Abstract
We genotyped a Somali population (n=85; age ≤ 30 years) for 617 cytokine and cytokine receptor SNPs using Illumina GoldenGate genotyping to determine associations with measles, mumps and rubella immunity. Overall, sixty-one significant associations (p≤0.01) were found between SNPs belonging to cytokine receptor genes regulating Th1 (IL12RB2, IL2RA and B) and Th2 (IL4R, IL10RB) immunity, and cytokine (IL1B, TNFA, IL6 and IFNB1) and cytokine receptor (IL1RA, IFNAR2, IL18R1, TNFRSF1A and B) genes regulating innate immunity, and variations in antibody levels to measles, mumps or rubella. SNPs within two major inflammatory cytokine genes, TNFA and IL6, demonstrated associations with measles-specific antibodies. Specifically, the minor allele variant of rs1799964 (TNFA -1211 C>T) was associated with primarily seronegative values (median EIA index values ≤0.87; p=0.002; q=0.23) in response to measles disease and/or vaccination. A heterozygous variant CT for rs2069849 (IL6 +4272C>T; Phe201Phe) was also associated with seronegative values and a lower median level of antibody response to measles disease and/or vaccination (p=0.004; q=0.36) or measles vaccination alone (p=0.008). Several SNPs within the coding and regulatory regions of cytokine and cytokine receptor genes demonstrated associations with mumps and rubella antibody levels, but were less informative as strong LD patterns and lower frequencies for minor alleles were observed among these SNPs. Our study identifies specific SNPs in innate immune response genes that may play a role in modulating antibody responses to measles vaccination and/or infection in Somali subjects.
Keywords: Polymorphisms, Cytokine, Cytokine receptors, Antibodies, MMR, Immunity
Introduction
Cytokines are important mediators in shaping both innate and adaptive immune responses, as well as eliciting recall immune responses to vaccines [1,2]. The ability of a vaccine to establish a long-lived memory response is dependent on the activation of the appropriate cytokine milieu for a particular pathogen. Although antibody levels are the “gold standard” for measuring protection, genetic factors that regulate the cytokine microenvironment in response to vaccination impact protective immunity by influencing antibody responses.
Single nucleotide polymorphisms (SNPs) are the most common genetic variations described in cytokine and cytokine receptor genes [3,4]. SNPs located in the coding regions of the genes can result in loss, abrogation, or altered function of the downstream protein by causing alterations in amino acid sequences and protein structure. Similarly, SNPs located in the promoter and regulatory regions of genes may modify the transcriptional activity of these genes. The influence of cytokine and cytokine receptor SNPs on corresponding gene activity and subsequent SNP-disease associations have been extensively reviewed [4–7] and can be accessed on-line as well (available at http://www.nanea.dk/cytokinesnps/: last accessed January, 2008).
Genetic heterogeneity in allele distribution can result in inter-individual variations in vaccine induced immunity. Functional polymorphisms at positions -1082, -819 and -592 in the IL10 promoter influence the immune response after vaccination with hepatitis B surface antigen (HBsAg) and hepatitis A virus (HAV). Individuals carrying the IL10 promoter ACC haplotype, which is associated with lower IL10 production, have anti-HBs titers twice as high as individuals that do not carry this haplotype [8]. However, the presence of the ACC haplotype suppressed levels of anti-HAV antibodies as compared with individuals carrying the GCC haplotpe [8]. We have previously demonstrated that specific SNPs in the IL2, IL10 and IL12RB genes are associated with variations in antibody and lymphoproliferative responses to measles vaccine in Caucasian subjects[9]. We have also identified SNPs in the IL10RA and IL12RB cytokine-receptor genes that were significantly associated with variations in immune responses to mumps vaccine [10].
Racial differences have been identified in inherited SNPs within the immunomodulatory genes including cytokines and cytokine receptors [11–16]. Genetic association studies aimed at determining the role of immune response gene SNPs in vaccine induced immunity have primarily been carried out in Caucasian populations. As a result, little or no information is available on SNP associations with vaccine immunity in other racial groups. In this hypothesis generating study, we examined a selected set of SNPs from cytokine and cytokine receptor genes regulating Th1 (IL2, IFNG and IL12A & B), Th2 (IL4 and IL10) and innate (IL1B, IL18, IL6, IFNA1, IFNB1 and TNFA) responses in a cohort of Somali subjects living in Olmsted County, Minnesota. Ours is the first study designed to examine associations between SNPs in these highly relevant immune function genes and antibody levels to measles, mumps and rubella. Our overall goal was to identify genetic determinants influencing antibody responsiveness in this minority population.
Materials and methods
Our study cohort consisted of 89 Somali subjects (≤ 30 years of age) vaccinated with a single dose of measles-mumps rubella (MMR) vaccine that were sampled from a previously recruited and described larger cohort of the Somali refugee community residing in Olmsted County, Minnesota. [17,18] We collected demographic information on vaccination status and disease history for measles, mumps and rubella with the help of a bilingual/bicultural physician assisted by a team of phlebotomists and investigators. The study was approved by the Mayo Clinic institutional review board. We obtained informed consent from adult participants and assent was obtained from minors along with their parent/guardian consent.
Measurement of antibody levels to measles, mumps and rubella
Measles, mumps and rubella-specific circulating IgG antibody levels were tested in duplicate for each subject’s serum sample using a whole virus specific IgG enzyme-linked immunosorbent assay (MEASELISA II, MUMPS ELISA II, and RUBELISA II, BioWhittaker, Walkersville, MD) as a part of a previously described study. [17,18] Measles and rubella serum IgG levels with enzyme immunoassay (EIA) index values ≤0.87 were considered seronegative, index values between 0.88–0.99 were considered seroequivocal and index values ≥1.0 were considered seropositive [19]. For mumps, EIA index values ≤0.89 were considered seronegative, 0.90–0.99 were considered seroequivocal and ≥1.0 were considered seropositive. The median coefficient of variation among duplicate sample testing in our laboratory was 6.6%.
