Abstract
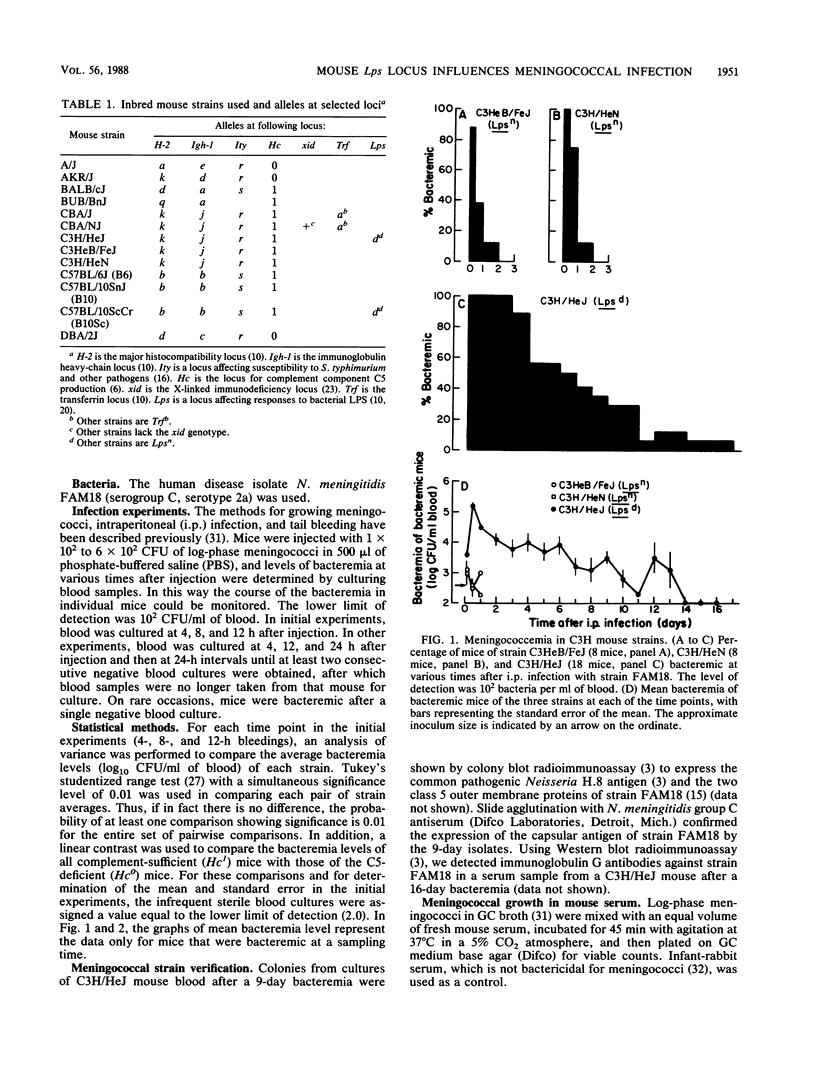
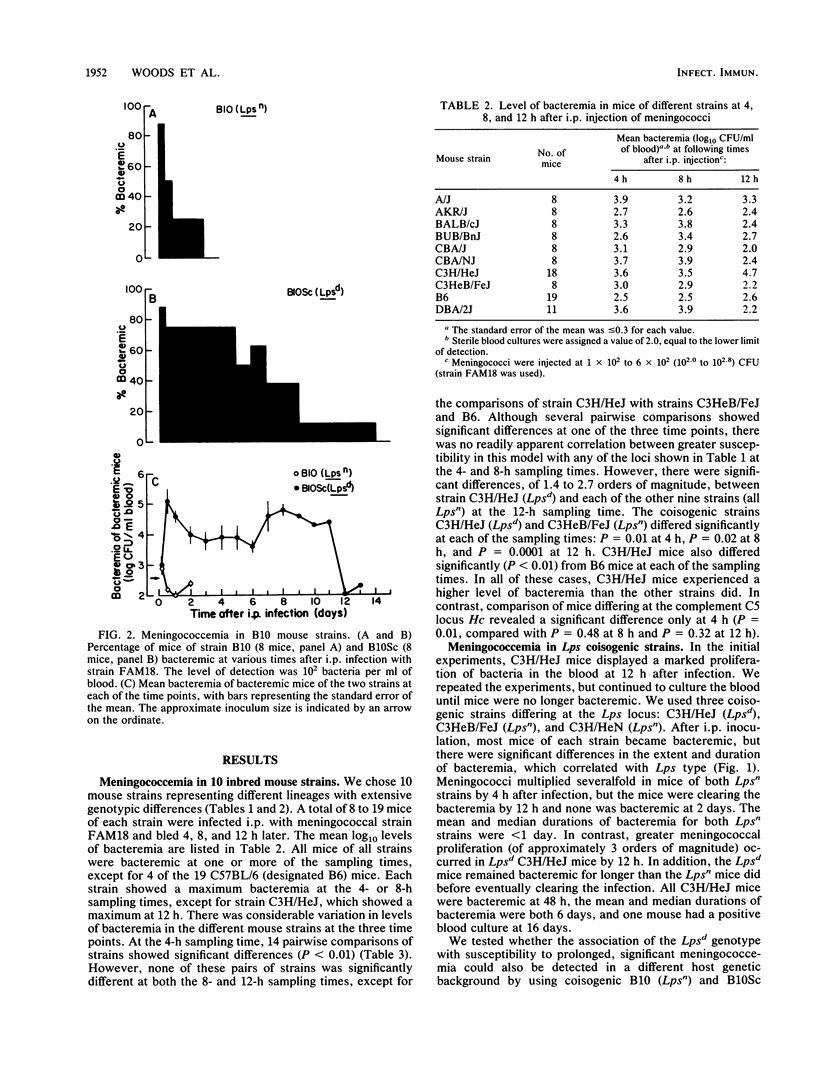
We surveyed a number of inbred mouse strains for susceptibility to meningococcemia. Mice of all strains became bacteremic after intraperitoneal injection of a serogroup C, serotype 2a human disease isolate, but the strains differed in levels of bacteremia, indicating influences of the host genome on susceptibility. There was no significant correlation between level of bacteremia and differences at major histocompatibility or immunoglobulin loci; the Salmonella susceptibility locus, Ity; the complement C5 locus, Hc; the antibody response locus, xid; or the transferrin locus, Trf. However, the Lps locus, which influences a range of host cellular responses to endotoxin and affects susceptibility to Salmonella typhimurium, did influence susceptibility to meningococcemia. There were significant differences in levels of bacteremia between C3H/HeJ (Lpsd) mice and each of the other strains (all Lpsn). We confirmed the association of the Lpsd genotype with susceptibility by using coisogenic strains from two widely separated mouse lineages: C3H and B10. Lpsd mice experienced a 1,000-fold proliferation of bacteria and were bacteremic for days before clearing the infection. In contrast, Lpsn mice cleared the bacteremia in less than 1 day. There was no difference in meningococcal growth in vitro in serum from C3H/HeJ and coisogenic C3H/HeN (Lpsn) mice, suggesting that the Lps-related difference in susceptibility may involve a cellular response.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauss F., Dröge W., Männel D. N. Tumor necrosis factor mediates endotoxic effects in mice. Infect Immun. 1987 Jul;55(7):1622–1625. doi: 10.1128/iai.55.7.1622-1625.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin W. H., Jr, Turnbough C. L., Jr, Posey B. S., Briles D. E. Salmonella typhimurium virulence genes necessary to exploit the Itys/s genotype of the mouse. Infect Immun. 1986 Mar;51(3):872–878. doi: 10.1128/iai.51.3.872-878.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. G., Black W. J., Nachamkin I., Stewart P. W. Monoclonal antibody that recognizes an outer membrane antigen common to the pathogenic Neisseria species but not to most nonpathogenic Neisseria species. Infect Immun. 1984 Mar;43(3):994–999. doi: 10.1128/iai.43.3.994-999.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoe I. W. The meningococcus and mechanisms of pathogenicity. Microbiol Rev. 1982 Jun;46(2):162–190. doi: 10.1128/mr.46.2.162-190.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy J. M., Sparkes B. G., Desrosiers M., Skamene E., Micusan V. V. Prevention of the enhancing effect of mucin and iron in mouse meningococcal infection. Can J Microbiol. 1983 Dec;29(12):1671–1674. doi: 10.1139/m83-255. [DOI] [PubMed] [Google Scholar]
