Abstract
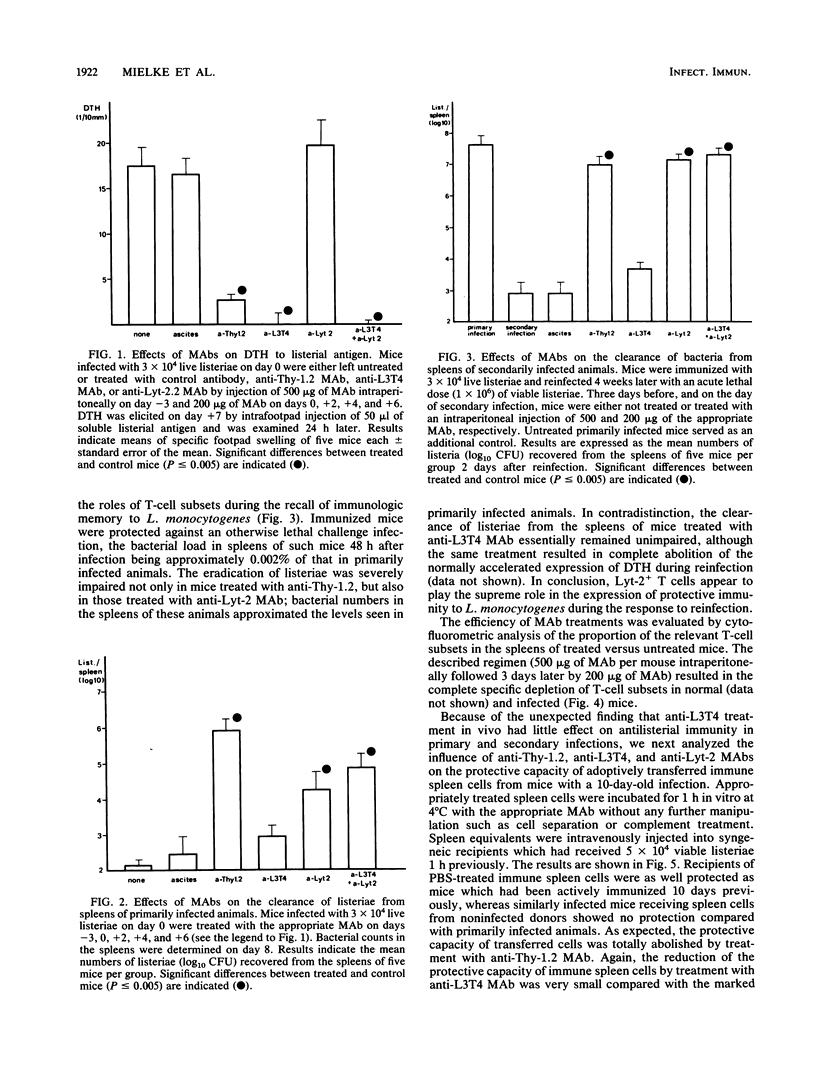
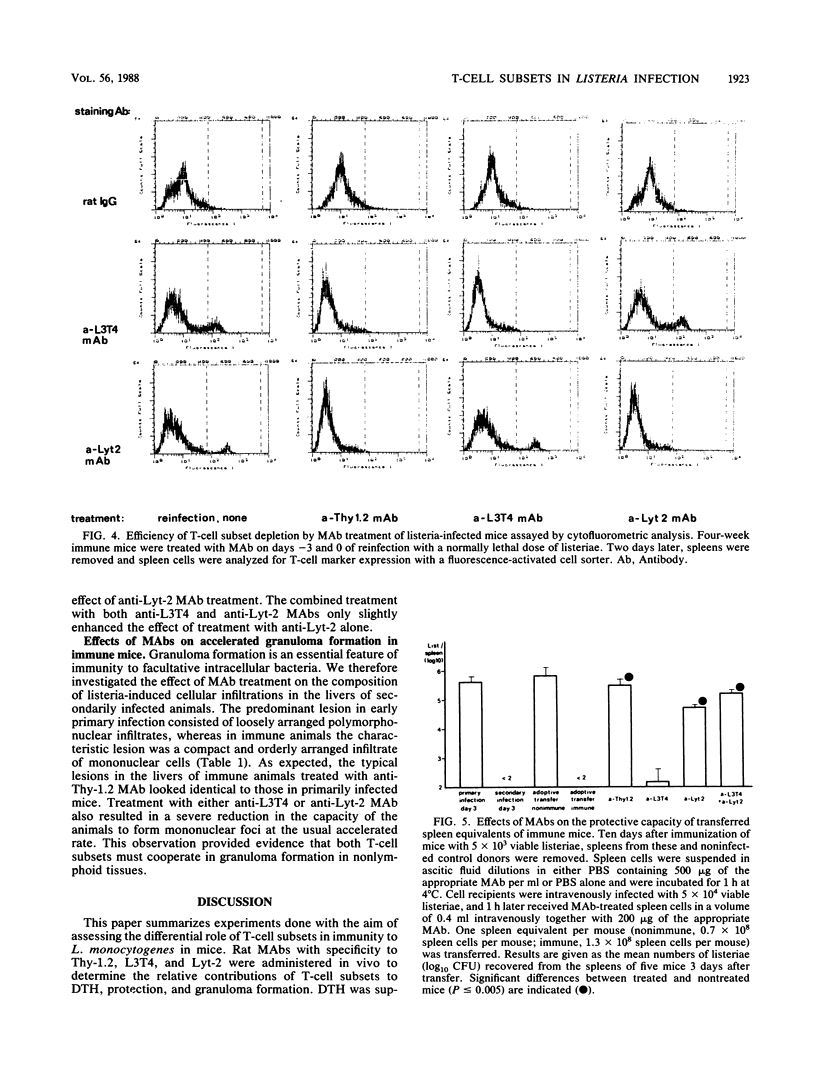
Immunity to Listeria monocytogenes was studied in mice treated with rat monoclonal antibodies (MAbs) specific for the Thy-1.2, L3T4, and Lyt-2 T-cell markers. Three characteristic T-cell-mediated phenomena were investigated. Delayed-type hypersensitivity (DTH) to listerial antigen was totally abolished in mice treated with anti-Thy-1.2 or anti-L3T4 MAbs, whereas anti-Lyt-2 MAb treatment had no effect, regardless of whether the MAb was given during the induction or the expression of DTH. On the other hand, the elimination of bacteria from the spleens of infected animals was inhibited only by the application of either anti-Thy-1.2 MAb or anti-Lyt-2 MAb. This could be shown most impressively during the secondary infection of immune mice with a normally lethal dose of listeriae. In this situation, treatment with anti-Lyt-2 MAb sufficed to completely abolish immunologic memory, whereas anti-L3T4 MAb had only a marginal effect on antibacterial protection. However, the accelerated development of mononuclear cell foci in the livers of immune mice was inhibited by the application of both anti-L3T4 MAb and anti-Lyt-2 MAb. It is concluded that in murine listeriosis, DTH and acquired immunity to reinfection are dissociable phenomena. Although DTH is a function of L3T4+ T lymphocytes, Lyt-2+ T cells are necessary and sufficient for the expression of acquired resistance to L. monocytogenes. The roles of the different T-cell subsets in granuloma formation warrant further investigation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. K., Hinrichs D. J. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J Immunol. 1987 Sep 15;139(6):2005–2009. [PubMed] [Google Scholar]
- Blanden R. V., Langman R. E. Cell-mediated immunity to bacterial infection in the mouse. Thymus-derived cells as effectors of acquired resistance to Listeria monocytogenes. Scand J Immunol. 1972;1(4):379–391. doi: 10.1111/j.1365-3083.1972.tb03304.x. [DOI] [PubMed] [Google Scholar]
- Cheers C., Sandrin M. S. Restriction in adoptive transfer of resistance to Listeria monocytogenes. II. Use of congenic and mutant mice show transfer to be H-2K restricted. Cell Immunol. 1983 Jun;78(2):199–205. doi: 10.1016/0008-8749(83)90274-5. [DOI] [PubMed] [Google Scholar]
