normal mice. Repeated freeze-thawing of the suspensions of AM before transfer to recipients significantly reduced the ability of the suspensions to induce neutrophil accumulation. This indicated that AM viability is necessary to cause a maximal neutrophil infiltration upon transfer of the AM. To determine the extent to which LPS-induced neutrophil accumulation depends on the presence of AM, the ability of LPS to elicit neutrophil accumulation when injected alone or together with AM into air pouches was determined. The injection of either AM or LPS alone caused few neutrophils to accumulate, whereas the injection of LPS and AM together caused a large number of neutrophils to accumulate. The results of this study indicate that LPS deposition in the lung can stimulate AM to induce neutrophil accumulation and that this may be the major mechanism by which LPS causes neutrophil accumulation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck G., Habicht G. S., Benach J. L., Miller F. Interleukin 1: a common endogenous mediator of inflammation and the local Shwartzman reaction. J Immunol. 1986 Apr 15;136(8):3025–3031. [PubMed] [Google Scholar]
- Bice D. E., Schnizlein C. T. Cellular immunity induced by lung immunization of Fischer 344 rats. Int Arch Allergy Appl Immunol. 1980;63(4):438–445. doi: 10.1159/000232660. [DOI] [PubMed] [Google Scholar]
- Billingham M. E. Cytokines as inflammatory mediators. Br Med Bull. 1987 Apr;43(2):350–370. doi: 10.1093/oxfordjournals.bmb.a072187. [DOI] [PubMed] [Google Scholar]
- Boyle M. D., Chiodo V. A., Lawman M. J., Gee A. P., Young M. Urokinase: a chemotactic factor for polymorphonuclear leukocytes in vivo. J Immunol. 1987 Jul 1;139(1):169–174. [PubMed] [Google Scholar]
- Brain J. D., Frank R. Alveolar macrophage adhesion: wash electrolyte composition and free cell yield. J Appl Physiol. 1973 Jan;34(1):75–80. doi: 10.1152/jappl.1973.34.1.75. [DOI] [PubMed] [Google Scholar]
- Colditz I. G., Movat H. Z. Kinetics of neutrophil accumulation in acute inflammatory lesions induced by chemotaxins and chemotaxinigens. J Immunol. 1984 Oct;133(4):2169–2173. [PubMed] [Google Scholar]
- Cybulsky M. I., Colditz I. G., Movat H. Z. The role of interleukin-1 in neutrophil leukocyte emigration induced by endotoxin. Am J Pathol. 1986 Sep;124(3):367–372. [PMC free article] [PubMed] [Google Scholar]
- Dunn M. M., Toews G. B., Hart D., Pierce A. K. The effects of systemic immunization of pulmonary clearance of Pseudomonas aeruginosa. Am Rev Respir Dis. 1985 Mar;131(3):426–431. doi: 10.1164/arrd.1985.131.3.426. [DOI] [PubMed] [Google Scholar]
- Harmsen A. G., Turney T. H. Inhibition of in vivo neutrophil accumulation by stress. Possible role of neutrophil adherence. Inflammation. 1985 Mar;9(1):9–20. doi: 10.1007/BF00915407. [DOI] [PubMed] [Google Scholar]
- Harris D. E., Cairns L., Rosen F. S., Borel Y. A natural model of immunologic tolerance. Tolerance to murine C5 is mediated by T cells, and antigen is required to maintain unresponsiveness. J Exp Med. 1982 Aug 1;156(2):567–584. doi: 10.1084/jem.156.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslett C., Worthen G. S., Giclas P. C., Morrison D. C., Henson J. E., Henson P. M. The pulmonary vascular sequestration of neutrophils in endotoxemia is initiated by an effect of endotoxin on the neutrophil in the rabbit. Am Rev Respir Dis. 1987 Jul;136(1):9–18. doi: 10.1164/ajrccm/136.1.9. [DOI] [PubMed] [Google Scholar]
- Issekutz A. C., Bhimji S., Bortolussi R. Effect of immune serum or polymyxin B on Escherichia coli-induced inflammation and vascular injury. Infect Immun. 1982 May;36(2):548–557. doi: 10.1128/iai.36.2.548-557.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz A. C., Bhimji S. Role for endotoxin in the leukocyte infiltration accompanying Escherichia coli inflammation. Infect Immun. 1982 May;36(2):558–566. doi: 10.1128/iai.36.2.558-566.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz A. C., Megyeri P., Issekutz T. B. Role for macrophage products in endotoxin-induced polymorphonuclear leukocyte accumulation during inflammation. Lab Invest. 1987 Jan;56(1):49–59. [PubMed] [Google Scholar]
- Magnusson K. E., Dahlgren C., Sjölander A. Effect of N-formylated methionyl-phenylalanine (FMP) and methionyl-leucyl-phenylalanine (FMLP) on gut permeability. A model of local inflammatory process. Inflammation. 1985 Dec;9(4):365–373. doi: 10.1007/BF00916336. [DOI] [PubMed] [Google Scholar]
- Meyrick B., Brigham K. L. Acute effects of Escherichia coli endotoxin on the pulmonary microcirculation of anesthetized sheep structure:function relationships. Lab Invest. 1983 Apr;48(4):458–470. [PubMed] [Google Scholar]
- Rehm S. R., Gross G. N., Pierce A. K. Early bacterial clearance from murine lungs. Species-dependent phagocyte response. J Clin Invest. 1980 Aug;66(2):194–199. doi: 10.1172/JCI109844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahin R. D., Engberg I., Hagberg L., Svanborg Edén C. Neutrophil recruitment and bacterial clearance correlated with LPS responsiveness in local gram-negative infection. J Immunol. 1987 May 15;138(10):3475–3480. [PubMed] [Google Scholar]
- Tsurufuji S., Yoshino S., Ohuchi K. Induction of an allergic air-pouch inflammation in rats. Int Arch Allergy Appl Immunol. 1982;69(3):189–198. doi: 10.1159/000233170. [DOI] [PubMed] [Google Scholar]
- Weinstein D. L., Lissner C. R., Swanson R. N., O'Brien A. D. Macrophage defect and inflammatory cell recruitment dysfunction in Salmonella susceptible C3H/HeJ mice. Cell Immunol. 1986 Oct 1;102(1):68–77. doi: 10.1016/0008-8749(86)90326-6. [DOI] [PubMed] [Google Scholar]