SNP selection
A list of cytokine (n=12) and corresponding cytokine receptor (n=19) genes with a documented role in viral immunity was generated after an extensive review of the literature as part of an ongoing population genetics study on vaccine response. The known SNPs in the cytokine and cytokine receptor genes were identified from public databases such as SNP500 Cancer database (Available at: http://snp500cancer.nci.nih.gov/home_1.cfm) and the NCBI SNP database (Available at: http://www.ncbi.nlm.nih.gov/projects/SNP/). A total of 768 SNPs were selected by prioritization based on their prevalence in the African American population (minor allele frequency >5%), and location within each gene (coding: synonymous or non-synonymous, 5′ or 3′ untranslated regions or introns) traversing 10 kB upstream and downstream for each gene. The nomenclature used for the description of the variants follows that described by den Dunnen and Antonarakis [20].
Genotyping methods
Genomic DNA was extracted from frozen blood clots using the Puregene extraction kit (Gentra Systems Inc., Minneapolis, MN) and was quantified using the Picogreen method (Molecular Probes, Carlsbad, CA).
We designed two 384-plex Illumina GoldenGate™ assays (Illumina Inc., San Diego, CA) for 768 candidate cytokine and cytokine receptor SNPs. All of the SNPs selected for the two custom oligo pooled assays had Illumina design scores > 0.6. DNA samples (n=89, 250 ng each) were genotyped following the Illumina protocol. Four samples failed due to inadequate DNA quality leaving only 85 subjects for final analysis. Genotype calls were made using the Genotyping module of the BeadStudio 2 software. Replicate and inheritance data of control genomic DNA samples (Corriel Trio DNA, Mother: 1347-02 NA11875, Father: 1347-01 NA10859, Daughter: 1347-08 NA11875 and two other genomic DNAs) were used to review and refine clustering. All our samples had Illumina 10% GenCall scores above 0.4 and call rates above 90%, which is accepted as standard initial laboratory quality assurance (QA).
Genotype data on SNPs were generated by BeadStudio and transferred electronically to a server from which data were exported into SAS for further analysis. Of the 768 SNPs included, 741 (96.48%) yielded genotype data and the study sample success rate was 98.81%. We also tested for SNP-specific deviation from the Hardy-Weinberg Equilibrium (HWE) using chi-square goodness-of-fit tests and excluded any SNP that displayed violations of HWE (p<0.001). Thirty SNPs failed the initial QA because of failure to amplify, poor clustering or multiple replicate errors and HWE violations. Further, we excluded all SNPs monomorphic for our study cohort leaving 617 SNPs for the final analysis.
Statistical Methods
Three outcomes were of primary interest: antibody response for measles, mumps and rubella, expressed in EIA optical density index units. Data were descriptively summarized using frequencies and percentages for categorical variables, and medians and inter-quartile ranges for continuous variables. SNPs were examined on a genotypic level, creating three levels for each SNP: homozygous major allele (AA), heterozygous (AB) and homozygous minor allele (BB). Estimates of pair-wise linkage disequilibrium (LD) based on the r2 statistics were also obtained using Haploview version 3.32 [21]. Associations of antibody levels with demographic and clinical variables were assessed using analysis of variance methods.
We examined associations between antibody levels and SNPs in the cytokine and cytokine receptor genes using analysis of covariance (ANCOVA) methods. Primary antigen-specific analyses included all 85 subjects and adjusted for the following set of potential confounding variables: age, gender, personal history of disease, and vaccination status. In order to identify associations due mainly to the effects of vaccine response, we ran a series of secondary analyses subset to all subjects age 30 and younger with no prior history of disease and documentation of a single vaccination, and adjusted for age, gender and time since vaccination. We tested all analyses for associations using a two degree-of-freedom SNP test, making no assumptions about the form of the genotypic associations. To protect against the probability of Type I errors, and as this was a hypothesis-generating study, p-values less than or equal to 0.01 were considered statistically significant. To better assess the effects of multiple testing, we supplemented the p-values from the primary ANCOVA models with their associated q-values [22,23]. Briefly, the q-values are based on the concept of false discovery rates and can be interpreted as the expected proportion of false positive results among all features at least as extreme as the observed result. All statistical tests were two-sided, and analyses were carried out using the SAS (SAS Institute, Inc., Cary, NC) software system.
Results
Demographic and clinical variables of Somali cohort
The demographic characteristics of the subjects are summarized in Table 1. The median age of the study subjects was 10.4 years with 55% being male. The seropositivity rates for measles, mumps and rubella antibodies were 88%, 86% and 96% respectively. A fraction of the cohort claimed prior measles, mumps or rubella disease (Table 1). We report associations between demographic variables and antibody responses to measles, mumps and rubella in Table 2. The antibody levels to mumps (p<0.001) and rubella (p=0.01) showed an association with age at enrollment (Table 2). Females had significantly higher median antibody responses to mumps (p=0.03) as compared to males (Table 2). Importantly, a history of disease did not influence antibody responses to any of the vaccines studied.
Table 1.
Demographic Characteristics in Somali Study Subjects
| Variable | No. (%) of Subjects* |
|---|---|
| Age of enrollment in years, Median (IQR) | 10.4 (6.7,18.5) |
| Gender | |
| Males | 47 (55) |
| Females | 38 (45) |
| Measles antibodies | |
| Negative or equivocal | 10 (12) |
| Positive | 75 (88) |
| Mumps antibodies | |
| Negative or equivocal | 12 (14) |
| Positive | 73 (86) |
| Rubella antibodies | |
| Negative or equivocal | 3(4) |
| Positive | 82 (96) |
| History of measles disease | |
| No | 73 (86) |
| Yes | 12 (14) |
| History of mumps disease | |
| No | 81 (95) |
| Yes | 4 (5) |
| History of rubella disease | |
| No | 84 (99) |
| Don’t know | 1 (1) |
| History of measles vaccination | |
| No | 9 (11) |
| Yes | 64 (75) |
| Don’t know | 12 (14) |
| History of mumps vaccination | |
| No | 9 (11) |
| Yes | 65 (76) |
| Don’t know | 11 (13) |
| History of rubella vaccination | |
| No | 9 (11) |
| Yes | 64 (75) |
| Don’t know | 12 (14) |
Data are number (percentage) of subjects unless indicated otherwise IQR- inter-quartile range
Table 2.