- Gervais F., Stevenson M., Skamene E. Genetic control of resistance to Listeria monocytogenes: regulation of leukocyte inflammatory responses by the Hc locus. J Immunol. 1984 Apr;132(4):2078–2083. [PubMed] [Google Scholar]
- Glode L. M., Rosenstreich D. L. Genetic control of B cell activation by bacterial lipopolysaccharide is mediated by multiple distinct genes or alleles. J Immunol. 1976 Dec;117(6):2061–2066. [PubMed] [Google Scholar]
- Goldschneider I., Gotschlich E. C., Artenstein M. S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969 Jun 1;129(6):1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood B. M., Oduloju A. J., Ade-Serrano M. A. Cellular immunity in patients with meningococcal disease and in vaccinated subjects. Clin Exp Immunol. 1979 Oct;38(1):9–15. [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Briles D. E., Edén C. S. Evidence for separate genetic defects in C3H/HeJ and C3HeB/FeJ mice, that affect susceptibility to gram-negative infections. J Immunol. 1985 Jun;134(6):4118–4122. [PubMed] [Google Scholar]
- Holbein B. E. Differences in virulence for mice between disease and carrier strains of Neisseria meningitidis. Can J Microbiol. 1981 Jul;27(7):738–741. doi: 10.1139/m81-113. [DOI] [PubMed] [Google Scholar]
- Holbein B. E. Iron-controlled infection with Neisseria meningitidis in mice. Infect Immun. 1980 Sep;29(3):886–891. doi: 10.1128/iai.29.3.886-891.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawula T. H., Aho E. L., Barritt D. S., Klapper D. G., Cannon J. G. Reversible phase variation of expression of Neisseria meningitidis class 5 outer membrane proteins and their relationship to gonococcal proteins II. Infect Immun. 1988 Feb;56(2):380–386. doi: 10.1128/iai.56.2.380-386.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissner C. R., Weinstein D. L., O'Brien A. D. Mouse chromosome 1 Ity locus regulates microbicidal activity of isolated peritoneal macrophages against a diverse group of intracellular and extracellular bacteria. J Immunol. 1985 Jul;135(1):544–547. [PubMed] [Google Scholar]
- Lowell G. H., Smith L. F., Artenstein M. S., Nash G. S., MacDermott R. P., Jr Antibody-dependent cell-mediated antibacterial activity of human mononuclear cells. I. K lymphocytes and monocytes are effective against meningococi in cooperation with human imune sera. J Exp Med. 1979 Jul 1;150(1):127–137. doi: 10.1084/jem.150.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melancon-Kaplan J., Gronvik K. O., Murgita R. A. Selective impairment of B cell function by Neisseria meningitidis. Cell Immunol. 1986 Jun;100(1):247–259. doi: 10.1016/0008-8749(86)90024-9. [DOI] [PubMed] [Google Scholar]