- Chen-Woan M., McGregor D. D., Noonan S. K. Isolation and characterization of protective T cells induced by Listeria monocytogenes. Infect Immun. 1986 May;52(2):401–407. doi: 10.1128/iai.52.2.401-407.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Woan M., Sajewski D. H., McGregor D. D. T-cell co-operation in the mediation of acquired resistance to Listeria monocytogenes. Immunology. 1985 Sep;56(1):33–42. [PMC free article] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Emmerling P., Finger H., Bockemühl J. Listeria monocytogenes infection in nude mice. Infect Immun. 1975 Aug;12(2):437–439. doi: 10.1128/iai.12.2.437-439.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr A. G., Dorf M. E., Unanue E. R. Secretion of mediators following T lymphocyte-macrophage interaction is regulated by the major histocompatibility complex. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3542–3546. doi: 10.1073/pnas.74.8.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr A. G., Kiely J. M., Unanue E. R. Macrophage-T cell interactions involving Listeria monocytogenes--role of the H-2 gene complex. J Immunol. 1979 Jun;122(6):2395–2404. [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Hussein S., Curtis J., Akuffo H., Turk J. L. Dissociation between delayed-type hypersensitivity and resistance to pathogenic mycobacteria demonstrated by T-cell clones. Infect Immun. 1987 Mar;55(3):564–567. doi: 10.1128/iai.55.3.564-567.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. Effective antibacterial protection induced by a Listeria monocytogenes-specific T cell clone and its lymphokines. Infect Immun. 1983 Mar;39(3):1265–1270. doi: 10.1128/iai.39.3.1265-1270.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hahn H. Biological functions of t cell lines with specificity for the intracellular bacterium Listeria monocytogenes in vitro and in vivo. J Exp Med. 1982 Jun 1;155(6):1754–1765. doi: 10.1084/jem.155.6.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., De Libero G. Listeria monocytogenes-reactive T lymphocyte clones with cytolytic activity against infected target cells. J Exp Med. 1986 Jul 1;164(1):363–368. doi: 10.1084/jem.164.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., Väth U., De Libero G. Specific lysis of Listeria monocytogenes-infected macrophages by class II-restricted L3T4+ T cells. Eur J Immunol. 1987 Feb;17(2):237–246. doi: 10.1002/eji.1830170214. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., Väth U., Müller I. Effective protection against Listeria monocytogenes and delayed-type hypersensitivity to listerial antigens depend on cooperation between specific L3T4+ and Lyt 2+ T cells. Infect Immun. 1985 Apr;48(1):263–266. doi: 10.1128/iai.48.1.263-266.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Simon M. M., Hahn H. Specific Lyt 123 cells are involved in protection against Listeria monocytogenes and in delayed-type hypersensitivity to listerial antigens. J Exp Med. 1979 Oct 1;150(4):1033–1038. doi: 10.1084/jem.150.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The relationship of delayed hypersensitivity to acquired cellular resistance. Br Med Bull. 1967 Jan;23(1):52–54. doi: 10.1093/oxfordjournals.bmb.a070516. [DOI] [PubMed] [Google Scholar]
- North R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973 Apr;7(1):166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- Näher H., Sperling U., Hahn H. H-2K-restricted granuloma formation by Ly-2+ T cells in antibacterial protection to facultative intracellular bacteria. J Immunol. 1985 Jan;134(1):569–572. [PubMed] [Google Scholar]
- Näher H., Sperling U., Takacs L., Hahn H. Dynamics of T cells of L3T4 and Ly 2 phenotype within granulomas in murine listeriosis. Clin Exp Immunol. 1985 Jun;60(3):559–564. [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Adoptive protection of the Mycobacterium tuberculosis-infected lung. Dissociation between cells that passively transfer protective immunity and those that transfer delayed-type hypersensitivity to tuberculin. Cell Immunol. 1984 Mar;84(1):113–120. doi: 10.1016/0008-8749(84)90082-0. [DOI] [PubMed] [Google Scholar]
- Sperling U., Kaufmann S. H., Hahn H. Production of macrophage-activating and migration-inhibition factors in vitro by serologically selected and cloned Listeria monocytogenes-specific T cells of the Lyt 1+2- phenotype. Infect Immun. 1984 Oct;46(1):111–115. doi: 10.1128/iai.46.1.111-115.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Althage A., Adler B., Blanden R. V., Davidson W. F., Kees U., Dunlop M. B., Shreffler D. C. H-2 restriction of cell-mediated immunity to an intracellular bacterium: effector T cells are specific for Listeria antigen in association with H-21 region-coded self-markers. J Exp Med. 1977 May 1;145(5):1353–1367. doi: 10.1084/jem.145.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]