Demographic Characteristics associated with Serum Antibody Levels in Somali Study Subjects
| Antibody levels, median (IQR) | |||
|---|---|---|---|
| Characteristics | Measles | Mumps | Rubella |
| Age at enrollment, years | |||
| Q1 (≤6) | 1.5 (1.1,21) | 1.8 (1.3,2.5) | 2.7 (2.5,3.0) |
| Q2 (7–10) | 1.7 (1.4,2.2) | 3.0 (1.5,3.6) | 2.9 (2.5,3.1) |
| Q3 (11–18) | 1.9 (1.6,2.7) | 3.7 (3.0,4) | 2.6 (2.7,2.8) |
| Q4 (>18) | 1.9 (1.6,2.1) | 3.1 (2.0,3.7) | 2.4 (2.0,2.7) |
| P value | 0.613 | <0.001 | 0.014 |
| Gender | |||
| Male | 1.7 (1.3,2.2) | 2.4 (1.4,3.6) | 2.6 (2.4,2.8) |
| Female | 1.8 (1.4,2.1) | 3.1 (2.3,3.9) | 2.7 (2.4,3.0) |
| P value | 0.361 | 0.032 | 0.956 |
| History of disease | |||
| No | 1.8 (1.4,2.2) | 3.0 (1.8,3.7) | 2.7 (2.4,2.9) |
| Yes | 1.9 (1.8,2.5) | 2.5 (1.3,3.8) | - |
| Don’t know | - | - | 2.9 (2.9,2.9) |
| P value | 0.319 | 0.797 | 0.599 |
| History of vaccination | |||
| No | 2.02 (1.03,3.41) | 1.77 (1.26,1.86) | 1.84 (1.45,2.65) |
| Yes | 2.95 (1.66,3.65) | 1.8 (1.37,2.18) | 2.65 (2.48,2.97) |
| Don’t know | 3.2 (2.86,4.01) | 1.76 (1.54,2.35) | 2.73 (2.51,2.95) |
| P value | p=0.108 | p=0.607 | p<0.0001 |
P-values calculated using analysis of variance methods.
Measles, mumps and rubella antibody levels are expressed in EIA index units
In addition to examining all 85 subjects, we examined a subset of subjects ≤ 30 years of age who had no history of measles, mumps or rubella disease and documentation of a single dose of measles, mumps and rubella vaccine, in order to study responses to vaccination alone. This resulted in 55, 60 and 64 subjects for measles, mumps and rubella vaccine respectively.
Cytokine and cytokine receptor SNPs associated with measles antibodies
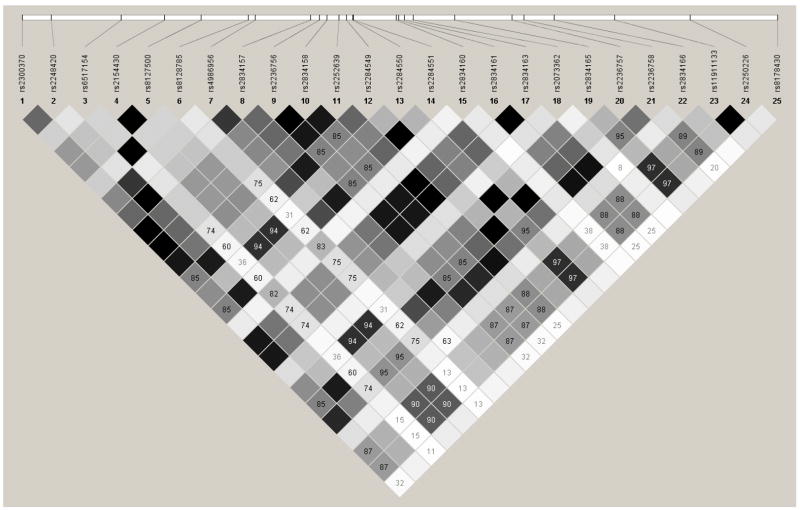
Overall, thirteen of the 617 SNPs regulating inflammatory (TNFA, IL6, TNFRSF1B, IFNAR2) and Th1 (IL2RB) immunity to measles were found to be significantly (p≤0.01) associated with an increase/decrease in measles-specific antibodies among all 85 subjects. The significant SNPs located in the coding, promoter and 5′ and 3′ untranslated region (UTR) (high priority by our SNP selection algorithm) are shown in Table 3. The CC variant of rs1799964 (TNFA -1211 C>T) was associated with significantly lower (p=0.002; q=0.23) levels of antibody to measles following disease or vaccination (Table 3). Heterozygous variant CT for rs2069849 (IL6 +4272C>T; Phe201Phe) was also associated with a lower (p=0.004; q=0.36) level of overall median antibody response to measles and a lower (p=0.008) median antibody to measles vaccination alone (Table 3). Further, a propensity toward seronegative status was seen with the presence of the heterozygous allele for this SNP; however, an allele dose response could not be established due to the lack of minor allele variants in our study cohort. We also found associations between intronic SNPs in cytokine (IL6 and TNFA) and cytokine receptor (IL2RB, IFNAR2 and TNFRSF1B) genes and measles-specific antibodies (Supplementary Table 1). However, these data should be interpreted with caution as six SNPs belonging to two LD blocks located in the IFNRA2 gene demonstrated strong linkage (r2= 1), suggesting that all of these findings were not independent observations (Figure 1). Further, four additional SNPs in the IL2RB gene were identified after subsetting to the 55 individuals who were vaccinated with a single dose of measles vaccine and had no history of measles disease (Supplementary Table 1).
Table 3.
