Abstract
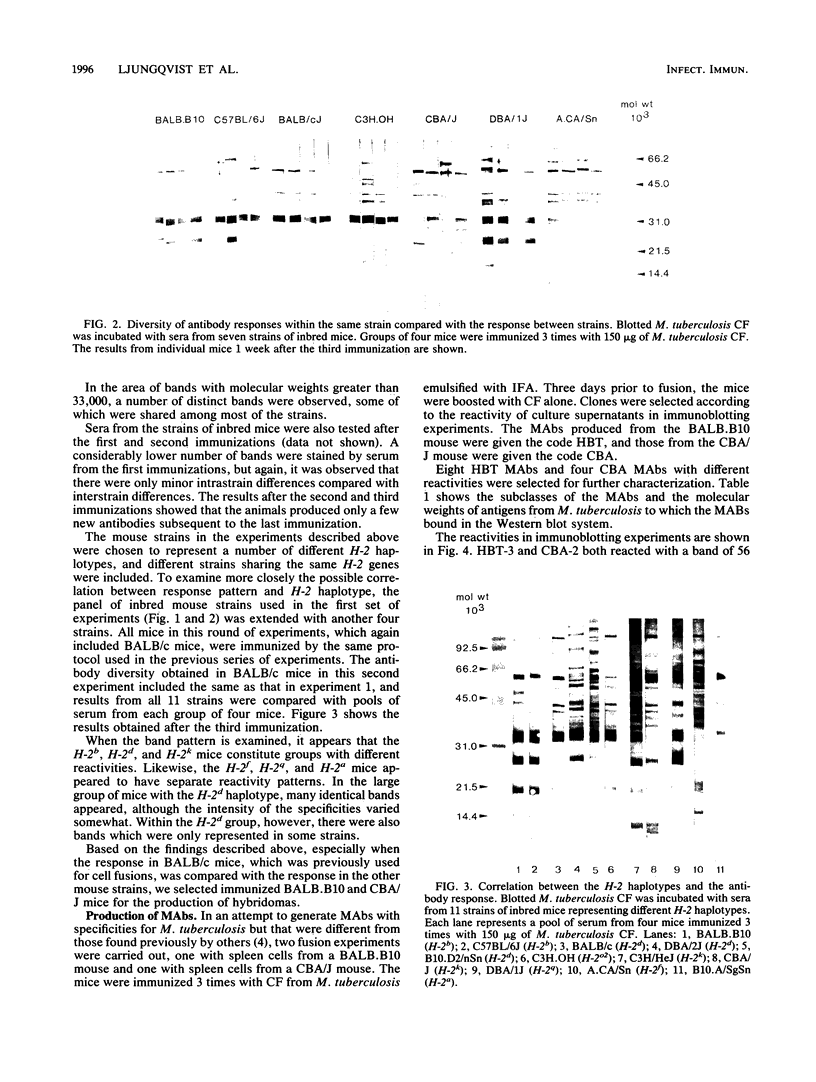
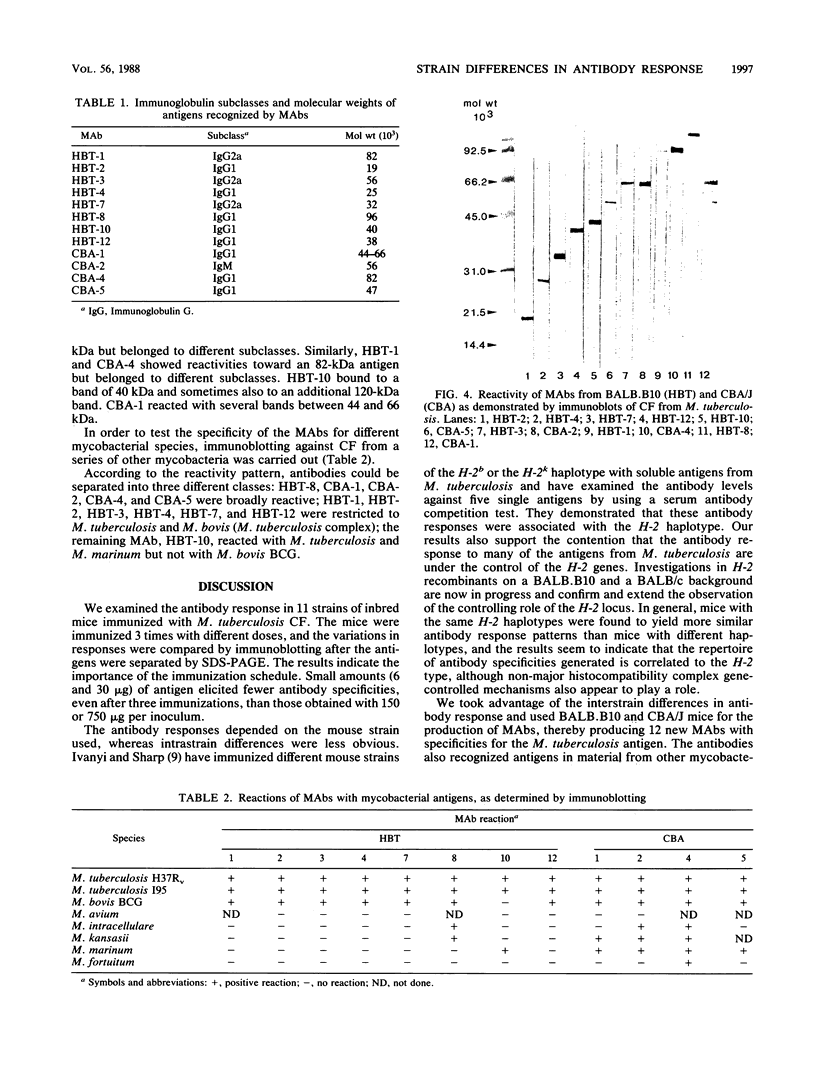
Eleven strains of inbred mice were immunized with a culture filtrate of Mycobacterium tuberculosis H37Rv, and the quality of the antibody responses was determined by immunoblotting. The quantity of mycobacterial antigen used for each immunization ranged from 6 to 750 micrograms per inoculum. The culture filtrate of M. tuberculosis was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrotransferred to nitrocellulose filters. Immunoblotting results were obtained with serum from the following 11 strains of immunized mice: C57BL/6J, BALB/cJ, BALB.B10, C3H.OH, A.CA/Sn, CBA/J, DBA/1J, DBA/2J, C3H/HeJ, B10.A/SgSn, and B10.D2/nSn. Mice were tested individually, and results from each mouse were compared after each immunization. It was found that sera from individual mice within the same strain differed only slightly in their immune response patterns. In contrast, major differences were seen when the reactivities of sera from different strains were compared. Hybridomas were obtained from cell fusions by using spleen cells from BALB.B10 and CBA/J mice. Twelve monoclonal antibodies were raised, which identified epitopes on molecules with different electrophoretic mobilities than those already described by other investigators. The monoclonal antibodies were characterized by immunoblotting with respect to their reactivities with culture filtrates from M. tuberculosis and six other mycobacterial species. One of the monoclonal antibodies (HBT-10) identified an epitope that was present in M. tuberculosis H37Rv but not in Mycobacterium bovis BCG.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen A. B., Yuan Z. L., Hasløv K., Vergmann B., Bennedsen J. Interspecies reactivity of five monoclonal antibodies to Mycobacterium tuberculosis as examined by immunoblotting and enzyme-linked immunosorbent assay. J Clin Microbiol. 1986 Mar;23(3):446–451. doi: 10.1128/jcm.23.3.446-451.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates A. R., Hewitt J., Allen B. W., Ivanyi J., Mitchison D. A. Antigenic diversity of Mycobacterium tuberculosis and Mycobacterium bovis detected by means of monoclonal antibodies. Lancet. 1981 Jul 25;2(8239):167–169. doi: 10.1016/s0140-6736(81)90355-x. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Gonchoroff N. J., Katzmann J. A., Olds G. R. Specificity of Mycobacterium tuberculosis antigen 5 determined with mouse monoclonal antibodies. Infect Immun. 