- Moeller G. R., Terry L., Snyderman R. The inflammatory response and resistance to endotoxin in mice. J Immunol. 1978 Jan;120(1):116–123. [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- O'Brien A. D., Metcalf E. S., Rosenstreich D. L. Defect in macrophage effector function confers Salmonella typhimurium susceptibility on C3H/HeJ mice. Cell Immunol. 1982 Mar 1;67(2):325–333. doi: 10.1016/0008-8749(82)90224-6. [DOI] [PubMed] [Google Scholar]
- O'Brien A. D., Rosenstreich D. L. Genetic control of the susceptibility of C3HeB/FeJ mice to Salmonella typhimurium is regulated by a locus distinct from known salmonella response genes. J Immunol. 1983 Dec;131(6):2613–2615. [PubMed] [Google Scholar]
- O'Brien A. D., Scher I., Metcalf E. S. Genetically conferred defect in anti-Salmonella antibody formation renders CBA/N mice innately susceptible to Salmonella typhimurium infection. J Immunol. 1981 Apr;126(4):1368–1372. [PubMed] [Google Scholar]
- Ross S. C., Densen P. Complement deficiency states and infection: epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency. Medicine (Baltimore) 1984 Sep;63(5):243–273. [PubMed] [Google Scholar]
- Ross S. C., Rosenthal P. J., Berberich H. M., Densen P. Killing of Neisseria meningitidis by human neutrophils: implications for normal and complement-deficient individuals. J Infect Dis. 1987 Jun;155(6):1266–1275. doi: 10.1093/infdis/155.6.1266. [DOI] [PubMed] [Google Scholar]
- Skamene E. Genetic regulation of host resistance to bacterial infection. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S823–S832. doi: 10.1093/clinids/5.supplement_4.s823. [DOI] [PubMed] [Google Scholar]
- Sparkes B. G. Dual effect of meningococcal antigens on a T cell dependent immune response. Can J Microbiol. 1983 Dec;29(12):1619–1625. doi: 10.1139/m83-248. [DOI] [PubMed] [Google Scholar]
- Sparkes B. G. Immunomodulating activity of meningococcal antigens. Can J Microbiol. 1983 Dec;29(12):1611–1618. doi: 10.1139/m83-247. [DOI] [PubMed] [Google Scholar]
- Watson J., Riblet R. Genetic control of responses to bacterial lipopolysaccharides in mice. II. A gene that influences a membrane component involved in the activation of bone marrow-derived lymphocytes by lipipolysaccharides. J Immunol. 1975 May;114(5):1462–1468. [PubMed] [Google Scholar]
- Woods J. P., Black J. R., Barritt D. S., Connell T. D., Cannon J. G. Resistance to meningococcemia apparently conferred by anti-H.8 monoclonal antibody is due to contaminating endotoxin and not to specific immunoprotection. Infect Immun. 1987 Aug;55(8):1927–1928. doi: 10.1128/iai.55.8.1927-1928.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect Immun. 1983 Apr;40(1):257–264. doi: 10.1128/iai.40.1.257-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