Statistically significant associations between SNPs in coding and regulatory regions of cytokine and cytokine receptor genes and humoral immunity to measles in Somali Study Subjects
| Gene | SNP ID | Position | Genotype | N | Median Ab EIA index units | Global P-value$ | Global P-value! | Q-value** |
|---|---|---|---|---|---|---|---|---|
| TNFA | rs1799964* | 5′ intergenic | TT/TC/CC | 50/30/5 | 1.7/2.0/0.7 | 0.002 | ns | 0.23 |
| IL6 | rs2069849* | Phe201Phe | CC/CT/TT | 80/5/0 | 1.8/0.8/- | 0.004 | 0.008 | 0.36 |
T-Thymine, C-Cytosine, ns- non-significant (p-value >0.01)
Minor allele heterozygous or minor allele homozygous genotypes resulted in median antibody EIA index value in seronegativity range
Analysis of covariance P-value on 85 subjects adjusted for age, gender, history of disease and vaccination status
Analysis of covariance P-value on a subset on 55 subjects that were vaccinated with single dose of measles vaccine and had no history of measles disease, after adjusting for age and gender
Corresponding q-value based on the distribution of p-values for all 85 subjects across all three antigens and all 617 SNPs of interest
Figure 1.
IFNAR2 gene
Cytokine and cytokine receptor SNPs associated with mumps antibodies
Five SNPs regulating Th1 (IL2RA), Th2 (IL4R) a inflammatory (IL1B) immunity in mumps were found to be significantly (p≤0.01) associated with variations in mumps humoral immunity among all 85 subjects, and an additional SNP regulating Th2 (IL10RB) was identified after subsetting to individuals vaccinated with a single dose of mumps vaccine and with no history of mumps disease (Supplementary Table 2). However, most of these associations had low frequency for the homozygous minor allele giving us less confidence in the results. Further, strong LD pattern (r2=1) was also observed among two SNPs (Supplementary Table 2) located in a LD block belonging to the IL1B gene. SNPs with significant associations located in the coding and regulatory regions are shown in Table 4. The heterozygous variant GA for rs2228149 (IL2RA +40606A>G) was associated (p=0.004; q=0.36) with primarily seronegative values for mumps as determined by the overall median antibody response to disease or vaccination (Table 4). Another synonymous SNP, rs1143634 (IL1B +3416A>G) was associated (p=0.007; q=0.46) with variations in the overall antibody level elicited post mumps vaccination/disease. However, these associations could not be confirmed in the subset of subjects without a history of mumps disease and who were vaccinated with a single dose of mumps vaccine. Further, no allele dose response relationships were observed for these SNPs. In addition, rs999788 (IL10RB -740C>T) was significantly associated (p=0.005) with variations in the median antibody response to mumps vaccination alone (Table 4). We also found significant (p≤0.01) associations between intronic SNPs in cytokine (IL1B) and cytokine receptor (IL2RA, IL4R and IL10RB) genes and mumps-specific antibodies (Supplementary Table 2)
Table 4.
Statistically significant associations between SNPs in coding and regulatory regions of cytokine and cytokine receptor genes and humoral immunity to mumps in Somali Study Subjects
| Gene | SNP ID | Position | Genotype | N | Median Ab EIA index units | Global P-value* | Global P-value! | Q-value** |
|---|---|---|---|---|---|---|---|---|
| IL2RA | rs2228149* | His172His | GG/GA/AA | 83/2/0 | 3.0/0.8/- | 0.004 | ns | 0.36 |
| IL1B | rs1143634 | Phe105Phe | GG/GA/AA | 44/40/1 | 2.4/3.3/2.3 | 0.007 | ns | 0.46 |
|
| ||||||||
| IL10RB | rs999788 | 5′ intergenic | CC/CT/TT | 49/10/1 | 2.8/3.5/1.8 | ns | 0.005 | |
A-Adenine, G-Guanine, T-Thymine, C-Cytosine, ns-non-significant (p-value >0.01)
Minor allele heterozygous or minor allele homozygous genotypes resulted in median antibody EIA index value in seronegativity range
Analysis of covariance P-value on 85 subjects adjusted for age, gender, history of disease and vaccination status
Analysis of covariance P-value on a subset on 60 subjects that were vaccinated with single dose of mumps vaccine and had no history of mumps disease, after adjusting for age and gender
- No subject for that genotype
Corresponding q-value based on the distribution of p-values for all 85 subjects across all three antigens and all 617 SNPs of interest
Cytokine and cytokine receptor SNPs associated with rubella antibodies
Overall, 12 SNPs regulating Th1 (IL2RA&B, IL12RB2) and inflammatory (IL6, TNFRSF1A, IFNB1) immunity to rubella were found to be significantly (p≤0.01) associated with variations in rubella-specific humoral immunity (Supplementary Table 3). Amongst these, eight associations had a single observation for the minor allele homozygous group and hence should be interpreted with caution as these significant p-values are not independent associations because they are in high LD with each other. Further, a strong LD association (r2≥0.95) between SNPs within IFNB1, IL2RA and IL18R1 genes was observed as well (Supplementary Table 3). The SNPs located in the coding and regulatory regions that are of higher relevance by our SNP selection algorithm are shown in Table 5. We found associations in an additional 16 SNPs in IL18R1, IL2RB, IL12RB2 and TNFRSF1B genes after subsetting to individuals vaccinated with a single dose of rubella vaccine and with no history of rubella disease (Supplementary Table 3).
Table 5.
