Editor:
Tumor cells were found in nests within soft tissue adherent to a percutaneously retrieved Günther Tulip inferior vena cava (IVC) filter (Cook, Bloomington, IN) in a 19-year-old man with metastatic osteosarcoma of the left lower extremity following a limb-sparing operation. The filter was in place for 14 days as prophylaxis for recurrent perioperative pulmonary emboli from a known distal superficial femoral deep vein thrombosis. Pathology favors hematogenous showering of metastatic cells during surgery, and the lack of tumor growth within the vessels suggests that direct tumor extension or bulk tumor embolism are unlikely.
This unexpected finding (Figure) despite normal angiography is concerning. Preremoval imaging or angiography may not detect clinically silent small caval thromboses. The safety of IVC filters in patients with cancer with potential hematogenous spread is questionable. Filter retrieval might shower tumor emboli during manipulation, and a filter may act as a nidus for local neoplastic growth. Caval thrombosis is a known complication of permanent filters, but the incidence of tumor thrombus is unknown.
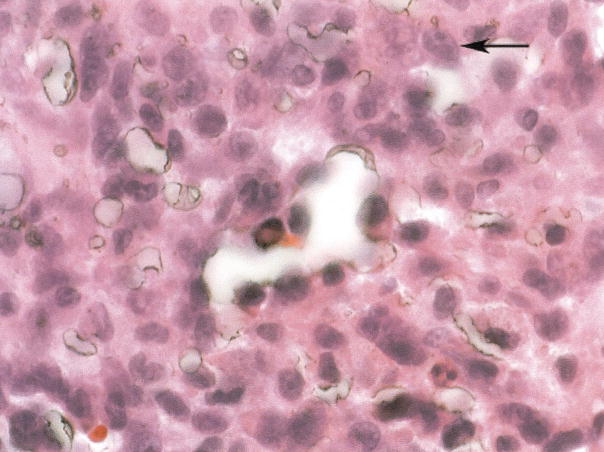
Figure.
The tightly adherent fragmentary tissue was scraped off the filter struts, fixed in formalin, and stained with hema-toxylin and eosin. Adherent to the filter is a dense fibrosis with clusters of tumor cells in nests. The tumor cells are pleomorphic in shape, have high nuclear/cytoplasm ratios, and are morphologically similar to the patient’s primary osteosarcoma. The tumor is incorporated within the organizing soft tissue surrounding the filter, does not appear to be infiltrating from the external area to the more central area of the specimen, and is not found within vessels, favoring a showering of metastatic cells during surgery, landing on a filter that was undergoing fibrinization as opposed to direct tumor extension or bulk tumor embolism. The arrow indicates tumor cells with prominent nucleoli, pleomorphic in shape, and with high nuclear/cytoplasm ratios.
Despite more than three decades of clinical use, controversy remains regarding indications for IVC filters (1). We acknowledge the existence of a consensus on several basic and sound indications (2). Although many authorities would disagree, our interpretation is that no true consensus exists among specialists regarding other indications for IVC filter placement. There are only a few randomized controlled trials assessing efficacy and safety (3). Most of the publications are retrospective studies and case reports. Study limitations include heterogeneities among patient populations, inclusion and exclusion criteria, evaluation criteria, and follow-up methodology. There are 150,000 deaths per year from venous thromboembolism, with 30,000–40,000 IVC filters inserted each year in the United States (4). Comprehensive, retrospective, multicenter statistical analysis could be facilitated by a national filter registry or a consensus conference.
In the past decade, there have been multiple studies on various temporary retrievable IVC filter devices, addressing feasibility and safety of retrieval for up to 134 days (5). These emerging devices have already further broadened the nebulous inclusion criteria spectrum, and practice patterns are changing as a result. Liberal interpretation of the indications for filters could include patient populations that were traditionally excluded from filter placement.
Unfortunately, filter placement has become common practice for a wide variety of unproven indications, despite the paucity of safety and efficacy data. Appropriate validation of indications for temporary retrievable IVC filters needs to be developed. Practice patterns for temporary retrievable IVC filters may represent a dangerous situation in which nonvalidated clinical practice outpaces hypothesis-driven scientific method.
References
- 1.Girard P, Stern JB, Parent F. Medical literature and vena cava filters: so far so weak. Chest. 2002;122:963–967. doi: 10.1378/chest.122.3.963. [DOI] [PubMed] [Google Scholar]
- 2.Grassi CJ, Swan TL, Cardella JF, et al. Quality improvement guidelines for percutaneous permanent inferior vena cava filter placement for the prevention of pulmonary embolism. SCVIR Standards of Practice Committee. J Vasc Interv Radiol. 2001;12:137–141. doi: 10.1016/s1051-0443(07)61818-1. [DOI] [PubMed] [Google Scholar]
- 3.Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prevention du Ris-que d’Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998;338:409–415. doi: 10.1056/NEJM199802123380701. [DOI] [PubMed] [Google Scholar]
- 4.Magnant JG, Walsh DB, Juravsky LI, Cronenwett JL. Current use of inferior vena cava filters. J Vasc Surg. 1992;16:701–706. doi: 10.1067/mva.1992.40474. [DOI] [PubMed] [Google Scholar]
- 5.Asch MR. Initial experience in humans with a new retrievable inferior vena cava filter. Radiology. 2002;225:835–844. doi: 10.1148/radiol.2252011825. [DOI] [PubMed] [Google Scholar]