1984 Jul;45(1):52–55. doi: 10.1128/iai.45.1.52-55.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis T. P., Buchanan T. M. Production and partial characterization of monoclonal antibodies to Mycobacterium leprae. Infect Immun. 1982 Jul;37(1):172–178. doi: 10.1128/iai.37.1.172-178.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanyi J., Krambovitis E., Keen M. Evaluation of a monoclonal antibody (TB72) based serological test for tuberculosis. Clin Exp Immunol. 1983 Nov;54(2):337–345. [PMC free article] [PubMed] [Google Scholar]
- Ivanyi J., Sharp K. Control by H-2 genes of murine antibody responses to protein antigens of Mycobacterium tuberculosis. Immunology. 1986 Nov;59(3):329–332. [PMC free article] [PubMed] [Google Scholar]
- Kadival G. V., Chaparas S. D. Production, characterization, and species specificity of five monoclonal antibodies to Mycobacterium tuberculosis. J Clin Microbiol. 1987 Jan;25(1):76–80. doi: 10.1128/jcm.25.1.76-80.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolk A. H., Ho M. L., Klatser P. R., Eggelte T. A., Kuijper S., de Jonge S., van Leeuwen J. Production and characterization of monoclonal antibodies to Mycobacterium tuberculosis, M. bovis (BCG) and M. leprae. Clin Exp Immunol. 1984 Dec;58(3):511–521. [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Minden P., Kelleher P. J., Freed J. H., Nielsen L. D., Brennan P. J., McPheron L., McClatchy J. K. Immunological evaluation of a component isolated from Mycobacterium bovis BCG with a monoclonal antibody to M. bovis BCG. Infect Immun. 1984 Nov;46(2):519–525. doi: 10.1128/iai.46.2.519-525.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds G. R., Sanson A. J., Daniel T. M. Characterization of Mycobacterium tuberculosis antigen 5 epitopes by using a panel of 19 monoclonal antibodies. J Clin Microbiol. 1987 Mar;25(3):471–475. doi: 10.1128/jcm.25.3.471-475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schou C., Yuan Z. L., Andersen A. B., Bennedsen J. Production and partial characterization of monoclonal hybridoma antibodies to Mycobacterium tuberculosis. Acta Pathol Microbiol Immunol Scand C. 1985 Dec;93(6):265–272. doi: 10.1111/j.1699-0463.1985.tb02955.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Gordon J. Immunoblotting and dot immunobinding--current status and outlook. J Immunol Methods. 1984 Sep 4;72(2):313–340. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]