Statistically significant associations between SNPs in coding and regulatory regions of cytokine and cytokine receptor genes and humoral immunity to rubella in Somali Study Subjects
| Gene | SNP ID | Position | Genotype | N | Median Ab EIA index unit | Global P-value* | Global P-value! | Q-value** |
|---|---|---|---|---|---|---|---|---|
| IFNB1 | rs7873167* | 3′ intergenic | AA/AC/CC | 60/24/1 | 2.7/2.6/0.1 | 0.001 | ns | 0.23 |
| IFNB1 | rs3885423* | 3′ intergenic | AA/AG/GG | 61/23/1 | 2.7/2.6/0.1 | 0.001 | ns | 0.23 |
| IFNB1 | rs1364613* | 3′ intergenic | AA/AC/CC | 61/23/1 | 2.7/2.6/0.1 | 0.001 | ns | 0.23 |
| IFNB1 | rs1364612* | 3′ intergenic | CC/CG/GG | 61/23/1 | 2.7/2.6/0.1 | 0.001 | ns | 0.23 |
| IL2RA | rs12722713 | 3′UTR | AA/AG/GG | 82/3/0 | 2.7/2.0/- | 0.001 | 0.001 | 0.23 |
| IL2RA | rs12722698 | Ile231Ile | GG/GA/AA | 82/3/0 | 2.7/2.0/- | 0.001 | 0.001 | 0.23 |
| IL2RB | rs228937 | 3′ intergenic | TT/TG/GG | 29/45/11 | 2.8/2.6/2.7 | 0.005 | ns | 0.37 |
| IL6 | rs2069824 | 5′ intergenic | TT/TC/CC | 71/14/0 | 2.7/2.5/- | 0.006 | ns | 0.46 |
| TNFRSF1A | rs4149650 | 3′UTR | AA/AG/GG | 80/5/0 | 2.6/3.2/- | 0.009 | 0.001 | 0.53 |
|
| ||||||||
| IL12RB2 | rs2307147 | Asp26Asp | TT/TC/CC | 55/9/0 | 2.6/3.1/- | ns | 0.005 | |
| IL18R1 | rs3732127 | 3′ UTR | CC/CT/TT | 31/27/6 | 2.6/2.8/2.5 | ns | 0.009 | |
A-Adenine, C-Cytosine, G-Guanine, T-Thymine, ns-non-significant (p-value >0.01)
Minor allele heterozygous or minor allele homozygous genotypes resulted in median antibody EIA index value in seronegativity range
Analysis of covariance P-value on 85 subjects adjusted for age, gender, history of disease and vaccination status
Analysis of covariance P-value on a subset on 64 subjects that were vaccinated with single dose of rubella vaccine and had no history of rubella disease, after adjusting for age and gender
- No subject for that genotype
Corresponding q-value based on the distribution of p-values for all 85 subjects across all three antigens and all 617 SNPs of interest
The homozygous minor allele variants for four SNPs located in the 3′ intergenic region of the IFNB1 gene were associated (p≤0.001; q=0.23) with an overall seronegative status in response to rubella disease or vaccination. However, these results had low subject representation for the homozygous minor allele and could not be confirmed in the subset cohort which had no history of rubella disease and who had received a single dose of rubella vaccine (Table 5). SNPs rs12722713 (IL2RA +49334A>G) and rs12722698 (IL2RA +44057A>G) that demonstrated strong LD and rs4149650 (TNFRSF1A+13452A>G), were associated with variations in both the overall humoral immunity to rubella virus from vaccination and/or disease, and humoral immunity to vaccination alone (Table 5). Additionally, rs228937 (IL2RB +19241G>T) and rs2069824 (IL6 -1650C>T) were associated with variations in overall humoral immunity to rubella, and rs2307147; (IL12RB2 +1234C>T) and rs3732127 (IL18R1 +34629C>T) were associated with rubella vaccine-induced humoral immunity (Table 5).
Further, the associations found between intronic SNPs in the cytokine (IFNB1, IL6 and cytokine receptor (IL2RA, IL2RB, IL12RB2, TNFRSF1A, TNFRSFIB and IL18R1) genes and rubella-specific antibodies in are shown in supplementary Table 3.
Discussion
Immune response to vaccines is a complex phenomenon which is controlled by numerous factors. Diverse ethnic and racial trends in the distribution of allelic frequencies for SNPs in the immunomodulatory genes have been identified [12,13]. These racial and ethnic genetic variations are associated with differences in disease susceptibility or outcome of immune response [24–26] In recent years, it has been shown that the genetic architecture of key regulatory molecules, such as cytokine and cytokine receptors, play an important role in regulation of the immune response. The exploration of the associations between correlates of immune response to vaccines and SNPs is of immense interest in the design of new vaccines. The data from this study suggests that specific SNPs present in cytokine and cytokine receptor genes involved in innate and adaptive immunity may be associated with variations in antibody responses to childhood vaccines, specifically measles, in a Somali population.
There are three major innate-response cytokines (TNFA, IL6 and IL1B) that are known to play a crucial role in infection, inflammation and immunity to various pathogens including viral diseases such as chronic hepatitis B virus (HBV) infection [26,27]. In our Somali cohort, we identified SNPs in the TNFA and the IL6 genes that were associated with seronegative status to measles immunity in response to single dose of measles vaccination and/or infection. The rs1799964 SNP which was associated with lower measles antibody response in our study is located in the 5′upstream regulatory region of the TNFA gene and is a part of a haplotype block containing five other SNPs [TNF-488A>G (rs1800629), TNF-418A>G (rs361525), LTA-379A>C (rs2239704), TNF -3448A>G (rs1800683) and rs2857713] [27]. This haplotype block covers all the common SNPs in the regulatory and coding regions with known functional importance from the TNFA and closely located lymphotoxin-alpha gene on chromosome 6p21 in African Americans [27], suggesting the potential role of SNPs in this haplotype block in modulating the host antibody responses indirectly by influencing their cytokine milieu. The TNFA gene is also located between the HLA-B and HLA-DR loci within the class III HLA region [28]. Based on the cell-type examined, the promoter region SNP rs1800629 in the TNFA gene has been shown to have mixed results, yielding both positive and negative allelic correlations with constitutive and inducible TNFa expression, and it is also strongly associated with higher HLA-DR3 expression [28,29].
It has been previously reported that genetic variations in the IL6 gene, which are predominant in African American populations, can alter expression of the co-stimulatory molecule B7 (CD80 and CD86) and hence influence the outcome of the host immune response [13,16,30]. The observed associations between SNPs in the TNFA, IL6 and IL1R and lower humoral immunity may be a result of alterations in immunological phenomenon, such as antigen presentation, co-stimulation and cytokine production. Polymorphisms in the coding and the regulatory regions of cytokine and cytokine receptor genes can directly or indirectly lead to an altered gene product and/or function. Further, interactions between cytokine/cytokine receptor SNPs, particularly those “tagged” with the genes controlling antigen processing and presentation such as HLA genes, can also cause phenotypic variability.
