Abstract
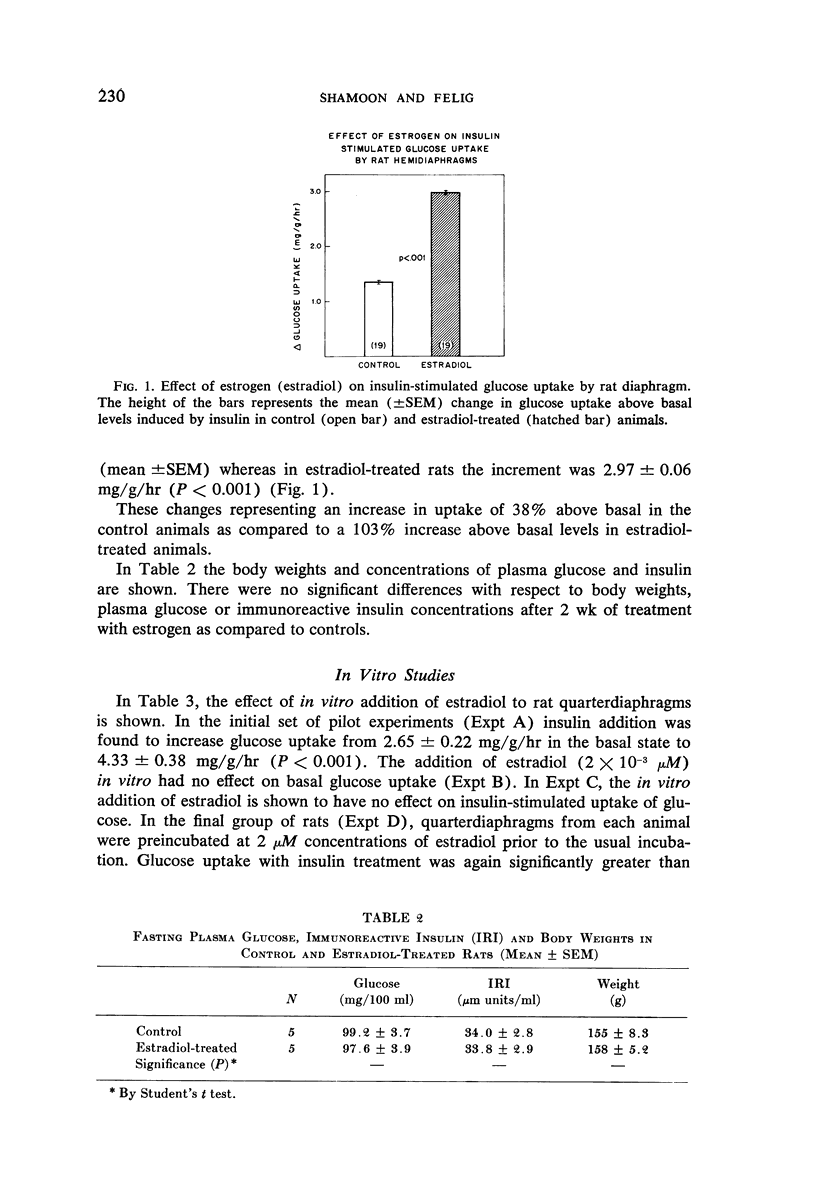
The isolated rat diaphragm was used to study the effects of 17β-estradiol on basal and insulin-mediated glucose uptake. Rats were injected with estradiol for 2 wk in daily doses of 10 μg/100 g of body weight and were compared to untreated control animals. Estrogen treatment resulted in a 16% decrease in basal glucose uptake by diaphragm muscle as compared to controls. In contrast, in the presence of insulin, glucose uptake by muscle increased 103% above basal in estradiol-treated animals as compared to a 38% rise in the control group. The absolute rate of glucose uptake induced by insulin in the estradiol treated animals (5.8 mg/g/hr) was 22% higher than in controls. These findings were not accompanied by changes in weight gain, plasma glucose and plasma immunoreactive insulin concentrations in the treated animals. In vitro incubation of diaphragm muscle with estradiol did not have an effect on basal or insulin-mediated glucose uptake.
The data indicate that treatment with naturally occurring estrogens increases muscle sensitivity to insulin-stimulated glucose uptake. These findings suggest that the carbohydrate intolerance associated with the administration of oral contraceptives may be related to the use of synthetic rather than natural estrogens and/or progestins in such preparations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck P. Contraceptive steroids: modifications of carbohydrate and lipid metabolism. Metabolism. 1973 Jun;22(6):841–855. doi: 10.1016/0026-0495(73)90056-5. [DOI] [PubMed] [Google Scholar]
- Brown-Grant K., Exley D., Naftolin F. Peripheral plasma oestradiol and luteinizing hormone concentrations during the oestrous cycle of the rat. J Endocrinol. 1970 Oct;48(2):295–296. doi: 10.1677/joe.0.0480295. [DOI] [PubMed] [Google Scholar]
- DESAULLES P. A., KRAEHENBUEHL C. COMPARISON OF THE ANTI-FERTILITY AND SEX HORMONAL ACTIVITIES OF SEX HORMONES AND THEIR DERIVATIVES. Acta Endocrinol (Copenh) 1964 Nov;47:444–456. doi: 10.1530/acta.0.0470444. [DOI] [PubMed] [Google Scholar]
- HOUSSAY B. A., FOGLIA V. G., RODRIGUEZ R. R. Production or prevention of some types of experimental diabetes by oestrogens or corticosteroids. Acta Endocrinol (Copenh) 1954;17(1-4):146–164. [PubMed] [Google Scholar]
- Javier Z., Gershberg H., Hulse M. Ovulatory suppressants, estrogens, and carbohydrate metabolism. Metabolism. 1968 May;17(5):443–456. doi: 10.1016/0026-0495(68)90067-x. [DOI] [PubMed] [Google Scholar]
- Jensen E. V., DeSombre E. R. Mechanism of action of the female sex hormones. Annu Rev Biochem. 1972;41:203–230. doi: 10.1146/annurev.bi.41.070172.001223. [DOI] [PubMed] [Google Scholar]
- SAIFER A., GERSTENFELD S. The photometric microdetermination of blood glucose with glucose oxidase. J Lab Clin Med. 1958 Mar;51(3):448–460. [PubMed] [Google Scholar]
- Spellacy W. N. A review of carbohydrate metabolism and the oral contraceptives. Am J Obstet Gynecol. 1969 Jun 1;104(3):448–460. doi: 10.1016/s0002-9378(16)34204-1. [DOI] [PubMed] [Google Scholar]
- WARDLAW A. C., MOLONEY P. J. The assay of insulin with anti-insulin and mouse diaphragm. Can J Biochem Physiol. 1961 Apr;39:695–712. doi: 10.1139/o61-071. [DOI] [PubMed] [Google Scholar]
- Wynn V., Doar J. W. Some effects of oral contraceptives on carbohydrate metabolism. Lancet. 1966 Oct 1;2(7466):715–719. doi: 10.1016/s0140-6736(66)92978-3. [DOI] [PubMed] [Google Scholar]