Several intronic SNPs in cytokine and cytokine receptor genes were associated with measles, mumps and rubella-specific antibodies in our subjects. Although no direct relevance or mechanism of action by which intronic SNPs influence gene function has yet been reported, indirect mechanisms of action have been proposed. For example, intronic SNPs can prevent correct splicing of introns resulting in premature stop codons or exon deletions and hence lead to the generation of cryptic splice sites or aberrant mRNA [31–34].
We have previously reported associations between specific SNPs in Th1 and Th2 cytokine/cytokine receptor genes and measles and mumps-specific immune responses following two doses of MMR vaccination in a cohort of Caucasians [9,10]. Several SNPs located in the IL2, IL10, and IL12RB2 genes were found to be significantly associated with measles vaccine induced antibody and lymphoproliferative responses [9]. Further, we identified minor allele variants for four SNPs located in the IL4R gene associated with higher levels of secreted IL4 following measles vaccination [9]. Similarly, we identified four SNPs in the IL10RA, IL12RB cytokine-receptor genes that were significantly associated with humoral and cellular responses to mumps vaccine [10]. These studies suggest an important role for both Th1 (IL2 and IL12) and Th2 (IL4 and IL10) cytokine and/or cytokine receptor genes in regulating the adaptive immune responses to measles and mumps vaccines. In this current study of Somali subjects, we confirmed that majority of the Th1 (IL2R, IL12RB) and Th2 (IL4R and IL10RB) cytokine receptor genes identified in Caucasians were also associated with humoral immune response to measles and mumps vaccine. However, we did not see overlap between individual SNPs within these cytokine/cytokine receptor genes in Caucasian and Somali populations. These results support the previous findings in the literature that genetic heterogeneity exists in immunomodulatory genes across distinct racial populations [11–16]. However, there may be other contributory factors to this disparity in our study such as small sample size, subject selection based on antibody and cellular response in the Caucasian cohort, number of MMR doses and the presence of disease/exposure history in a subset of the Somali subjects.
Amongst the Th1/Th2 cytokines significantly associated with variations in immune response to MMR vaccine, IL4, IL10 and IL12 are central immunoregulatory cytokines with important effects on antibody producing B-cells [35–38]. IL4 is a pleiotropic cytokine that drives naive CD4 T cells to differentiate into Th2 phenotype [35]. IL10 is a broad anti-inflammatory cytokine produced by the Th2 and regulatory T cells. Its suppressive role is mediated by inhibiting macrophage and dendritic cell functions by interfering with the production of pro-inflammatory cytokines such as IL12 and reducing the cell surface expression of co-stimulatory and MHC class II molecules, which may result in decreased antibody responses [36,37]. IL12 is another multifunctional cytokine that bridges innate and adaptive immunity by polarizing naïve CD4 T cells to differentiate and proliferate into the Th1 phenotype [38]. Genetic variations in these Th1/Th2 cytokines, in combination with innate-response cytokines such as IL6 and TNFA, may result in variations in antibody response to viral vaccines by influencing multiple mechanisms such as antigen presentation, co-stimulation, cell differentiation and proliferation, and cytokine secretion.
There are several limitations to this study. Our study is based on a relatively small number of subjects. Therefore, we had a very low representation of subjects for the homozygous minor allele group in some SNPs, giving us less confidence in these results and limiting our ability to draw firm conclusions. Further, it is also possible we missed other important associations due to the limitations in power resulting from a small sample size. Assuming a Type I error rate of 0.01, a two-sided test of hypothesis, a minor allele frequency of 0.2, and a dominant genotypic effect, we would have 36% power to detect an effect size of 0.5 standard deviations with our study cohort of 85 subjects, and 22% power to detect the same effect size with 55 subjects, i.e. those with no previous history of measles disease and one prior measles vaccination. In addition, many tests were performed; hence the possibility of false positive results exists. Our primary analyses examined 617 SNPs across three antigens for all 85 subjects, resulting in a total of 1854 tests. Assuming independent tests of association, we would expect about 18.5 tests to be statistically significant at the p=0.01 level. We found a total of 31 significant tests, which is 1.7-fold more than expected. This gives us some confidence that at least some of our results are not false positives. However, not all of these tests can be considered independent as some SNPs are in linkage disequilibrium. This makes it difficult to explicitly enumerate the exact range for the number of expected associations. A larger sample size consistent with current guidelines for significant P-values in SNP association studies for candidate genes in humans is critical to validating our preliminary results [39,40]. Our study was also limited to measuring whole virus-specific antibodies due to cost effectiveness instead of neutralizing antibodies, which is a better correlate of protective antibody titers [41].
In conclusion, studies such as ours are important in understanding associations between polymorphisms in immune response genes and inter-individual variations in immune response to vaccines. Further, as the directed design of new vaccines becomes increasingly informed by immunogenetic considerations, race and ethnicity-specific immune response gene polymorphism data will be important [42].
Supplementary Material
Acknowledgments
We thank the subjects who participated in this study. We acknowledge the efforts of the research fellows, nurses and students from the Mayo Vaccine Research Group. We thank Julie Cunningham, Ph.D and Yanhong Wu, Ph. D for assistance with genotyping in the Mayo Advanced Genomic Technology Center. We thank Mahamoud Jimale, M.D. for assistance in subject recruitment. We thank Cheri Hart for her editorial assistance.
Footnotes
Dr. Gregory A. Poland is the chair of a DMSB for non-measles based vaccines in development by Merck Research Laboratories.
This work was supported by NIH grants N01-AI-40065, AI48793 and AI33144.
Requests for reprints should be directed to Dr. Gregory A. Poland via e-mail at poland.gregory@mayo.edu
References
- 1.Haring JS, Badovinac VP, Harty JT. Inflaming the CD8+ T cell response. Immunity. 2006;25:19–29. doi: 10.1016/j.immuni.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Chabalgoity JA, Baz A, Rial A, Grille S. The relevance of cytokines for development of protective immunity and rational design of vaccines. Cytokine Growth Factor Rev. 2007;18:195–207. doi: 10.1016/j.cytogfr.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Keen LJ. The extent and analysis of cytokine and cytokine receptor gene polymorphism. Transpl Immunol. 2002;10:143–146. doi: 10.1016/s0966-3274(02)00061-8. [DOI] [PubMed] [Google Scholar]
- 4.Hollegaard MV, Bidwell JL. Cytokine gene polymorphism in human disease: online databases, Supplement 3. Genes Immun. 2006;7:269–276. doi: 10.1038/sj.gene.6364301. [DOI] [PubMed] [Google Scholar]
- 5.Bidwell J, Keen L, Gallagher G, Kimberly R, Huizinga T, McDermott MF, Oksenberg J, McNicholl J, Pociot F, Hardt C, D’Alfonso S. Cytokine gene polymorphism in human disease: on-line databases. Genes Immun. 1999;1:3–19. doi: 10.1038/sj.gene.6363645. [DOI] [PubMed] [Google Scholar]
- 6.Bidwell J, Keen L, Gallagher G, Kimberly R, Huizinga T, McDermott MF, Oksenberg J, McNicholl J, Pociot F, Hardt C, D’Alfonso S. Cytokine gene polymorphism in human disease: on-line databases: Supplement 1. Genes Immun. 2001;2:61–70. doi: 10.1038/sj.gene.6363733. [DOI] [PubMed] [Google Scholar]
- 7.Haukim N, Bidwell JL, Smith AJ, Keen LJ, Gallagher G, Kimberly R, Huizinga T, McDermott MF, Oksenberg J, McNicholl J, Pociot F, Hardt C, D’Alfonso S. Cytokine gene polymorphism in human disease: on-line databases: Supplement 2. Genes Immun. 2002;3:313–330. doi: 10.1038/sj.gene.6363881. [DOI] [PubMed] [Google Scholar]
- 8.Hohler T, Reuss E, Freitag CM, Schneider PM. A functional polymorphism in the IL-10 promoter influences the response after vaccination with HBsAg and hepatitis A. Hepatology. 2005;42:72–76. doi: 10.1002/hep.20740. [DOI] [PubMed] [Google Scholar]
- 9.Dhiman N, Ovsyannikova IG, Cunningham JM, Vierkant RA, Kennedy RB, Pankratz VS, Poland GA, Jacobson RM. Associations between measles vaccine immunity and single nucleotide polymorphisms in cytokine and cytokine receptor genes. J Infect Dis. 2007;195:21–29. doi: 10.1086/510596. [DOI] [PubMed] [Google Scholar]
- 10.Ovsyannikova IG, Jacobson RM, Dhiman N, Vierkant RA, Pankratz VS, Poland GA. HLA and cytokine receptor gene polymorphisms associated with heterogeneous immune responses to mumps viral vaccine. Pediatrics. 2008;121:e1091–1099. doi: 10.1542/peds.2007-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassan MI, Aschner Y, Manning CH, Xu J, Aschner JL. Racial differences in selected cytokine allelic and genotypic frequencies among healthy, pregnant women in North Carolina. Cytokine. 2003;21:10–16. doi: 10.1016/s1043-4666(02)00489-1. [DOI] [PubMed] [Google Scholar]
- 12.Martin AM, Athanasiadis G, Greshock JD, Fisher J, Lux MP, Calzone K, Rebbeck TR, Weber BL. Population frequencies of single nucleotide polymorphisms (SNPs) in immuno-modulatory genes. Hum Hered. 2003;55:171–178. doi: 10.1159/000073201. [DOI] [PubMed] [Google Scholar]
- 13.Girnita DM, Webber SA, Ferrell R, Burckart GJ, Brooks MM, McDade KK, Chinnock R, Canter C, Addonizio L, Bernstein D, Kirklin JK, Girnita AL, Zeevi A. Disparate distribution of 16 candidate single nucleotide polymorphisms among racial and ethnic groups of pediatric heart transplant patients. Transplantation. 2006;82:1774–1780. doi: 10.1097/01.tp.0000250656.33731.08. [DOI] [PubMed] [Google Scholar]
- 14.Lazarus R, Klimecki WT, Palmer LJ, Kwiatkowski DJ, Silverman EK, Brown A, Martinez F, Weiss ST. Single-nucleotide polymorphisms in the interleukin-10 gene: differences in frequencies, linkage disequilibrium patterns, and haplotypes in three United States ethnic groups. Genomics. 2002;80:223–228. doi: 10.1006/geno.2002.6820. [DOI] [PubMed] [Google Scholar]
- 15.Lazarus R, Vercelli D, Palmer LJ, Klimecki WJ, Silverman EK, Richter B, Riva A, Ramoni M, Martinez FD, Weiss ST, Kwiatkowski DJ. Single nucleotide polymorphisms in innate immunity genes: abundant variation and potential role in complex human disease. Immunol Rev. 2002;190:9–25. doi: 10.1034/j.1600-065x.2002.19002.x. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann SC, Stanley EM, Cox ED, DiMercurio BS, Koziol DE, Harlan DM, Kirk AD, Blair PJ. Ethnicity greatly influences cytokine gene polymorphism distribution. Am J Transplant. 2002;2:560–567. doi: 10.1034/j.1600-6143.2002.20611.x. [DOI] [PubMed] [Google Scholar]
- 17.St Sauver JL, Jacobson RM, Vierkant RA, Jacobsen SJ, Green EM, Poland GA. Association of parental vaccination reports with measles, mumps, and rubella protective antibody levels: comparison of Somali immigrant, Hispanic immigrant, and US children in Rochester, Minnesota. Mayo Clinic Proceedings. 2002;77:241–245. doi: 10.4065/77.3.241. [DOI] [PubMed] [Google Scholar]
- 18.Nysse LJ, Pinsky NA, Bratberg JP, Babar-Weber AY, Samuel TT, Krych EH, Ziegler AW, Jimale MA, Vierkant RA, Jacobson RM, Poland GA. Seroprevalence of antibody to varicella among Somali refugees. Mayo Clin Proc. 2007;82:175–180. doi: 10.4065/82.2.175. [DOI] [PubMed] [Google Scholar]
- 19.Boteler WL, Luipersbeck PM, Fuccillo DA, O’Beirne AJ. Enzyme-linked immunosorbent assay for detection of measles antibody. J Clin Microbiol. 1983;17:814–818. doi: 10.1128/jcm.17.5.814-818.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.den Dunnen JT, Antonarakis SE. Nomenclature for the description of human sequence variations. Hum Genet. 2001;109:121–124. doi: 10.1007/s004390100505. [DOI] [PubMed] [Google Scholar]
- 21.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 22.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Storey JD. A direct approach to false discovery rates. J R Statist Soc B. 2002;64:479–498. [Google Scholar]
- 24.Selvaraj P, Sriram U, Mathan KS, Reetha AM, Narayanan PR. Tumour necrosis factor alpha (−238 and −308) and beta gene polymorphisms in pulmonary tuberculosis: haplotype analysis with HLA-A, B and DR genes. Tuberculosis (Edinb) 2001;81:335–341. doi: 10.1054/tube.2001.0307. [DOI] [PubMed] [Google Scholar]
- 25.McGuire W, Hill AV, Allsopp CE, Greenwood BM, Kwiatkowski D. Variation in the TNF-alpha promoter region associated with susceptibility to cerebral malaria. Nature. 1994;371:508–510. doi: 10.1038/371508a0. [DOI] [PubMed] [Google Scholar]
- 26.Hohler T, Kruger A, Gerken G, Schneider PM, Meyer zum Buschenefelde KH, Rittner C. A tumor necrosis factor-alpha (TNF-alpha) promoter polymorphism is associated with chronic hepatitis B infection. Clin Exp Immunol. 1998;111:579–582. doi: 10.1046/j.1365-2249.1998.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belfer I, Buzas B, Hipp H, Dean M, Evans C, Lorincz I, Max MB, Goldman D. Haplotype structure of inflammatory cytokines genes (IL1B, IL6 and TNF/LTA) in US Caucasians and African Americans. Genes Immun. 2004;5:505–512. doi: 10.1038/sj.gene.6364118. [DOI] [PubMed] [Google Scholar]
- 28.Kroeger KM, Carville KS, Abraham LJ. The -308 tumor necrosis factor-alpha promoter polymorphism effects transcription. Mol Immunol. 1997;34:391–399. doi: 10.1016/s0161-5890(97)00052-7. [DOI] [PubMed] [Google Scholar]
- 29.Brinkman BM, Zuijdeest D, Kaijzel EL, Breedveld FC, Verweij CL. Relevance of the tumor necrosis factor alpha (TNF alpha) −308 promoter polymorphism in TNF alpha gene regulation. J Inflamm. 1995;46:32–41. [PubMed] [Google Scholar]
- 30.Hutchings A, Guay-Woodford L, Thomas JM, Young CJ, Purcell WM, Pravica V, Perrey C, Hutchinson IV, Benfield MR. Association of cytokine single nucleotide polymorphisms with B7 costimulatory molecules in kidney allograft recipients. Pediatr Transplant. 2002;6:69–77. doi: 10.1034/j.1399-3046.2002.1o444.x. [DOI] [PubMed] [Google Scholar]
- 31.Silverman TA, Noguchi M, Safer B. Role of sequences within the first intron in the regulation of expression of eukaryotic initiation factor 2 alpha. J Biol Chem. 1992;267:9738–9742. [PubMed] [Google Scholar]
- 32.Law AJ, Kleinman JE, Weinberger DR, Weickert CS. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum Mol Genet. 2007;16:129–141. doi: 10.1093/hmg/ddl449. [DOI] [PubMed] [Google Scholar]
- 33.Aretz S, Uhlhaas S, Sun Y, Pagenstecher C, Mangold E, Caspari R, Moslein G, Schulmann K, Propping P, Friedl W. Familial adenomatous polyposis: aberrant splicing due to missense or silent mutations in the APC gene. Hum Mutat. 2004;24:370–380. doi: 10.1002/humu.20087. [DOI] [PubMed] [Google Scholar]
- 34.Yu Y, Panhuysen C, Kranzler HR, Hesselbrock V, Rounsaville B, Weiss R, Brady K, Farrer LA, Gelernter J. Intronic variants in the dopa decarboxylase (DDC) gene are associated with smoking behavior in European-Americans and African-Americans. Hum Mol Genet. 2006;15:2192–2199. doi: 10.1093/hmg/ddl144. [DOI] [PubMed] [Google Scholar]
- 35.De Vries JE, Carballido JM, Aversa G. Receptors and cytokines involved in allergic TH2 cell responses. J Allergy Clin Immunol. 1999;103:S492–S496. doi: 10.1016/s0091-6749(99)70166-1. [DOI] [PubMed] [Google Scholar]
- 36.Wu K, Bi Y, Sun K, Wang C. IL-10-producing type 1 regulatory T cells and allergy. Cell Mol Immunol. 2007;4:269–275. [PubMed] [Google Scholar]
- 37.O’Garra A, Vieira P. T(H)1 cells control themselves by producing interleukin-10. Nat Rev Immunol. 2007;7:425–428. doi: 10.1038/nri2097. [DOI] [PubMed] [Google Scholar]
- 38.Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, Parmiani G, Anichini A. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13:4677–4685. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- 39.Freimer NB, Sabatti C. Guidelines for association studies in Human Molecular Genetics. Hum Mol Genet. 2005;14:2481–2483. doi: 10.1093/hmg/ddi251. [DOI] [PubMed] [Google Scholar]
- 40.Ioannidis JP, Trikalinos TA, Ntzani EE, Contopoulos-Ioannidis DG. Genetic associations in large versus small studies: an empirical assessment. Lancet. 2003;361:567–571. doi: 10.1016/S0140-6736(03)12516-0. [DOI] [PubMed] [Google Scholar]
- 41.Chen RT, Markowitz LE, Albrecht P, Stewart JA, Mofenson LM, Preblud SR, Orenstein WA. Measles antibody: Reevaluation of protective titers. J Infect Dis. 1990;162:1036–1042. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- 42.Poland GA, Ovsyannikova IG, Jacobson RM, Smith DI. Heterogeneity in vaccine immune response: The role of immunogenetics and the emerging field of vaccinomics. Clin Pharmacol Ther. 2007;82:653–664. doi: 10.1038/sj.clpt.6100415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.